Published online Nov 15, 2018. doi: 10.4251/wjgo.v10.i11.398
Peer-review started: July 1, 2018
First decision: July 17, 2018
Revised: September 25, 2018
Accepted: October 7, 2018
Article in press: October 7, 2018
Published online: November 15, 2018
Gastric cancer (GC) is the third most common cancer-related cause of death worldwide. In locally advanced tumors, neoadjuvant chemotherapy has recently been introduced in most international Western guidelines. For metastatic and unresectable disease, there is still debate regarding correct management and the role of surgery. The standard approach for stage IV GC is palliative chemotherapy. Over the last decade, an increasing number of M1 patients who responded to palliative regimens of induction chemotherapy have been subsequently undergone surgery with curative intent. The objective of the present review is to analyze the literature regarding this approach, known as “conversion surgery”, which has become one of the most commonly adopted therapeutic options. It is defined as a treatment aiming at an R0 resection after chemotherapy in initially unresectable tumors. The 13 retrospective studies analyzed, with a total of 411 patients treated with conversion therapy, clearly show that even if standardization of unresectable and metastatic criteria, post-chemotherapy resectability evaluation and timing of surgery has not yet been established, an R0 surgery after induction chemotherapy with partial or complete response seems to offer superior survival results than chemotherapy alone. Additional larger sample-size randomized control trials are needed to identify subgroups of well-stratified patients who could benefit from this multimodal approach.
Core tip: Conversion surgery is defined as a surgical treatment with the goal of R0 resection in initially unresectable gastric cancer patients after response to chemotherapy. Although the heterogeneity of metastatic disease factors makes it difficult to identify true prognostic variables, a survival benefit has been demonstrated in several reports. Further prospective large-scale studies seem to be necessary to improve patient selection and to validate this promising multimodal therapy.
- Citation: Zurleni T, Gjoni E, Altomare M, Rausei S. Conversion surgery for gastric cancer patients: A review. World J Gastrointest Oncol 2018; 10(11): 398-409
- URL: https://www.wjgnet.com/1948-5204/full/v10/i11/398.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i11.398
Gastric cancer (GC) is known to be the third most common cancer-related cause of death worldwide[1]. Surgical treatment with adequate extended lymphadenectomy is associated with good outcomes in early stages. However, in advanced GC, prognosis remains poor. Neoadjuvant chemotherapy (NAC) has been suggested for resectable, locally advanced GC based on well-known Randomized Controlled Trial (RCT)s[2,3]. Despite many enrolled patients having lower esophagus or esophagogastric junction involvement and surgery not always including a standard extended lymphadenectomy, there was a survival advantage of NAC plus surgery compared to surgery alone. Therefore, NAC, or preferably preoperative chemotherapy, has been recently introduced as an option in most treatment guidelines[4-9].
The SEER database shows that one third of Western patients with GC have unresectable disease, and different strategies have recently been adopted to manage advanced unresectable cancer[10]. Generally, in these cases, surgery is upfront considered as a palliative treatment for obstruction or bleeding.
Palliative chemotherapy remains the main treatment strategy of IV stage GC patients[11]. Although the median survival time (MST) of these patients has improved due to development of new chemotherapeutics agents, it is still unsatisfactory. Therefore, patients who demonstrated a response to chemotherapy have begun to be subsequently surgically treated with curative intent. This approach in stage IV patients, called “conversion surgery”, is becoming one of the most common therapeutic options discussed in the literature over the last decades. The aim of the present review was to define the effective usefulness of this strategy, to identify its crucial aspects and to highlight critical issues and implications for future perspectives.
We analyzed articles published in English from 1997 to 2017 using the following key words: Conversion surgery, conversion therapy, R0 resection stage IV GC, unresectable GC. We excluded case reports and case series, ultimately obtaining 13 articles for 13 studies. We first analyzed stage IV factors singularly to define major current therapeutic strategies for any selected patient, and then, we considered oncological outcomes of palliative chemotherapy through experiences derived from several trials. Therefore, we focused on the emerging role of conversion therapy as a new treatment option for metastatic gastric cancer patients.
Stage IV GC is a heterogeneous biological condition with a mixture of distant metastases, including hematologic, lymph nodal and/or peritoneal. To reduce this heterogeneity, the Japanese Gastric Cancer Association (JGCA) and the Union Internationale Contre le Cancer (UICC) minimized differences between their classifications and categorized similar groups[12-16]. However, these systems do not seem sufficient to derive any significant clinical suggestions.
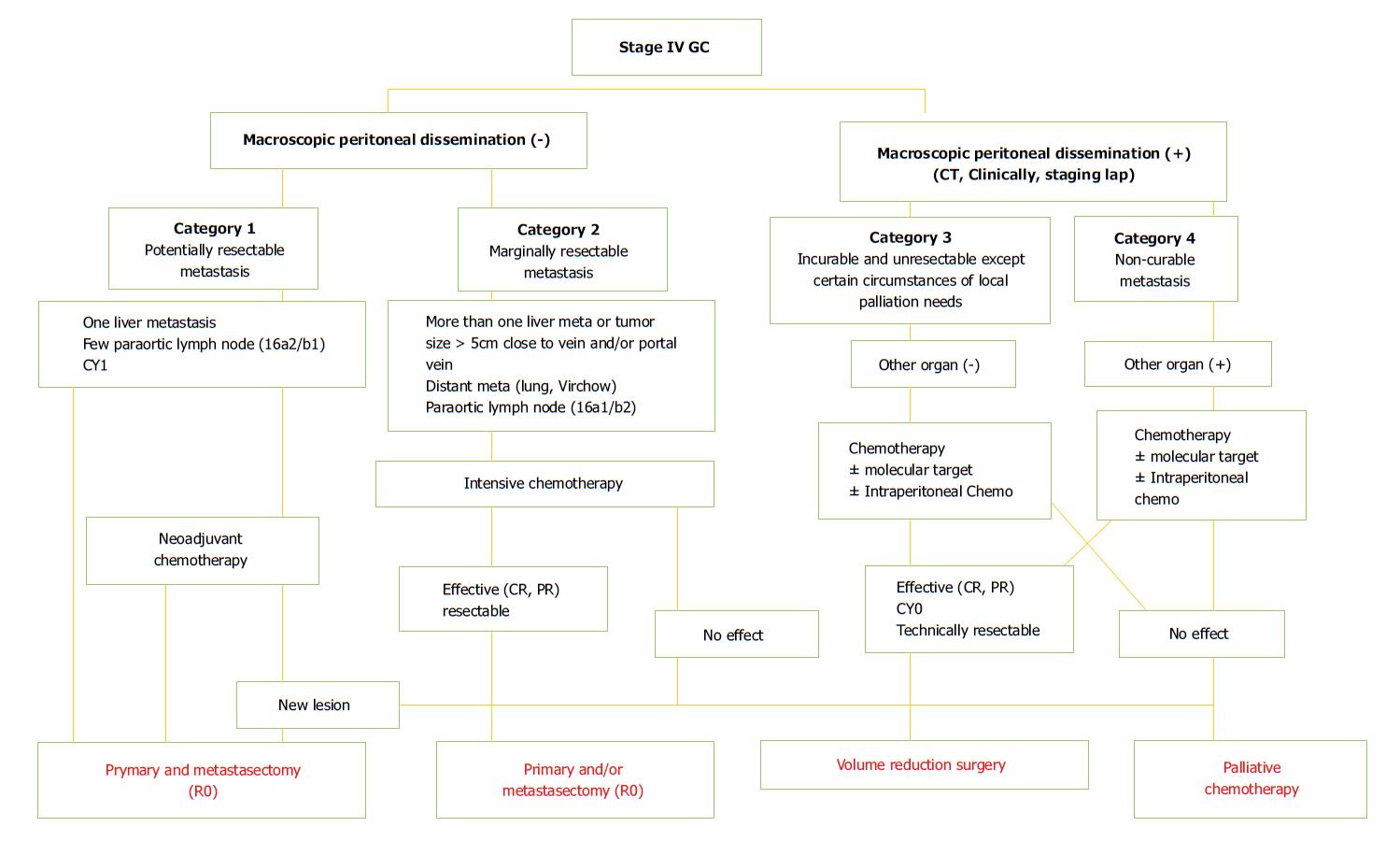
In the recent classification introduced by Yoshida et al[17] with the proposal to identify objective principles for conversion surgery, stage IV patients were subdivided into 4 new categories (Figure 1). Initially, the presence of macroscopic peritoneal dissemination is considered as a different biological and prognostic finding compared with hematological metastases. Patients without peritoneal involvement belong to category 1 (potentially resectable metastases) and category 2 (marginally resectable metastases). Patients with macroscopic peritoneal metastases are stratified into category 3 (unresectable except certain situations) and category 4 (incurable metastases). Below we highlight different critical aspects in terms of staging, treatment and prognosis of different potential metastatic patterns in stage IV GC.
Synchronous peritoneal carcinomatosis (PC) is the most frequent site of metastasis in stage IV GC. PC occurs in 14%-43% of GC patients and represents 35% of all synchronous metastases[18,19]. The prognosis of PC in GC is worse than that for other metastatic sites[20,21]. Peritoneal dissemination of GC is a dynamic multistep process that involves several molecules acting in a coordinated way. As reported in a recent review by Kanda et al[22], there are 4 steps in peritoneal dissemination: (1) migration to the abdominal cavity after detachment of cells from the tumor; (2) adaptation to the abdominal microenvironment; (3) adhesion to mesothelial cells and invasion of the baseline membrane; and (4) growth and angiogenesis of the tumor. These molecular mechanisms are very challenging because identification of a single pathway is not necessarily correlated with disease prognosis.
Survival of patients with PC is poor, despite the progress of chemotherapy. Hence, PC is often considered a determinant for a “real” curative treatment possibility, and several scoring systems on extension of PC have been validated to accurately discriminate treatment options, stratify patients prognosis, and, consequently, correct statistical analyses[23-25]. Okabe et al[26] noted that in curatively (R0) resected patients, after disappearance of limited peritoneal dissemination treated with induction therapy (S-1 plus cisplatin), MST was significantly longer (43.2 mo) than in patients who underwent non-curative resection (12.6 m), as well as in patients without surgery (10.3 m). To increase chemotherapy efficacy for PC, the literature suggests an additional benefit of hyperthermic intraperitoneal administration of drugs (hyperthermic intraperitoneal chemotherapy, HIPEC)[27-31].
Recent advances in multimodal treatment for patients with peritoneal dissemination are highlighted by Ishigami et al[32] in the PHOENIX-GC trial that, although failing to show statistical superiority for intraperitoneal paclitaxel plus systemic chemotherapy, suggested possible clinical benefit for this treatment option. In a systematic review of 10 studies considering 441 patients treated with cytoreductive surgery plus HIPEC, a median overall survival of 15 mo after radical (R0) cytoreduction was shown by Gill et al[33]. Consistently, the phase III randomized trial by Yang et al[34] and the GYMSSA trial reported improved survival rates with surgery plus HIPEC compared with surgery alone[35].
Many patients with stage IV GC have multiple metastatic sites. Usually, the first site of metastasis occurring through the hematogenous pathway is the liver. Systemic chemotherapy is a standard treatment approach for GC patients with liver metastases[36], recommended by both the National Comprehensive Cancer Network (NCCN) Guidelines and the Japanese Guidelines[37,38]. Surgical resection has been recently reported to prolong survival in highly selected patients[39-41]. Li et al[42] reported a 100% response rate after chemotherapy with weekly DCF regimen before curative gastrectomy in 8 patients. A multidisciplinary approach, including surgery in selected GC patients when the liver is the only site of metastasis, is associated with interesting results[43]. However, treatment of synchronous or metachronous hepatic metastases is not well standardized in GC patients. Once combined with gastrectomy and extended lymphadenectomy, there are no differences in 5-year survival after resection of synchronous and metachronous liver metastases[44]. Considering metachronous metastases, patients submitted to surgery benefit from better selection and exhibit good survival over short and medium terms[45]. Surgical treatment of the best subgroups of candidates can achieve good results that should encourage surgeons and medical oncologists[41,46].
A proper lymphadenectomy during surgical resection is a milestone for GC treatment. Patients with para-aortic lymph node (PAN) metastases, or bulky nodes around the hepatic, splenic, or celiac arteries are considered unresectable. Some retrospective studies demonstrated the presence of PAN metastases in greater than 20% of patients undergoing D2 + PAN dissection, and 5-year survival rates of patients with PAN metastases do not exceed 20%[47,48]. Furthermore, a phase III trial JCOG9501 comparing D2 nodal dissection with or without PAN dissection for GC concluded that prophylactic PAN dissection does not improve survival rates[49]. Interestingly, patients with macroscopic metastases in these nodes were excluded from analysis, resulting in a low incidence of metastatic n° 16 nodes in patients receiving PAN dissection. This “selection bias” left open the issue of prognostic efficacy of removal of PAN station in PAN metastatic patients[50]. On the other hand, since 2000, three phase II trials (JCOG0001, JCOG0405 and JCOG1002) have explored preoperative/induction chemotherapy and PAND gastrectomy for bulky N2/N3 gastric cancer[51-54]. The JCOG0001 study reported a low 3-year survival rate (27%) after 2-3 cycles of irinotecan and cisplatin followed by surgery. Conversely, the JCOG0405 trial demonstrated an excellent response rate (up to 64.7%) with 3-year survival of 58.8% in patients who received 2-3 cycles of cisplatin and S-1 before surgery. Similarly, in the JCOG1002 study, among 52 eligible patients, 48 underwent surgery, 44 with R0 resection (84.6%), after 2-3 cycles of docetaxel, cisplatin and S-1 with a pathological response rate of 50%.
As specified above, according to current guidelines, palliative chemotherapy is the main strategy for treatment of stage IV GC patients. These cases have always represented the ideal setting for use of many new combinations of chemotherapeutic agents, both in Japan and in Western countries[55-67]. The median overall survival observed in these studies varies between 3 and 17 mo. In the SPIRIT trial, an overall survival of 13 mo was reported using S-1 plus cisplatin, which is defined as the standard treatment for metastatic GC in Japan[56]. In Western countries, the treatment most commonly used for metastatic GC is a combination of chemotherapy regimens, including fluoropyrimidine plus a platinum agent, though epirubicin or docetaxel can also be combined[64,66]. Recent developments in chemotherapeutic and molecular targeted agents have added new clinical issues in the management of incurable GC. As reported in the ToGA trial, Trastuzumab plus chemotherapy in HER2-positive patients improved overall median survival from 11.1 to 13.8 mo[60]. In addition, histological biomarkers have been identified to predict survival among GC patients[68]. Recently, palliative chemotherapy seemed further validated compared with palliative surgery by results of the REGATTA trial. In fact, although some authors emphasized the beneficial role of palliative gastrectomy[69,70], in this RCT, Fujitani et al[71] demonstrated no survival benefit for palliative gastrectomy prior to chemotherapy in advanced GC patients with a single non-curative factor. However, the methodological biases of the REGATTA trial negatively affect reliability of its results and weaken its potential clinical implications[72]. Therefore, at the moment, for stage IV GC patients, we have no strong evidence to consider the results of palliative chemotherapy satisfactory. On the other hand, we also have no reliable data to suggest definitely abandoning surgery.
The heterogeneous presentation of stage IV GC characteristics makes it difficult to identify the best therapeutic strategy for these tumors due to their different biological behaviors. On the other hand, given the poor results achieved with chemotherapy alone, in order to further improve survival of these patients, new therapeutic approaches have been considered. Based on experiences of the multidisciplinary treatment of metastatic colorectal cancer, in the last 2 decades, many studies have been conducted to evaluate efficacy of the combination of chemotherapy and surgery for stage IV GC. Surgical resection for advanced tumors has historically been called “radical”, “salvage”, “adjuvant” or “secondary” gastrectomy. More specifically, the concept of conversion surgery has been recently treated by Yoshida[17] to define a treatment aiming to R0 resection after chemotherapy in initially unresectable patients.
Tables 1 and 2 show patient characteristics and treatment options analyzed in the considered studies, as well as survival results. Below, we discuss in chronological order the main results of these studies, with particular focus on potential prognostic factors in conversion surgery strategy.
| Reference | Period | Population (conversion surgery) | Median - age | Unresectable criteria | Chemotherapy | Surgery | Lymphadenectomy (D2 or more) | Ro | |||||
| P1 | H1 | Cy1 | PAN/N3 | T4 | Other | ||||||||
| Nakajima et al[73], 1997 | 1989-1995 | 30 (19) | 53 | 9 (30%) | 11 (37%) | 23 (77%) | 8 (27%) | 3 (10%) | FLEP | NS | NS | 9 (30%) | |
| Yano et al[74], 2002 | May 1994-Dec 1999 | 34 (14) | 54.4 (31-73) | 26 (76%) | 4 (12%) | 10 (3.4%) | 12 (35%) | 1 (0.3%) | FEMTXP or THP-FLPM | NS | NS | 8 (24%) | |
| Satoh et al[75],2012 | May 2003-Mar 2008 | 51 (44) | 63 (35-79) | 24 (49%) | 3 (6%) | 12 (23%) | 7 (14%) | 5 (10%) | S1 + Cisplatin | TG (58%) | 82% | 26 (51%) | |
| DG (21.5%) | |||||||||||||
| Kanda et al[76],2012 | Apr 2000-Mar 2008 | 31(28) | 65.5 (49-79) | 7 (25%) | 4 (14.3%) | 15 (54%) | 9 (32%) | S1 + Cisplatin or Paclitaxel or Irinotecan | TG (42.89%) | 96.30% | 26 (93%) | ||
| DG (57.1%) | |||||||||||||
| Han et al[77], 2014 | Jan 2000-Dec 2009 | 34 (34) | 56 (28-71) | 7 (14%) | 5 (10%) | 15 (29.4%) | 7 (14%) | 5-FU + Platinum or 5-FU + Platinum + Taxane | NS | NS | 26 (76.5%) | ||
| Kim et al[78], 2014 | Jan 2003-Dec 2012 | 43 (18) | 52.8 (32-72) | 43 (100%) | 5-FU + Cisplatin or S1 + Cisplatin | TG (72.2%) | 100% | 10 (55%) | |||||
| DG (27.7%) | |||||||||||||
| Fukuchi et al[79], 2015 | Feb 2003-Dec 2013 | 151 (40) | 66 (31-79) | 11 (28%) | 5 (13%) | 3 (8%) | 6 (15%) | 26 (65%) | S1 + Cisplatin or S1 + Paclitaxel | TG (72.5%) | NS | 32 (80%) | |
| DG (27.5%) | |||||||||||||
| Kinoshita et al[80], 2015 | Apr 2006-Mar 2012 | 57 (34) | 65 (30-78) | 15 (26%) | 18 (32%) | 23 (40%) | 2 (3.5%) | DCS | TG (64.7%) | 50% | 27 (79%) | ||
| DG (26.5%) | |||||||||||||
| Sato et al[81], 2017 | Dec 2002-Apr 2014 | 100 (33) | 63 (26-78) | 33 (33%) | 29 (29%) | 61 (61%) | 14 (14%) | 11 (11%) | DCS I line, CPT-11 II line | TG (84.8%) | 100% | 28 (85%) | |
| DG (12.1%) | |||||||||||||
| Einama et al[82],2017 | Jan 2009-Dec 2015 | 10 | 70.5 (59-86) | 3 (30%) | 1 (10%) | 1 (10%) | 4 (40%) | 1 (10%) | S1 + CDDP or DOC | TG (40%) | 100% | 10 (100%) | |
| DG (30%) | |||||||||||||
| Mieno et al[83],2017 | Oct 2006-Dec 2012 | 31 (31) | 63 (35-78) | 25% | 16% | 58% | 26% | DCS + DS (Docetaxel-S1) in responder patients | TG (74.2%) | 77% | 23 (74%) | ||
| DG (22.6%) | |||||||||||||
| Yamaguchi et al[84], 2017 | 2001-2013 | 259 (84) | 61.7 (21-78) | 35 (41%) | 37 (44%) | 34 (40%) | DCS or S1 or S1 + Cisplatin or S1 + Taxane | TG (82.1%) | NS | 43 (51%) | |||
| DG (17.9%) | |||||||||||||
| Morgagni et al[85], 2018 | Apr 2005-Aug 2016 | 73 (22) | 69 (59-74) | Epirubicin + Cisplatinum + 5-FU or Oxaliplatin + 5-FU or Docetaxel + Oxaliplatin + 5-FU or Other | TG (72.7%) | 91.90% | 22 (100%) | ||||||
| DG (22.7%) | |||||||||||||
| Reference | Years | OS (rate) | MST (mo) | ||||
| CHT | CHT + surgery | CHT | CHT + surgery | ||||
| R1/R2 | R0 | R1/R2 | R0 | ||||
| Nakajima et al[73], 1997 | 2/3-yr | 4.7 | 6.5 | ||||
| 5-yr | 55.6 | ||||||
| Yano et al[74], 20022 | 2/3-yr | ||||||
| 5-yr | |||||||
| Satoh et al[75], 2012 | 2/3-yr | 43 | 751 | 19.2 | |||
| 5-yr | |||||||
| Kanda et al[76], 2012 | 2/3-yr | 0 | 45.9 | 29 | |||
| 5-yr | 34.4 | ||||||
| Han et al[77], 2014 | 2/3-yr | 41.4 | 7.8 | 22.9 | |||
| 5-yr | |||||||
| Kim et al[78], 2014 | 2/3-yr | 0 | 0 | 50 | 8 | 18 | 37 |
| 5-yr | |||||||
| Fukuchi et al[79], 2015 | 2/3-yr | 14 | 30 | 62 | |||
| 5-yr | 1 | 15 | 49 | ||||
| Kinoshita et al[80], 2015 | 2/3-yr | 0 | 16 | 63.5 | 9.6 | 29.9 | |
| 5-yr | |||||||
| Sato et al[81], 2017 | 2/3-yr | 18.7 | 15.7 | 21.7 | 47.9 | ||
| 5-yr | 0 | 0 | 48.6 | ||||
| Einama et al[82], 2017 | 2/3-yr | 29 | |||||
| 5-yr | |||||||
| Mieno et al[83], 2017 | 2/3-yr | 56.9 | 73.1 | 56.1 | |||
| 5-yr | |||||||
| Yamaguchi et al[84], 2017 | 2/3-yr | 11.3 | 21.2 | 41.3 | |||
| 5-yr | |||||||
| Morgagni et al[85], 2018 | 2/3 yr | 0 | 39.4 | 14 | 383 | ||
| 5 yr | |||||||
Probably, the first report of conversion surgery was in 1997 by Nakajima et al[73]. Thirty patients with incurable GC were treated with combined chemotherapy and radical surgery. Survival of patients with curative resection was 55.6% at 5 years. Long-term survivors were exclusively found among patients with distant metastatic lymph nodes. PC and extra-abdominal lesions did not respond to chemotherapy and, hence, did not reach surgery[73].
Yano et al[74] analyzed 34 patients with inoperable GC who underwent NAC. Eight patients among 14 who received salvage surgery exhibited curative resection. Histological type, T4 as non-curative factors, clinical response, and salvage surgery were significant prognostic factors. T4 unresectable lesions and para-aortic node metastases showed high dissolution rates after chemotherapy, whereas peritoneal and distant metastases did not[74]. A study on combined treatment with S-1 plus cisplatin followed by gastrectomy and post-operative S-1 for stage IV GC was conducted by Satoh et al[75]. Their results showed that 26 patients among 44 who received preoperative chemotherapy underwent R0 surgical resection. Interestingly, all 12 patients with pre-cy1 as a single pre-stage IV factor achieved R0 resection with a 2-year OS of 75%[75].
In 2012, Kanda et al[76] reported a good response rate to S-1 chemotherapy in patients with incurable GC who were submitted to secondary surgery. Twenty-six patients of 28 underwent R0 resection. The results showed that 1-, 3-and 5-year survival were 82.1, 45.9 and 34.4%, respectively. Multivariate analysis revealed histological lesion length to be the only significant prognostic factor[76]. According to reports from Han et al[77], 22/34 M1 patients with one initial metastatic site who responded to induction chemotherapy exhibited good survival outcomes after R0 resection, with resection rates of 88% and 44% for one and two metastatic sites, respectively. MST of R0 was 22.9 mo, with a 3-year overall survival of 41.4%. Concerning gastric cancer patients with peritoneal seeding, Kim et al[78] published results of 18 conversion patients in which 10 received R0 resection after chemotherapy. MST and 3-year OS of R0 patients were 37 mo and 50%, respectively. Unexpectedly, 8 patients who received non-curative resection had longer survival rates than did other patients who continued chemotherapy[78].
Fukuchi et al[79] reported a series of 40 out of 151 patients who underwent conversion surgery. In 32 of them, it was possible to perform R0 resection with a 5-year OS of 49% (MST: 62 mo). By multivariate analysis, the presence of just one non-curative factor and R0 resection were significant independent predictors for good OS[79].
Kinoshita et al[80] analyzed the effects of conversion gastrectomy after docetaxel, cisplatin and S-1 (DCS) combined chemotherapy. Of 57 patients, 42 were categorized as unresectable, while 15 patients were potentially resectable cases, with a single incurable factor (16 a2-b1 metastases or < 3 peripheral liver metastases). The 3-year OS rate of potentially resectable cases was 92.9%, compared with 35.1% of unresectable cases[80].
In a multi-institutional retrospective study, Sato et al[81] highlighted pathological response as a significant independent predictor for OS. He determined that 33/100 patients were able to undergo conversion therapy. Almost eighty-five of them received an R0 resection after DCS chemotherapy with a pathological response rate of 78.8%. Five-year OS in R0 patients was 48.6%[81].
Ten patients with one incurable factor were retrospectively analyzed by Einama et al[82]. All cases were considered resectable after chemotherapy, achieving R0 resection. The authors reported a longer survival of surgical patients compared with those who received chemo alone (MST 29 mo). Non-invasive macroscopic type, higher differentiation, and absence of peritoneal dissemination were all favorable survival predictors[82].
Another study concerning conversion surgery after combination chemotherapy of docetaxel, cisplatin, and S-1 from Mieno et al[83] reported that 74.2% of the study population (23/31) underwent R0 resection in patients with stage IV GC initially deemed unresectable. Fifty-eight point one percent of patients had extra regional lymph node as unresectable factor[83].
In a study by Yamaguchi et al[84], 84 patients among 259 with stage IV GC received conversion surgery after chemotherapy. Patients were classified into four categories previously published by the same authors[17]. Survival results of this series rose from 24.7 to 31.0 of MST. Patients who underwent R0 resection had an MST of 41.3 mo[84]. Recently, Morgagni et al[85] reported a Western series of 22 patients among 73 unresectable subjects who underwent R0 resection after induction chemotherapy. Gastrectomy plus HIPEC was performed in 9 patients. The 1- and 3-year survival rates were 63.6% and 39.4%, respectively[85].
Gastric cancer is known to be a heterogeneous disease. Dissemination may occur directly to the peritoneum, through the hematogenous and lymphatic systems. Moreover, the method whereby cancer cells enter into the portal circulation varies, resulting in significant variability of metastatic patients both for the site and the amount of tumor. Consequently, few metastatic patients are eligible for conversion surgery. Moreover, frequent coexistence of different factors of incurability make it difficult to identify true prognostic variables, as well as the rate of response to chemotherapeutic treatments. Despite progress in chemotherapy providing significant hope with new drug agents, the response rates of metastatic GC patients remain unsatisfactory with non-optimal patient compliance. The definition of initial unresectable criteria and post-chemotherapy resectability has yet to be established. In many cases, the line between neoadjuvant and induction chemotherapy remains unclear. Therefore, analysis of experiences on conversion surgery in stage IV GC is very challenging due to the heterogeneity of series, makes it very difficult to compare results from different studies. Furthermore, the majority of analyzed studies have been performed in Eastern Asia (only one in Italy). As such, this could represent a potential bias for reliable evaluation independent of differences in chemotherapy schedules, quality of surgery, and patient biology, for example. Undoubtedly, the Regatta trial taught us that even a palliative gastrectomy increases patient morbidity compared with chemotherapy alone. Hence, a strict selection of patients who could potentially benefit from conversion surgery seems mandatory. Yoshida et al[17] proposed a biological classification to stratify all stage IV GC patients to respond to this need (Figure 1). Probably, long-term survivors can be found mostly in the first three categories, though the small number of patients in the first category can be explained by this unusual condition. Actually, these patients are likely to benefit from NAC.
Although analyzed studies were retrospective and limited with respect to number of patients enrolled, the possibility of curative resection seems a crucial aspect. The literature reports R0 resection rates ranging from 24%-100% (Table 1), and these numbers are closely correlated with prognosis (Table 2). Thus, the survival benefit derived from R0 resections might justify a predictable increase in morbidity compared with survival from medical therapy alone. Interestingly, even non-curative resection often results in superior survival compared to chemotherapy alone. Consistent with this suggestion from the literature, quality of life (QOL) after conversion (even if non curative) surgery remains an intriguing issue to be analyzed. In this regard, a meta-analysis conducted by Lasithiotakis et al[86] underlined the relevant role of QOL outcomes after palliative gastrectomy.
Consistent with considerations by Yoshida et al[17], the presence of only one-site of metastasis is one of the most important prognostic factors according to most analyzed studies. In this literature review, lymph node metastases and positive cytology on peritoneal washing as unresectable factors are also related to better prognoses after conversion surgery when partial or complete response to chemo was observed. In this regard, while the more reliable (and later) evaluation of pathological response was demonstrated to be correlated with survival after conversion therapy, we have no unquestionable prognostic data and no objective criteria for clinical response assessment. Indeed, another determining factor is the detection of the best timing to operate (or to decide to not operate). Generally, surgery occurs when the tumor decreases in sizes and before it develops any drug resistance. For this determinant decision making step, cooperation between oncologists and surgeons is mandatory for general management of patients (and not the tumor alone). Regarding type of surgery and extension of lymphadenectomy, total or distal gastrectomy (also with multivisceral approach) aiming at R0 resection was generally associated with D2 or more extended lymphadenectomy. We believe that a proper and standardized D2 lymphadenectomy could achieve optimal results with acceptable morbidity/mortality. Finally, whether chemotherapy is required after an R0 resection is an issue that needs clarification.
In conclusion, the survival efficacy of conversion surgery may dramatically improve when combined with targeted chemotherapy. Perhaps new cytotoxic and molecular targeted agents and progress in sensitive molecular biomarker development could shift treatment from standardized to personalized, leading to further improved outcomes. The promising results of this multimodal therapy are increasingly gaining the attention of medical and surgical oncologists in planning further studies. Although it seems hard to design a valuable trial due to the difficulty of enrolling patients, it appears mandatory to demonstrate the effectiveness of this strategy in stage IV GC patients, or at least in well-selected and stratified stage IV patient subgroups. On the other hand, given that long-time survivors exist, we are convinced that the multidisciplinary discussion should always be recommended on a case-by-case basis. In conclusion, it is well known that some decades ago patients affected by unresectable GC represented a large population on whom medical oncologists applied new and promising therapies without great success. Today, the strategy of conversion surgery induces oncologists to consider that surgery could still have a role, even after almost “hopeless” systemic therapy.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Arigami T, Barreto SG, Caboclo JF, Fujita T, Ierardi E S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19854] [Article Influence: 2206.0] [Reference Citation Analysis (18)] |
| 2. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4120] [Cited by in F6Publishing: 4276] [Article Influence: 237.6] [Reference Citation Analysis (0)] |
| 3. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1216] [Cited by in F6Publishing: 1378] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1575] [Cited by in F6Publishing: 1810] [Article Influence: 258.6] [Reference Citation Analysis (0)] |
| 5. | Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449-1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 399] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 7. | Meyer HJ, Hölscher AH, Lordick F, Messmann H, Mönig S, Schumacher C, Stahl M, Wilke H, Möhler M. [Current S3 guidelines on surgical treatment of gastric carcinoma]. Chirurg. 2012;83:31-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi57-vi63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 9. | Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 10. | Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK (eds). SEER Cancer Statistics Review, 1975-2008. National Cancer Institute, Bethesda, MD. 2011; Available from: https://seer.cancer.gov/csr/1975_2008/. [Cited in This Article: ] |
| 11. | Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Union for International Cancer Control (UICC). TNM Classification of Malignant Tumours. 8th edition. Wiley-Blackwell. 2016;. [Cited in This Article: ] |
| 13. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 14. | Hermanek P. The second English edition of the Japanese Classification of Gastric Carcinoma. A Western commentary. Gastric Cancer. 1999;2:79-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 15. | Sasako M, Aiko T. Reply to Professor Hermanek’s comments on the new Japanese classification of gastric carcinoma. Gastric Cancer. 1999;2:83-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Zurleni T, Gjoni E, Ballabio A, Casieri R, Ceriani P, Marzoli L, Zurleni F. Sixth and seventh tumor-node-metastasis staging system compared in gastric cancer patients. World J Gastrointest Surg. 2013;5:287-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19:329-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 18. | Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE, de Hingh IH. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 19. | Abbasi SY, Taani HE, Saad A, Badheeb A, Addasi A. Advanced gastric cancer in jordan from 2004 to 2008: a study of epidemiology and outcomes. Gastrointest Cancer Res. 2011;4:122-127. [PubMed] [Cited in This Article: ] |
| 20. | Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395-2403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 389] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 21. | Kim JG, Ryoo BY, Park YH, Kim BS, Kim TY, Im YH, Kang YK. Prognostic factors for survival of patients with advanced gastric cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2008;61:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Kanda M, Kodera Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J Gastroenterol. 2016;22:6829-6840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 99] [Cited by in F6Publishing: 101] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 23. | Sugarbaker TA, Chang D, Koslowe P, Sugarbaker PH. Patterns of spread of recurrent intraabdominal sarcoma. Cancer Treat Res. 1996;82:65-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Gilly FN, Carry PY, Sayag AC, Brachet A, Panteix G, Salle B, Bienvenu J, Burgard G, Guibert B, Banssillon V. Regional chemotherapy (with mitomycin C) and intra-operative hyperthermia for digestive cancers with peritoneal carcinomatosis. Hepatogastroenterology. 1994;41:124-129. [PubMed] [Cited in This Article: ] |
| 25. | Japanese research Society for Gastric Cancer. The general rules for the gastric cancer study in surgery and pathology. 12th ed. Tokyo: Kanehara Shuppan 1993; . [Cited in This Article: ] |
| 26. | Okabe H, Ueda S, Obama K, Hosogi H, Sakai Y. Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol. 2009;16:3227-3236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Fujimoto S, Shrestha RD, Kokubun M, Ohta M, Takahashi M, Kobayashi K, Kiuchi S, Okui K, Miyoshi T, Arimizu N. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg. 1988;208:36-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 158] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Yonemura Y, Fujimura T, Nishimura G, FallaR , Sawa T, Katayama K, Tsugawa K, Fushida S, Miyazaki I, Tanaka M. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery. 1996;119:437-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F, Elias D; Association Française de Chirurgie. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370-2377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 303] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 30. | Hall JJ, Loggie BW, Shen P, Beamer S, Douglas Case L, McQuellon R, Geisinger KR, Levine EA. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg. 2004;8:454-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Yang XJ, Li Y, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J Surg Oncol. 2010;101:457-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, Imamoto H, Kodera Y, Uenosono Y, Amagai K. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J Clin Oncol. 2018;36:1922-1929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 33. | Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, Schiller D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104:692-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 34. | Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 449] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 35. | Rudloff U, Langan RC, Mullinax JE, Beane JD, Steinberg SM, Beresnev T, Webb CC, Walker M, Toomey MA, Schrump D. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol. 2014;110:275-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 36. | Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 459] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 37. | Ajani JA, Barthel JS, Bekaii-Saab T, Bentrem DJ, D’Amico TA, Das P, Denlinger C, Fuchs CS, Gerdes H, Hayman JA. Gastric cancer. J Natl Compr Canc Netw. 2010;8:378-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1723] [Cited by in F6Publishing: 1852] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 39. | Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, Ahn JB, Roh JK, Noh SH, Chung HC. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19:1146-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, Katai H, Kosuge T, Sasako M. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol. 2007;95:534-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Takemura N, Saiura A, Koga R, Arita J, Yoshioka R, Ono Y, Hiki N, Sano T, Yamamoto J, Kokudo N. Long-term outcomes after surgical resection for gastric cancer liver metastasis: an analysis of 64 macroscopically complete resections. Langenbecks Arch Surg. 2012;397:951-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | Li ZY, Tang L, Zhang LH, Bu ZD, Wu AW, Wu XJ, Zong XL, Wu Q, Shan F, Li SX. Weekly docetaxel and cisplatin plus fluorouracil as a preoperative treatment for gastric cancer patients with synchronous multiple hepatic metastases: a pilot study. Med Oncol. 2010;27:1314-1318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Gadde R, Tamariz L, Hanna M, Avisar E, Livingstone A, Franceschi D, Yakoub D. Metastatic gastric cancer (MGC) patients: Can we improve survival by metastasectomy? A systematic review and meta-analysis. J Surg Oncol. 2015;112:38-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Markar SR, Mikhail S, Malietzis G, Athanasiou T, Mariette C, Sasako M, Hanna GB. Influence of Surgical Resection of Hepatic Metastases From Gastric Adenocarcinoma on Long-term Survival: Systematic Review and Pooled Analysis. Ann Surg. 2016;263:1092-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 45. | Tiberio GA, Ministrini S, Gardini A, Marrelli D, Marchet A, Cipollari C, Graziosi L, Pedrazzani C, Baiocchi GL, La Barba G. Factors influencing survival after hepatectomy for metastases from gastric cancer. Eur J Surg Oncol. 2016;42:1229-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Schildberg CW, Croner R, Merkel S, Schellerer V, Müller V, Yedibela S, Hohenberger W, Peros G, Perrakis A. Outcome of operative therapy of hepatic metastatic stomach carcinoma: a retrospective analysis. World J Surg. 2012;36:872-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono H, Nagahori Y, Hosoi H, Takahashi M, Kito F. Comparison of surgical results of D2 versus D3 gastrectomy (para-aortic lymph node dissection) for advanced gastric carcinoma: a multi-institutional study. Ann Surg Oncol. 2006;13:659-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Fujimura T, Nakamura K, Oyama K, Funaki H, Fujita H, Kinami S, Ninomiya I, Fushida S, Nishimura G, Kayahara M. Selective lymphadenectomy of para-aortic lymph nodes for advanced gastric cancer. Oncol Rep. 2009;22:509-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 698] [Cited by in F6Publishing: 720] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 50. | Roviello F, Pedrazzani C, Marrelli D, Di Leo A, Caruso S, Giacopuzzi S, Corso G, de Manzoni G. Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol. 2010;36:439-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, Oshita H, Ito S, Kawashima Y, Fukushima N. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 52. | Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 219] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 53. | Katayama H, Ito S, Sano T, Takahari D, Mizusawa J, Boku N, Tsuburaya A, Terashima M, Sasako M; Stomach Cancer Study Group of the Japan Clinical Oncology Group. A Phase II study of systemic chemotherapy with docetaxel, cisplatin, and S-1 (DCS) followed by surgery in gastric cancer patients with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1002. Jpn J Clin Oncol. 2012;42:556-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, Kawashima Y, Kinoshita T, Terashima M, Nashimoto A. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer. 2017;20:322-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 55. | Takashima A, Boku N, Kato K, Nakamura K, Mizusawa J, Fukuda H, Shirao K, Shimada Y, Ohtsu A. Survival prolongation after treatment failure of first-line chemotherapy in patients with advanced gastric cancer: combined analysis of the Japan Clinical Oncology group trials JCOG9205 and JCOG9912. Gastric Cancer. 2014;17:522-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1320] [Cited by in F6Publishing: 1365] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 57. | Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140:319-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 58. | Ryu MH, Baba E, Lee KH, Boku N, Park YI, Hyodo I. Phase III trial of a 3-weekly versus 5-weekly schedule of S-1 plus cisplatin (SP) combination chemotherapy for first-line treatment of advanced gastric cancer (AGC): SOS study. J Clin Oncol. 2013;31:LBA4024. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 60. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4615] [Cited by in F6Publishing: 4876] [Article Influence: 348.3] [Reference Citation Analysis (1)] |
| 61. | Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968-3976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 795] [Cited by in F6Publishing: 887] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 62. | Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JG, Piedbois P, Paillot B. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648-2657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 382] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 63. | Ajani JA, Buyse M, Lichinitser M, Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha R, Carrato A. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: Results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer. 2013;49:3616-3624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1331] [Cited by in F6Publishing: 1410] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 65. | Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, Wenczl M, Goker E, Cisar L, Wang K. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 66. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR; Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1579] [Cited by in F6Publishing: 1611] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 67. | Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 544] [Cited by in F6Publishing: 591] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 68. | Sekikawa A, Fukui H, Zhang X, Maruo T, Tsumura T, Okabe Y, Wakasa T, Osaki Y, Chiba T, Tomita T. REG Iα is a biomarker for predicting response to chemotherapy with S-1 plus cisplatin in patients with unresectable stage IV gastric cancer. Br J Cancer. 2013;108:395-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Hartgrink HH, Putter H, Klein Kranenbarg E, Bonenkamp JJ, van de Velde CJ; Dutch Gastric Cancer Group. Value of palliative resection in gastric cancer. Br J Surg. 2002;89:1438-1443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 70. | Sun J, Song Y, Wang Z, Chen X, Gao P, Xu Y, Zhou B, Xu H. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13:577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 71. | Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 72. | D’Ugo D, Cananzi FC, Persiani R, Agnes A, Biondi A. REGATTA trial: a call for the USA and Europe. Lancet Oncol. 2016;17:261-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Nakajima T, Ota K, Ishihara S, Oyama S, Nishi M, Ohashi Y, Yanagisawa A. Combined intensive chemotherapy and radical surgery for incurable gastric cancer. Ann Surg Oncol. 1997;4:203-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Yano M, Shiozaki H, Inoue M, Tamura S, Doki Y, Yasuda T, Fujiwara Y, Tsujinaka T, Monden M. Neoadjuvant chemotherapy followed by salvage surgery: effect on survival of patients with primary noncurative gastric cancer. World J Surg. 2002;26:1155-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Satoh S, Okabe H, Teramukai S, Hasegawa S, Ozaki N, Ueda S, Tsuji A, Sakabayashi S, Fukushima M, Sakai Y. Phase II trial of combined treatment consisting of preoperative S-1 plus cisplatin followed by gastrectomy and postoperative S-1 for stage IV gastric cancer. Gastric Cancer. 2012;15:61-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Kanda T, Yajima K, Kosugi S, Ishikawa T, Ajioka Y, Hatakeyama K. Gastrectomy as a secondary surgery for stage IV gastric cancer patients who underwent S-1-based chemotherapy: a multi-institute retrospective study. Gastric Cancer. 2012;15:235-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Han DS, Suh YS, Kong SH, Lee HJ, Im SA, Bang YJ, Kim WH, Yang HK. Outcomes of surgery aiming at curative resection in good responder to induction chemotherapy for gastric cancer with distant metastases. J Surg Oncol. 2013;107:511-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Kim SW. The result of conversion surgery in gastric cancer patients with peritoneal seeding. J Gastric Cancer. 2014;14:266-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Fukuchi M, Ishiguro T, Ogata K, Suzuki O, Kumagai Y, Ishibashi K, Ishida H, Kuwano H, Mochiki E. Prognostic Role of Conversion Surgery for Unresectable Gastric Cancer. Ann Surg Oncol. 2015;22:3618-3624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (2)] |
| 80. | Kinoshita J, Fushida S, Tsukada T, Oyama K, Okamoto K, Makino I, Nakamura K, Miyashita T, Tajima H, Takamura H. Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol. 2015;41:1354-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 81. | Sato Y, Ohnuma H, Nobuoka T, Hirakawa M, Sagawa T, Fujikawa K, Takahashi Y, Shinya M, Katsuki S, Takahashi M. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer. 2017;20:517-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 82. | Einama T, Abe H, Shichi S, Matsui H, Kanazawa R, Shibuya K, Suzuki T, Matsuzawa F, Hashimoto T, Kohei N. Long-term survival and prognosis associated with conversion surgery in patients with metastatic gastric cancer. Mol Clin Oncol. 2017;6:163-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Mieno H, Yamashita K, Hosoda K, Moriya H, Higuchi K, Azuma M, Komori S, Yoshida T, Tanabe S, Koizumi W. Conversion surgery after combination chemotherapy of docetaxel, cisplatin and S-1 (DCS) for far-advanced gastric cancer. Surg Today. 2017;47:1249-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, Tanabe K, Ohdan H. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21:315-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 85. | Morgagni P, Solaini L, Framarini M, Vittimberga G, Gardini A, Tringali D, Valgiusti M, Monti M, Ercolani G. Conversion surgery for gastric cancer: A cohort study from a western center. Int J Surg. 2018;53:360-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 86. | Lasithiotakis K, Antoniou SA, Antoniou GA, Kaklamanos I, Zoras O. Gastrectomy for stage IV gastric cancer. a systematic review and meta-analysis. Anticancer Res. 2014;34:2079-2085. [PubMed] [Cited in This Article: ] |