
Abstract
Aims: Percutaneous coronary intervention (PCI) of saphenous vein grafts (SVG) is associated with worse outcomes as compared with native coronary arteries. Previous studies comparing drug-eluting stents (DES) with bare metal stents (BMS) for PCI of SVG have yielded inconsistent results. Therefore, we aimed to perform a comprehensive meta-analysis of randomised trials comparing DES versus BMS for SVG PCI.
Methods and results: Randomised trials that reported clinical outcomes and compared DES versus BMS for PCI of SVG were included. Summary estimates risk ratios (RRs) were constructed using a DerSimonian and Laird model. The primary outcome was major adverse cardiac events (MACE). Five trials with 1,535 patients were analysed. At a mean of 24.5 months, there was no difference in the risk of MACE (22.7% in the DES group versus 33.5% in the BMS group; RR 0.75, 95% CI: 0.49-1.16), all-cause mortality (9.5% versus 5.6%; RR 1.30, 95% CI: 0.77-2.18), cardiac mortality (5.5% versus 3.6%; RR 1.29, 95% CI: 0.53-3.15), myocardial infarction (7.1% versus 11.7%; RR 0.70, 95% CI: 0.40-1.22), stent thrombosis (2.5% versus 6.1%; RR 0.73, 95% CI: 0.26-2.03), and target vessel revascularisation (11.7% versus 24.4%; RR 0.66, 95% CI: 0.41-1.04) between DES and BMS.
Conclusions: Data from randomised trials indicate that DES are safe for PCI for SVG disease. The risk of MACE, mortality, myocardial infarction, stent thrombosis or target vessel revascularisation was not statistically significantly different between the devices. Novel therapies are needed in the management of this subset of patients.
Abbreviations
BMS: bare metal stents
CABG: coronary artery bypass graft
CI: confidence interval
DAPT: dual antiplatelet therapy
DES: drug-eluting stents
MACE: major adverse cardiac events
MI: myocardial infarction
PCI: percutaneous coronary intervention
RR: risk ratio
SVG: saphenous vein graft
TVR: target vessel revascularisation
Introduction
Saphenous vein graft (SVG) stenosis is relatively common after coronary artery bypass graft (CABG) surgery, with a prevalence of ~50% at five years after surgery1. Since repeat CABG carries a higher risk of complications, percutaneous coronary intervention (PCI) has emerged as the preferred management. A previous analysis of the United States National Cardiovascular Data Registry has shown that PCI of SVG represents approximately 6% of the total PCI volume2. However, PCI of SVG is associated with a higher risk of short- and long-term adverse outcomes compared with native coronary arteries3, which might be related to fibromuscular hyperplasia, excessive wall shear stress, accelerated atherosclerosis and suture site inflammatory response4. Drug-eluting stents (DES) have been shown to be superior to bare metal stents (BMS) in reducing the risk of restenosis and the need for future revascularisation in native coronary arteries5,6; however, there have been concerns that the superiority of DES may not be the same for SVG disease. In a randomised trial comparing both devices, DES were associated with an increased risk of late mortality and stent thrombosis compared with BMS7. Subsequent randomised trials did not demonstrate these risks8-10. In addition, observational and real-world studies have yielded discrepant findings regarding the safety and efficacy of DES as compared to BMS for SVG PCI11-19. Moreover, previous meta-analyses have also shown inconsistent results, partly due to the inclusion of observational studies, which could be prone to bias20-25. More recently, the results of two large randomised trials comparing both devices have been presented (Jeger RV. Drug-eluting vs. bare metal stents in saphenous vein grafts: The prospective randomized BASKET-SAVAGE trial. Presented at the European Congress of Cardiology, Rome, Italy. August 2016; Brilakis ES. Drug-eluting stents vs. bare metal stents In saphenous Vein graft Angioplasty (DIVA). Presented at the European Congress of Cardiology, Barcelona, Spain. August 2017). Therefore, we aimed to perform a comprehensive meta-analysis of randomised trials comparing DES versus BMS for SVG PCI.
Methods
DATA SOURCES
Electronic databases including Medline, Web of Science, and the Cochrane Register of Controlled Trials (CENTRAL) were searched without language restriction from inception up to August 2017 using the keywords and medical subject headings “bare metal stent”, “drug-eluting stent”, and “saphenous vein graft”. The major scientific cardiovascular sessions were also searched using the same keywords. This meta-analysis was registered at the PROSPERO international prospective register of systematic reviews (CRD42017074438)26. This meta-analysis was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines27.
SELECTION CRITERIA AND DATA EXTRACTION
Trials that randomised patients with SVG disease undergoing PCI to either DES (irrespective of the type of DES) versus BMS were included. We required that trials reported clinical outcomes. Data from the longest available reported follow-up time were preferentially used. Two independent authors (I.Y. Elgendy and A.N. Mahmoud) extracted data on study design, sample size, intervention strategies, outcomes, and other study characteristics from the included studies. Discrepancies were resolved by consensus. The number of clinical events in each arm was tabulated.
OUTCOMES AND DEFINITIONS
The primary outcome was major adverse cardiac events (MACE) as defined per the individual trials. We preferentially used the composite of cardiac mortality (or all-cause mortality), myocardial infarction (MI) or target vessel revascularisation (TVR) as the definition of MACE whenever available. The secondary outcomes included all-cause mortality, cardiac mortality, MI, TVR, and stent thrombosis.
QUALITY ASSESSMENT
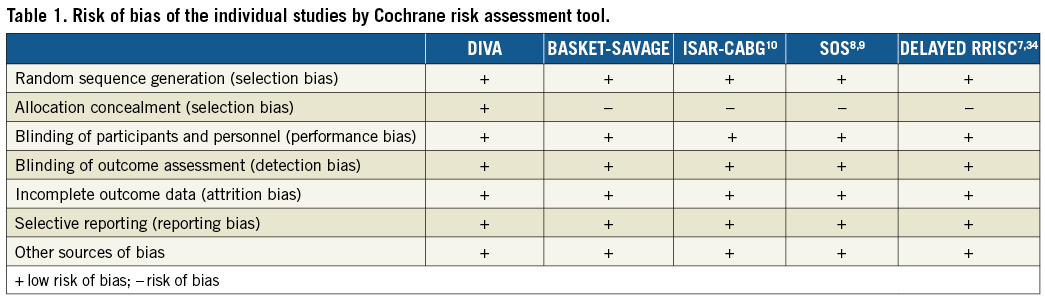
The Cochrane Collaboration’s tool for assessing the risk of bias was used to assess the risk of bias of the individual studies. This tool consists of seven points (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias) and tests for selection, performance, detection, attrition, reporting and other biases, respectively28.
The overall quality of evidence for the primary outcome was evaluated using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) tool. The GRADE tool ranks four levels of quality (high, moderate, low and very low) depending on the type of studies included in the assessment of each outcome. The quality of evidence could be reduced according to five factors: 1) limitations in the design and implementation of available studies (i.e., high likelihood of bias), 2) indirectness of evidence (indirect population, intervention, control, and outcomes), 3) unexplained heterogeneity or inconsistency of results, 4) imprecision of results (i.e., wide confidence intervals), and 5) high probability of publication bias.
STATISTICAL ANALYSIS
Outcomes were assessed with an intention-to-treat analysis. Since we anticipated a significant degree of heterogeneity due to the different types of DES assessed and the large time span in which the trials were conducted, we used random effects summary risk ratios (RR) based on a DerSimonian and Laird model29. Statistical heterogeneity was calculated using the I2 statistic30. Egger’s method was used to estimate publication bias31. All p-values were two-tailed, with statistical significance set at 0.05; confidence intervals (CI) were calculated at the 95% level for the overall estimates effect. All analyses were performed using Stata software, version 14 (StataCorp, College Station, TX, USA). To determine the impact of certain patient and angiographic characteristics on the risk of MACE, random effects meta-regression analyses were pre-specified in relation to age, acute coronary syndrome, diabetes mellitus, age of graft, the use of embolic protection devices, and publication/presentation year32. For the outcome of MACE, a sensitivity analysis was performed excluding unpublished studies, as well as a subgroup analysis comparing early (i.e., six to 12 months) versus late (i.e., 12 months) events. In addition, we performed a sensitivity analysis by excluding one trial at a time to explore the influence of each study on the degree of heterogeneity.
Results
INCLUDED STUDIES
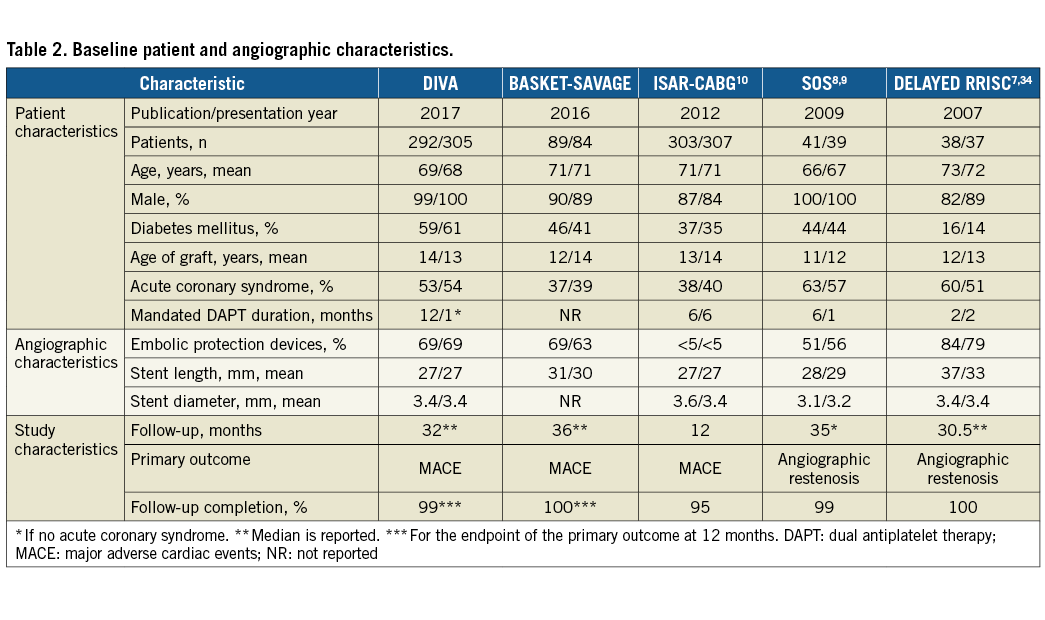
The electronic search yielded 137 articles that were screened by reviewing the title and/or abstract. We identified five trials that met our inclusion criteria (Figure 1)7,9,10. Two of these trials were only presented at major conferences (i.e., BASKET-SAVAGE and DIVA). One trial (i.e., SOS) reported the outcomes at both 12 months and at a median of 35 months8,9; thus, we utilised data from the longer report for the overall analysis9. The SOS trial had also reported retrospective extended follow-up data for some of the included patients in the major participating centre; however, we excluded this report since this does not represent randomised data33. Similarly, the DELAYED RRISC trial reported outcomes at six months, and at a median of 30.5 months7,34, so we included the second report for the overall analysis7. One study was a post hoc analysis of a large trial, which was not specific enough to compare both devices for SVG PCI and had an unbalanced number of patients in each arm; it was thus excluded35. Finally, five trials with 1,535 patients (763 patients in the DES group and 772 patients in the BMS group) were included in this meta-analysis7-10,34. All the included studies were deemed to be of low risk of bias (Table 1). All the studies were conducted at multiple centres except for DELAYED RRISC which was a single-centre trial7. The weighted mean follow-up duration was 24.5 months (standard error = 6.6 months). Four trials evaluated first-generation DES: the BASKET-SAVAGE and SOS trials used paclitaxel-eluting stents9, and the DELAYED RRISC tested sirolimus-eluting stents7. The ISAR-CABG trial utilised sirolimus-eluting stents, paclitaxel-eluting stents, and biodegradable sirolimus-eluting stents10. Only the DIVA trial utilised mainly second-generation DES (89% of the patients randomised to the DES arm). The duration of dual antiplatelet therapy (DAPT) was variable, ranging from one to six months after BMS implantation, and two to 12 months after DES implantation (only the DELAYED RRISC mandated clopidogrel for at least two months, whereas the remainder of the trials mandated it for at least six months). The baseline patient and angiographic characteristics of the included studies are summarised in Table 2.
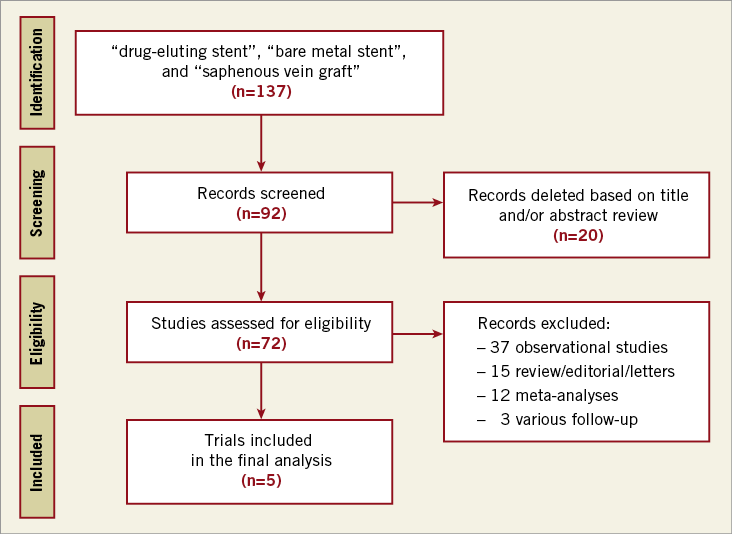
Figure 1. Summary of how the systematic search was conducted and how eligible studies were identified (PRISMA flow diagram).
MAJOR ADVERSE CARDIAC EVENTS
Three of the included studies reported the composite of cardiac mortality, MI, or TVR. The ISAR-CABG trial defined MACE as all-cause mortality, MI or target lesion revascularisation10, and DELAYED RRISC defined MACE as all-cause mortality, MI, or TVR7. The risk of MACE was similar in both groups (22.7% [95% CI: 13.8-31.7%] in the DES group versus 33.5% [95% CI: 19.0-47.9%] in the BMS group; RR 0.75, 95% CI: 0.49-1.16, I2=77%, p=0.20) (Figure 2). There was no evidence of publication bias using Egger’s test (p=0.76). The level of evidence was moderate using the GRADE method. Meta-regression analysis did not identify a difference in treatment effect based on age, acute coronary syndrome, diabetes mellitus, age of graft, the use of embolic protection devices, and publication/presentation year (p=0.40, 0.52, 0.51, 0.33, 0.36, and 0.75, respectively). Sensitivity analysis after excluding unpublished studies did not show any significant differences between the two devices (RR 0.77, 95% CI: 0.43-1.37, p=0.37, I2=82%). Subgroup analysis showed a lower risk of MACE with DES in early follow-up (i.e., six to 12 months) (RR 0.57, 95% CI: 0.36-0.89, p=0.01, I2=46%) but not in late follow-up (i.e., >12 months) (RR 0.77, 95% CI: 0.43-1.39, p=0.38, I2=82%). However, the interaction by follow-up duration was not significant (pinteraction=0.35) (Figure 3). The sensitivity analysis excluding each trial at a time suggested that no single study had a major influence on the degree of heterogeneity (I2 ranged from 70-82%).
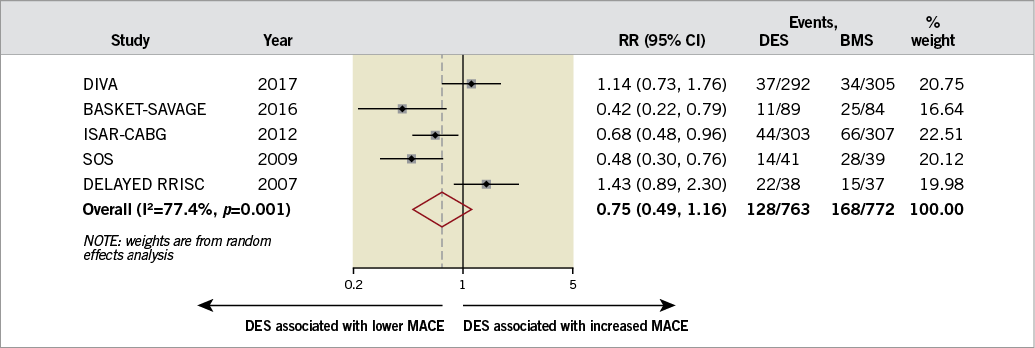
Figure 2. Summary plot for major adverse cardiac events. The relative size of the data markers indicates the weight of the sample size from each study. BMS: bare metal stents; CI: confidence interval; DES: drug-eluting stents; MACE: major adverse cardiac events; RR: risk ratio
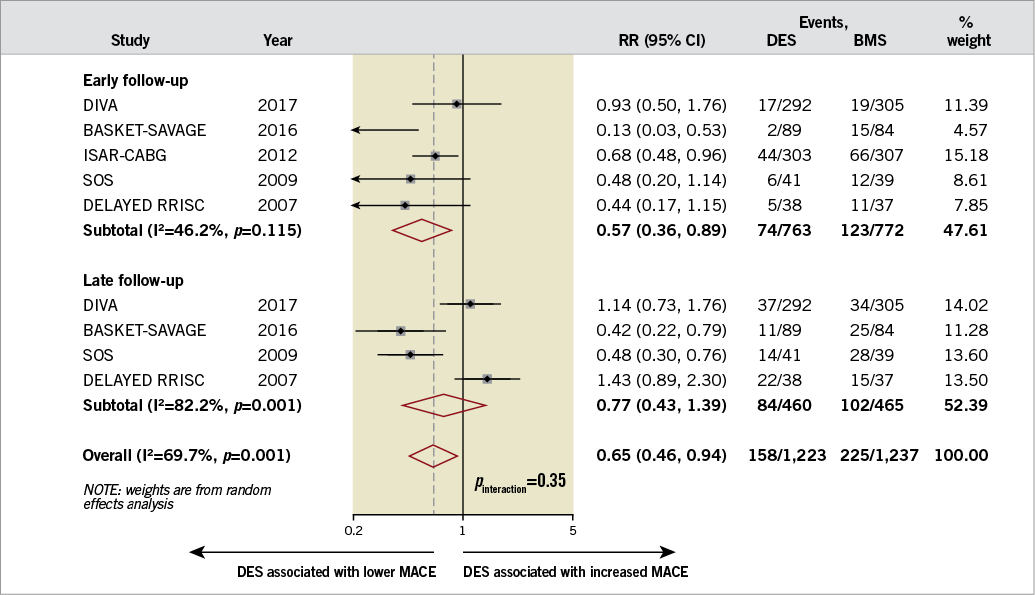
Figure 3. Summary plot for major adverse cardiac events according to early (six to 12 months) versus late (>12 months) follow-up. The relative size of the data markers indicates the weight of the sample size from each study. BMS: bare metal stents; CI: confidence interval; DES: drug-eluting stents; MACE: major adverse cardiac events; RR: risk ratio
SECONDARY OUTCOMES
The risk of all-cause mortality (9.5% [95% CI: 4.8-14.2] in the DES group versus 5.6% [95% CI: 3.7-7.4] in the BMS group; RR 1.30, 95% CI: 0.77-2.18, p=0.33, I2=34%) and cardiac mortality (5.5% [95% CI: 1.6-9.4] in the DES group versus 3.6% [95% CI: 0.3-6.9] in the BMS group; RR 1.29, 95% CI: 0.53-3.15, p=0.57, I2=34%) was similar in both groups. Similarly, there was no difference in the risk of MI (7.1% [95% CI: 3.7-10.4] in the DES group versus 11.7% [95% CI: 5.6-17.7] in the BMS group; RR 0.70, 95% CI: 0.40-1.22, I2=55%, p=0.20), stent thrombosis (2.5% [95% CI: –0.2-5.2] in the DES group versus 6.1% [95% CI: –1.2-13.2] in the BMS group; RR 0.73, 95% CI: 0.26-2.03, I2=20%, p=0.55), and TVR (11.7% [95% CI: 6.8-16.6] versus 24.4% [95% CI: 14.5-34.3]; RR 0.66, 95% CI: 0.41-1.04, I2=63%, p=0.07) between the two groups. Table 3 summarises the summary estimates for the outcomes. There was no evidence of publication bias for all the secondary outcomes using Egger’s test. Figure 4 demonstrates the incidence of the outcomes assessed in this meta-analysis.
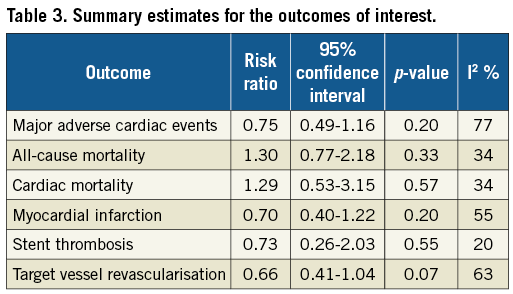
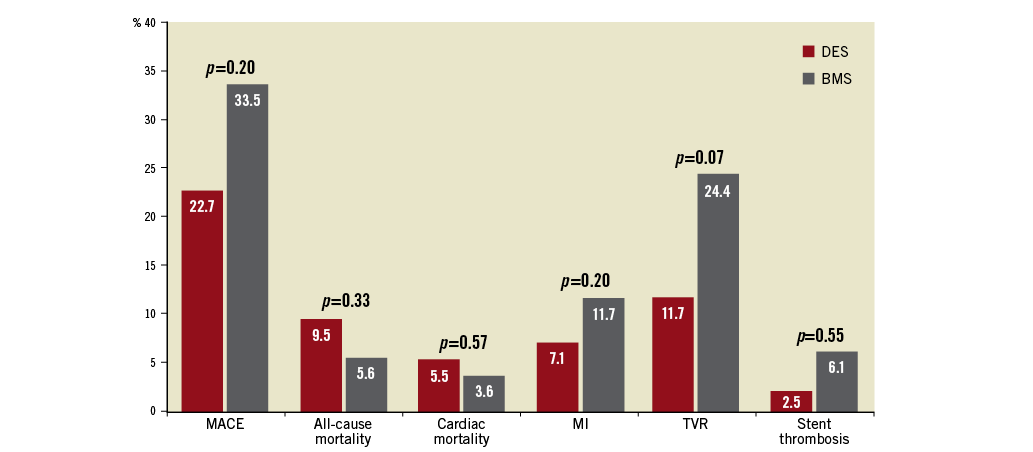
Figure 4. Bar chart summarising the incidences of all the outcomes assessed in this meta-analysis.
Discussion
In this updated and comprehensive meta-analysis of five high-quality randomised trials including 1,535 patients undergoing PCI for SVG disease, we demonstrated that there was no statistically significant difference between DES and BMS in the risk of MACE, all-cause mortality, cardiac mortality, MI, stent thrombosis or TVR. Meta-regression analysis did not identify any difference in the summary estimate based on various baseline patient and angiographic characteristics. There was no difference in the risk of MACE stratified by early (i.e., six to 12 months) versus late (i.e., >12 months) follow-up between the groups. The quality of evidence for MACE was moderate given the high level of heterogeneity among the included studies but with no evidence of publication bias. The sensitivity analysis excluding each trial at a time suggested that no single study had a major influence on the degree of heterogeneity.
Randomised trials have confirmed that DES are associated with a lower risk of restenosis as compared with BMS for native coronary arteries. However, SVG disease represents a different pathophysiological perspective which portends a higher risk of complications3. DELAYED RRISC was the first randomised trial to compare both devices in patients undergoing PCI for SVG disease7,34. At six months, DES had lower risk of restenosis34; however, this benefit was lost on longer follow-up7. Moreover, mortality was higher with DES at a median of 30.5 months (although many of the deaths were non-cardiac and surprisingly no patient died in the BMS group)7. These findings have led to concerns with DES use for SVG disease. Of note, the mortality rate in this trial was remarkably high as compared to the other trials, which could possibly be due to the shorter duration of DAPT mandated in this trial36. Subsequent randomised trials (i.e., SOS, ISAR-CABG, and BASKET-SAVAGE) also utilised first-generation DES, yet showed a lower risk of restenosis with DES, and did not replicate the safety concerns that were observed in the DELAYED RRISC trial8-10. These findings are reflected in the recommendations of the 2014 European Society of Cardiology guidelines on myocardial revascularisation37. However, the small sample size of these trials, as well as the premature termination of the BASKET-SAVAGE trial due to slow enrolment, are important limitations. In addition, these studies included routine angiographic follow-up which might have magnified the benefit which was noted with DES. Furthermore, first-generation DES are associated with higher adverse events (namely restenosis and stent thrombosis)38. With the more abundant use of second-generation DES and longer DAPT duration (as compared with the DELAYED RRISC trial), the DIVA trial showed no difference in outcomes between DES and BMS. The results of the DIVA trial were in contrast to the ISAR-CABG trial which had enrolled the largest number of patients. Although the ISAR-CABG trial exclusively utilised first-generation DES which are more prone to restenosis, as compared with the second-generation DES which were used in the DIVA trial, the follow-up duration in the ISAR-CABG trial was remarkably short (i.e., 12 months in ISAR-CABG, as opposed to 32 months in the DIVA trial). This longer duration could represent the expected time-related graft degeneration. This is further supported by the subgroup analysis which showed that MACE was lower with DES at the earlier follow-up (i.e., six to 12 months), but not at the late follow-up (i.e., beyond 12 months).
Prior meta-analyses have demonstrated conflicting results regarding the safety and efficacy of DES as compared with BMS for PCI of SVG disease20-25. Some of these analyses have suggested that DES use is associated with better outcomes, including mortality23-25. However, these meta-analyses were predominately composed of observational studies, which are prone to selection and ascertainment bias. Two prior meta-analyses included only randomised data and demonstrated that DES are associated with a lower risk of TVR, with no difference in the risk of MACE, mortality, and MI39,40. Compared with these meta-analyses, the present meta-analysis is the largest performed to date comparing the efficacy and safety of DES compared with BMS. Moreover, we used the Cochrane Collaboration tool and the GRADE methodology to assess the quality of the included trials, as well as the primary outcome.
Limitations
Despite the robust methodology, this meta-analysis is not without limitations. First, there was a remarkable degree of heterogeneity in all the outcomes assessed. We attempted to mitigate this by using a random effects model. In addition, we performed various sensitivity, subgroup, and meta-regression analyses for the outcome of MACE to explore the heterogeneity. Second, the studies that were included in this meta-analysis span over ten years. There have been advances in antithrombotic and antiplatelet therapy across these years. However, we noted no difference in the summary estimates according to the publication/presentation year. Third, the majority of the studies evaluated first-generation DES which are not frequently used. A sensitivity analysis limited to second-generation DES could not be performed since only one trial utilised second-generation DES. However, some observational data comparing first- and second-generation DES for PCI of SVG disease showed no differences in long-term outcomes between the devices41. Fourth, lack of patient-level data precluded a careful evaluation of the patient and lesion characteristics that could benefit from DES. Thus, we performed multiple study level meta-regression analyses and demonstrated that none of these baseline or angiographic characteristics had an influence on the outcomes. Fifth, the differential use of clopidogrel between treatment arms of the trials could have resulted in bias in favour of DES. Finally, the definition of MACE was not similar in all the included trials. Therefore, we used a consistent definition (i.e., composite of cardiac mortality, MI, or TVR) whenever reported.
Conclusions
Data from randomised trials indicate that DES are safe for PCI for SVG disease. The risk of MACE, mortality, myocardial infarction, stent thrombosis or target vessel revascularisation was not statistically significantly different between the devices. Novel therapies, such as treatment of the native coronary artery lesions, are needed in the management of this subset of patients.
| Impact on daily practice Management of patients with saphenous vein graft failure remains challenging. This updated meta-analysis of randomised trials suggests that drug-eluting stents appear to be safe in patients undergoing percutaneous coronary intervention for saphenous vein graft revascularisation; however, drug-eluting stents are not superior to bare metal stents in reducing the risk of adverse events. Future studies need to investigate other novel therapies for the management of these patients. |
Conflict of interest statement
E. Brilakis reports receiving consulting/speaker honoraria from Abbott Vascular, Amgen, Asahi, CSI, Elsevier, GE Healthcare, and Medicure, and research support from Boston Scientific and Osprey; his spouse was an employee of Medtronic. A. Bavry reports receiving an honorarium from the American College of Cardiology. The other authors have no conflicts of interest to declare.




