Review Article Open Access
Application of the SERS Spectroscopy to the Study of Catalytic Reactions by Means of Mono and Bimetallic Nanoparticles
Maurizio Muniz-Miranda*
Chemistry Department “Ugo Schiff”, University of Florence, Via Lastruccia 3, 50019 Sesto Fiorentino, Italy
- *Corresponding Author:
- Maurizio Muniz-Miranda
Chemistry Department “Ugo Schiff”, University of Florence
Via Lastruccia 3, 50019 Sesto Fiorentino, Italy
Tel: +39-055-457-30
E-mail: maurizio.muniz@unifi.it
Received date: September 30, 2015; Accepted date: October 13, 2015; Published date: October 20, 2015
Citation: Muniz-Miranda M (2015) Application of the SERS Spectroscopy to the Study of Catalytic Reactions by Means of Mono and Bimetallic Nanoparticles. J Anal Bioanal Tech 6:286. doi:10.4172/2155-9872.1000286
Copyright: © 2015 Muniz-Miranda M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Surface-enhanced Raman scattering (SERS) has long since found wide application in the field of heterogeneous catalysis for both chemical and photochemical reactions. Actually, this technique allows the in-situ detection of reactants or products or by-products in reactions that take place at the surface of nanostructured metals like silver, gold and copper. Silver nanoparticles, when activated by adsorption of chloride anions, are able to catalyze reactions for adsorbed molecules, in addition to promote higher SERS enhancements. Bimetallic nanoparticles, made of silver as SERS-active metal and another metal with strong performance in heterogeneous catalysis like palladium, or metal oxide such as titania, extremely active in photoreactions, allow monitoring the time evolution of different reactions by observing the changes observed in the SERS bands of the adsorbed species.
Keywords
SERS spectroscopy; Catalytic reactions; Metal nanoparticles
Introduction
Nanoparticles have unique properties, different from those of both isolated molecules and bulk materials. This is due, at least in part, to their great capacity to adsorb molecules or ions, by virtue of their large surface development. Such species can establish strong interactions with the "active sites" of the nanoparticle surface, seen as structural defects of the same surface with special chemical valences, that is, with the possibility of forming bonds different depending on the different species that are close to them. The formation of surface complexes demonstrates the reactivity of the nanostructured surface, which can catalyze chemical reactions as well as interact with biological systems. From here possible applications of the nanoparticles themselves are deriving, especially in the field of heterogeneous catalysis for both chemical and photochemical reactions. In addition, nanoparticles composed of metals have particular optical properties, due to the presence of “localized plasmons”. When invested by an appropriate electromagnetic wave, conduction electrons of a microscopic or macroscopic portion of a metal oscillate freely at the so-called plasma frequency and the metal appears as a reflective surface. When instead they are trapped in a nanometric portion of a metal their ability of movement is limited by the surface and the metal appears colored. The collective excitations of the conduction electrons belonging to a metal nanoparticle take the name of localized plasmons. These latter are basic to have huge enhancements of the Raman signal of molecules adhering to the surface of metal nanoparticles. In Raman scattering, a photon is scattered with energy different from that of the exciting radiation, depending on the vibrational modes of the molecule itself. Analyzing the frequency-shifts of the Raman signal of a molecule with respect to the frequency of the incident radiation, it is possible to obtain a set of vibrational bands, which constitute a valid fingerprint of the molecular material.
Raman scattering, however, has very weak intensity, so it is very difficult, under normal conditions, to observe the Raman response of a limited number of molecules. Nevertheless, the strong localization of the electromagnetic field associated with the localized plasmons allows obtaining an enhancement of several orders of magnitude (usually up to 107 factors) of the Raman response in the so called SERS (surfaceenhanced Raman scattering) effect [1], when molecules are adsorbed on nanostructured surfaces of metals with high optical reflectivity, such as Ag, Au, Cu. In addition to this mechanism, also a chemical enhancement contribution to the Raman signal of the adsorbed molecule can be effective, due to the perturbation of the molecular polarizability because of the formation of chemical complexes of the molecule itself with the active sites of the metallic surface. This latter contribution provides enhancement factors only up to 102 but gives rise to sizeable changes in both positions and intensities of the SERS bands with respect to those observed in the normal Raman spectrum of the non-adsorbed molecules. Hence, by means of the SERS enhancement Raman spectroscopy allows identifying, at trace level, reactants or products or by-products of catalytic reactions occurring where they take place, on the surface of the metal nanoparticles. Unfortunately, the metals providing efficient SERS enhancement for adsorbed molecules are not generally effective catalysts. Silver, which is by far the most suitable metal to provide SERS intensification, is not generally able to promote reactions of this kind. Therefore, efforts have been spent to "activate" silver nanoparticles, because they could guarantee maximum SERS enhancement and at the same time act as catalysts in reduction reactions. In order to extend the range of possible reactions, we have also manufactured bimetallic nanoparticles, made of silver as SERS-active metal and another metal with strong performance in heterogeneous catalysis like palladium, or metal oxide such as titania, extremely active in photoreactions. This article, therefore, shows the main results obtained over decades by me and my co-workers in this field of research, along with future developments of the SERS technique in the study of catalytic processes.
Activation of Silver Nanoparticles
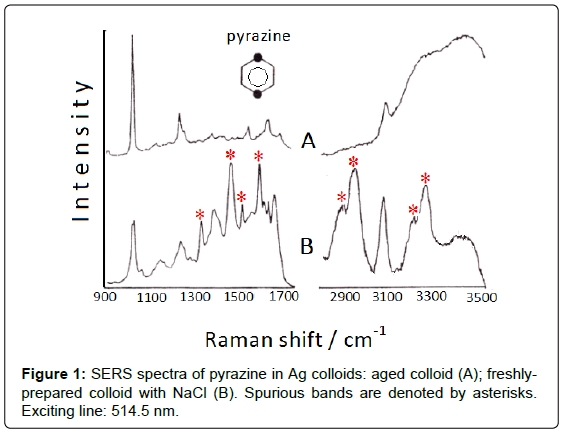
Addition of small amount of halide anions, as Cl- or Br-, which are strongly adsorbed on silver colloids, induces the formation of positive charges on the surface of the Ag nanoparticles, which thus become “anion-activated” [2]. Actually, these anions produce a strong enhancement of the SERS signal of the ligand and can also promote catalytic reactions. Hence, unexpected bands in the SERS spectra can appear, as observed for diazines (pyrazine, pyrimidine, pyridazine) adsorbed on Ag colloids activated by addition of NaCl [3]; in particular, the SERS spectrum of pyrazine (Figure 1) shows many intense bands that are attributable to di-hydro-pyrazine and the correspond dimer, as ascertained by means of FAB (Fast Atom Bombardment). As shown in Figure 1B, these bands are observed in the 1300-1700 cm-1 region as well as in the 2800-3400 cm-1 region, where the NH and CH stretching vibrations of aliphatic groups occur. However, a residual amount of borohydride, used as reductant of silver ions to obtain the colloidal silver, is necessary for these reduction processes. Actually, all the “spurious” bands disappear by addition of pyrazine to one-week aged colloid, when borohydride is totally transformed into borate (Figure 1A).
Aromatic nitro-derivatives (Ar-NO2) can undergo reduction in the presence of reductants, giving rise to aromatic amino-derivatives or azo-derivatives, according to the following reactions, which involve six or four electrons, respectively:
Ar-NO2 → Ar-NH2 (amino-derivative)
Ar-NO2 → Ar-NH-OH → Ar-N=N-Ar (azo-derivative)
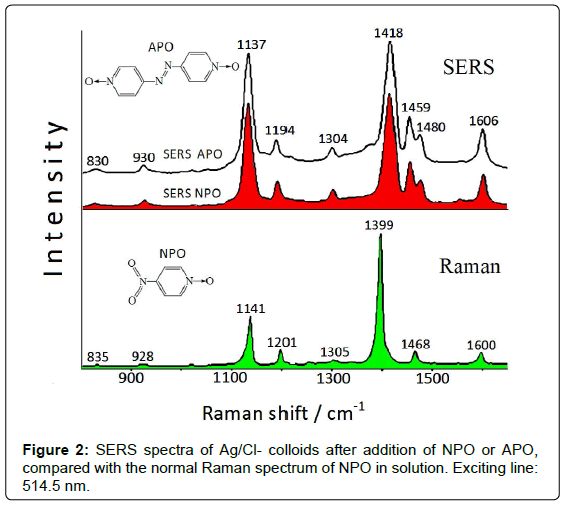
If nitroarenes are adsorbed on Ag colloids, these reactions can occur on the metal substrate by catalytic effect of the nanostrutured surface, as denoted by SERS spectroscopy. The SERS spectrum obtained in freshly-prepared Ag colloid after addition of 4-nitro-(pyridine N-oxide) (NPO) shows bands that cannot be ascribed to NPO [4], but are attributable to 4,4’-azobis-(pyridine N-oxide) (APO), obtained by reduction of the nitrogroup of NPO. The Raman and SERS spectra of NPO are reported in Figure 2.
This reaction is very likely due to the presence of residual borohydride, used as reducing agent in the preparation of the Ag colloidal dispersions. As a confirmation of this catalytic reaction, the SERS spectrum of APO (Figure 2) results identical to that obtained by adding NPO to the Ag colloid. This latter, however, has to be activated by addition of NaCl, because no SERS signal is instead observed in free-chloride colloid. This probably is due to the presence of species (borate anions), deriving from borohydride used as reductant in the colloid preparation, on the metal surface, which impair the adsorption of ligand molecules.
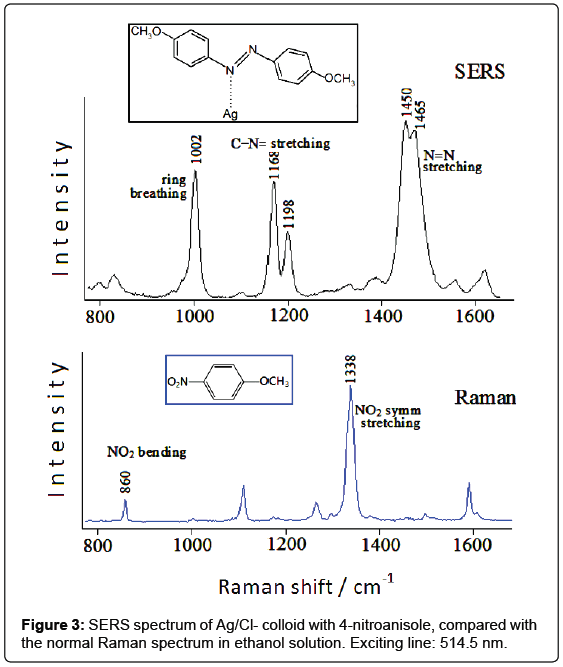
A similar situation is found by analyzing the SERS spectrum of silver colloids after addition of 4-nitroanisole [5], compared with the Raman spectrum of this molecule in solution. This latter (Figure 3) is dominated by the band at 1338 cm-1, ascribed to the symmetric stretching mode of the nitro group. Other intense bands occur around 1600 cm-1, as well as at 1296 cm-1 and 1108 cm-1, attributable to the ring stretching mode of the ring C=C, to the stretching mode of the ring-oxygen bond and to the stretching mode of the methyl-oxygen bond, respectively. Finally, the band at 860 cm-1 can be assigned to the bending vibration of the nitro group.
In the chloride-free Ag colloid no Raman band is detected; in the silver colloid activated by chloride anions, instead, strong SERS bands occur at 1002, 1168, 1198, 1450, 1465 cm-1 (Figure 3). It is evident that most of these bands cannot be related to 4-nitroanisole; moreover, the bands due to the nitrogroup, observed in the Raman spectra of 4-nitroanisole at 1338 and 860 cm-1, are completely absent in the SERS spectrum. This points out to a catalytic reduction of the nitro group by laser irradiation on the metal surface. In the case of 4-nitroanisole in Ag colloid, a possible guideline to recognize the species formed at the metal surface consists in the comparison of the SERS spectrum with the Raman spectra of the different reduction products of nitrobenzene, as reported in the literature [6]: the observed SERS bands appear quite similar to those of azobenzene, which exhibits intense Raman bands between 1440 and 1500 cm-1 and between 1140 and 1200 cm-1. By considering the formation of a dimeric azo-derivative, the most intense SERS bands observed at 1450 e 1465 cm-1 are attributable to the stretching mode of the N=N bond mixed with other vibrational modes, those at 1168 and 1198 cm-1 to stretching modes of the C-N=bonds, whereas that at 1002 cm-1 can be reasonably assigned to the breathing mode of the aromatic rings. However, an important role is played by the energy of the laser exciting line: if the IR radiation at 1064 nm is used as Raman exciting line, instead of the green light at 514.5 nm, the SERS spectrum does not show the bands attributable to the azoderivative, but other ones corresponding to the Raman bands of 4-nitroanisole. In conclusion, the colloid activation with chloride anions allows observing SERS bands attributable to an azo-derivative formed on the metal surface by photoreduction of nitro group.
The SERS activation of the silver colloids by adsorption of chloride or bromide anions is usually attributed to two possible mechanisms: (i) an increase of the local electromagnetic field by aggregation of silver colloidal particles induced by the addition of electrolytes; (ii) the formation of surface complexes involving ligand molecules, halide anions and positively charged active sites of the silver surface [5]. Actually, the adsorption of ligand molecules can be favored by adding halide anions, because these latter are able to free the active sites of the silver surface from the species deriving from the preparation of the Ag colloids, for example borate ions formed by the oxidation of borohydride with silver ions or also with water, when silver is completely reduced. In our case, this activation can be hardly attributed to an increase of the electromagnetic enhancement due to colloidal aggregation, because this latter is not visible in the UV-visible absorption spectra of our colloids by addition of chloride anions or ligand molecules, but, instead, could derive by both desorption of borate ions and formation of charge-transfer complexes between adsorbed ligand molecules and positively charged active sites of the silver surface. A confirmation of the presence of these charged Ag adatoms was obtained by XPS spectra [7], by observing a non-negligible amount of Ag(I) on the surface of Ag/Cl- colloidal particles. As a conclusion, the surface-active sites of the Ag nanoparticles become more reactive, increasing the chemical SERS enhancement and also the efficiency for catalytic reactions involving the adsorbed molecules.
Bimetallic Nanoparticles
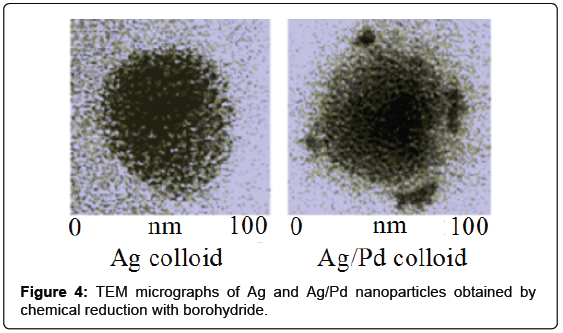
Most catalysts are formed by SERS-inactive nanostructured materials; thus, many efforts have been made to extend the application of the SERS technique from silver, gold or copper to more catalytically important metals such as palladium. Actually, palladium clusters have found widespread application in several classes of catalytic reactions. Ten years ago bimetallic nanoparticles composed by silver and palladium have been prepared [8] by successive metal reduction with borohydride and analyzed showing optical and catalytic properties different from those of monometallic nanoparticles. One hour after the preparation of the Ag colloid, palladium nitrate was added in order to have a mixed Ag/Pd colloid with 96:4 molar ratio. With larger palladium content, the colloidal suspensions showed instability and drastic quenching of the SERS activity. The Ag/Pd nanoparticles have palladium clusters adhering to the surface of spheroidal silver particles, as shown by TEM measurements (Figure 4).
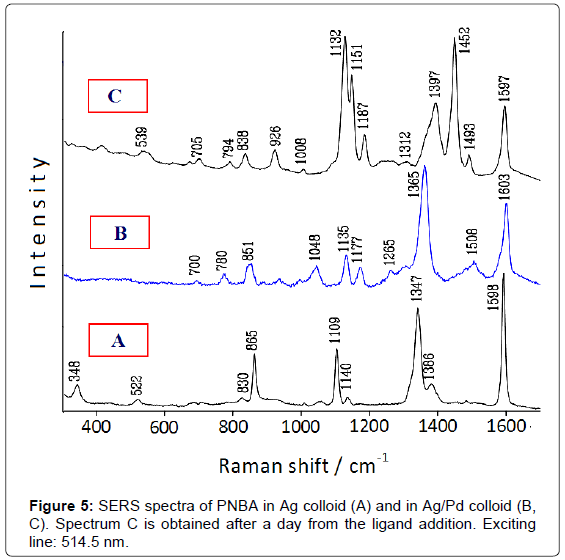
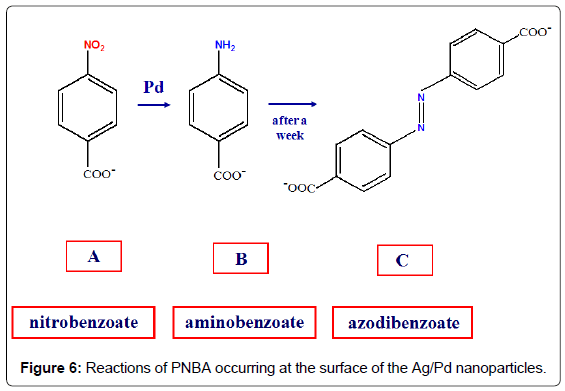
The catalytic performance of these bimetallic nanoparticles is tested by comparing the SERS spectrum of 4-nitrobenzoic acid (PNBA) in Ag/Pd colloid with that in pure Ag colloid [9]. This latter (Figure 5A) shows only bands attributable to the carboxylate anion of PNBA [10], whereas that in Ag/Pd colloid (Figure 5B) corresponds to the SERS spectrum of 4-amino-benzoate (PABA), as reported in the literature [11]. After 1 day, however, this latter spectrum drastically changes and strong SERS bands, attributable to the azo-dibenzoate anion [11], are detected (Figure 5C).
Hence, the SERS spectroscopy allows monitoring the catalytic processes occurring at the surface of the bimetallic nanoparticles, as shown in Figure 6: first, the reduction of the nitrogroup of PNBA to produce PABA; then, the oxidation of PABA with dimerization and formation of azo-dibenzoate in the presence of atmospheric oxygen [9]. These reactions are catalysed by palladium, because they do not occur in pure silver colloids, as observed by SERS. Also in this case the presence of borohydride is necessary for the reduction of PNBA in Ag/Pd colloids, because the SERS spectrum in aged Ag/Pd hydrosol, when the residual borohydride is almost completely transformed into borate, does not show the presence of reaction products, but closely corresponds to that observed in pure Ag colloid.
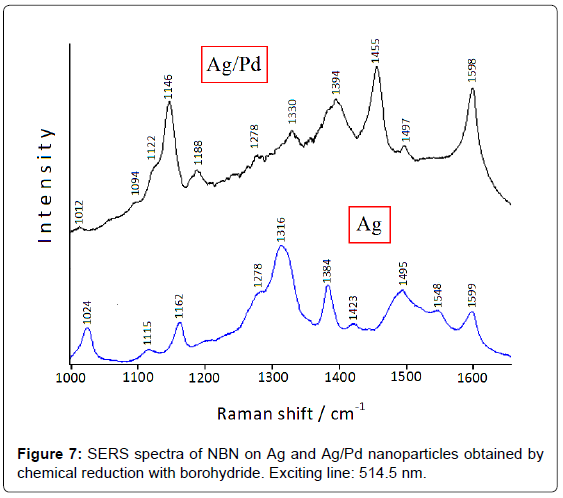
However, the colloidal instability of these bimetallic systems can represent a problem for monitoring catalytic reactions [4]; on the other hand, the use of colloid stabilizers could impair the adsorption of ligands or the catalytic activity of the metal substrate. Hence, Ag/ Pd colloidal nanoparticles have been deposited on satin-finished quartz plates to avoid particle aggregation [12]. The catalytic reduction of the nitrogroup is detected by comparing the SERS spectra of 4-nitrobenzonitrile (NBN) adsorbed on both Ag and Ag/Pd nanoparticles immobilized on quartz [13], as shown in Figure 7: the NO2 symmetric stretching band observed on silver at 1316 cm-1 dramatically decreases in intensity on Ag/Pd substrate, whereas very intense bands occur at 1455 and 1146 cm-1. These latter are attributable to the stretching modes of the N=N and C-N= bonds of the azo-derivative, respectively, in close analogy with 4-nitrobenzoate, which is reduced to azo-dibenzoate in Ag/Pd hydrosol, as shown in Figure 5.
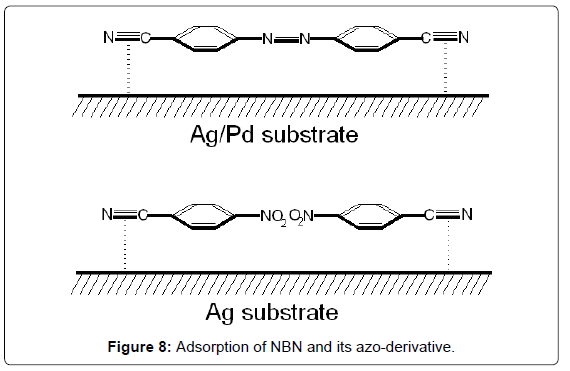
Figure 8 shows the adsorptions of NBN and its azo-derivative, 4,4’-azobis-(benzonitrile), on Ag and Ag/Pd surfaces, respectively, by interaction of the nitrile groups with the metal colloidal surface.
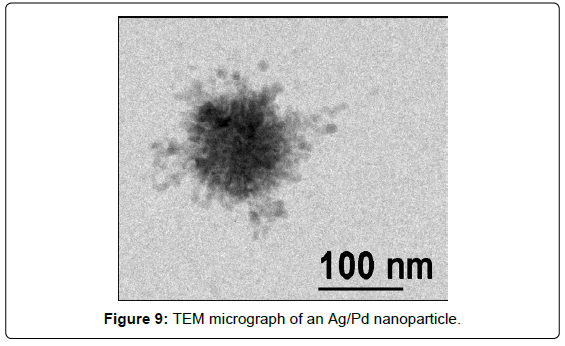
A new method to obtain SERS-active Ag/Pd colloidal nanoparticles can be proposed, consisting in a two-step procedure [14]: first, silver nanoparticles are obtained in water suspension by pulsed laser ablation; then, palladium nitrate is added to the silver colloid, producing by galvanic replacement Pd clusters outside the nanoparticle Ag core (Figure 9).
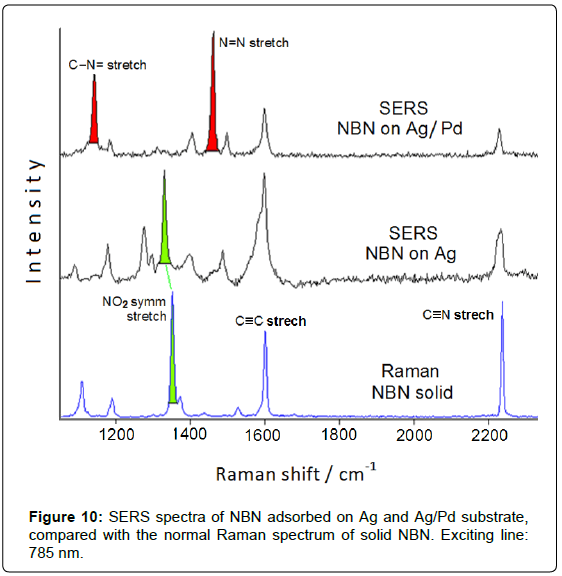
It is important to emphasize that these bimetallic nanoparticles are not capped by surfactants or organic stabilizers and are free from the presence of reducing agents and corresponding reduction by-products, which could interfere in the SERS detection of the adsorbed organic species. This is quite important for monitoring catalytic reactions occurring at the metal surface. The SERS and catalytic efficiency are checked by adsorption of NBN: the SERS spectrum obtained on Ag/Pd nanoparticles deposited on aluminium plate (Figure 10) evidences the presence of the azo-derivative, as well as observed on Ag/Pd colloidal nanoparticles obtained by chemical reduction with borohydride (Figure 7).
In general, the use of SERS-active bimetallic substrates is very useful in monitoring the catalytic reactions that take place on the surface of the nanoparticles, as above extensively shown. However, two problems that may arise in this investigation are to be considered:
1) The bimetallic colloidal suspensions usually show larger instability compared to that of pure silver colloids and this can impair an efficient reproducibility of the SERS data;
2) The introduction of catalyzing but non-SERS metals as palladium in the formation of the bimetallic nanoparticles provokes a sizeable quenching of the Raman enhancement; therefore it is necessary that both the amount and the timing of the addition of this metal are carefully assessed, in order to ensure a satisfactory catalytic activity for the bimetallic platforms, along with a SERS response sufficient to the detection of reactants and products.
Silver/Titania Nanoparticles
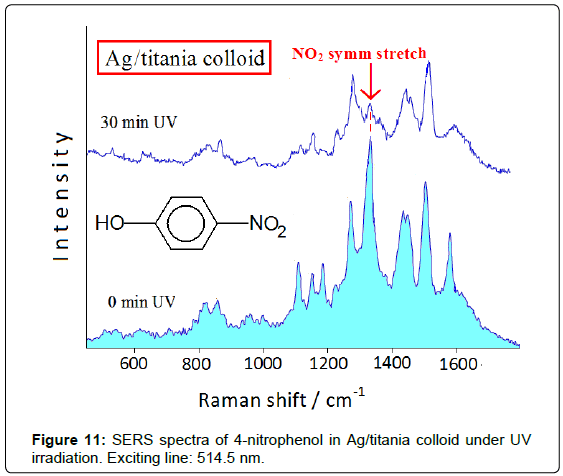
Thin films of titanium oxide (titania), formed of nanosized particles in the anatase form [15], show very high photocatalytic efficiency under UV irradiation. It is possible to combine the catalyzing properties of titania and the spectroscopic ones of SERS-active metals by doping colloidal titania with Ag nanoclusters obtained by reduction of silver ions with borohydride [16]. In this way the SERS spectroscopy allows studying the photocatalytic reactions occurring at the Ag/ titania surface, by observing the spectroscopic changes under UV irradiation and recognizing the adsorbed reaction products. The degradation of nitrophenols represents a big challenge for the environmental chemistry, mainly regarding 4-nitrophenol that rapidly soaks the soil, where it is accumulated with devastating effects for the environment. Figure 11 shows the spectral changes in the SERS spectrum of 4-nitrophenol adsorbed on Ag/titania colloid under UV irradiation (254 nm). The symmetric stretching band of the nitro group at about 1330 cm-1 decreases in intensity up to disappearing with the irradiation time, along with a marked lowering of the overall SERS enhancement. This effect is not observed in pure Ag hydrosol after irradiation, because in this case the SERS bands do not undergo modification in both positions and intensities. The spectral changes occurring by UV irradiation can be related to the catalyzing action of the Ag/titania nanoparticles, which provide by UV irradiation the electrons involved in the reduction of nitro group to amino group. The drastic decrease of the SERS enhancement under UV irradiation can be explained by considering that 4-aminophenol, produced by effect of the Ag/ titania nanoparticles, does not strongly interact with the substrate, as, instead, occurs when the electron-drawing nitro group is present. This Ag/titania nanocomposites exhibit high stability in time, in both the absence and the presence of ligand, as well as during the UV irradiation, as monitored by UV-visible absorption spectra, where the plasmon resonance band of the nanosized silver at 410 nm does not undergo any change. This highlights the photocatalytic effect of the UV irradiation on the adsorbate, as shown by the SERS spectra, and not to possible modifications of the substrate.
Conclusion
This work wants to illustrate the potential of the SERS spectroscopy applied to the processes of heterogeneous catalysis, which occur at the nanostructured surface of metals or metal oxides. Actually, this technique combines high chemical specificity, due to the set of vibrational bands belonging to the molecular adsorbate, and high sensitivity, due to both electromagnetic and chemical enhancement mechanisms of the Raman signal. This study was focused on the optical and catalytic properties of silver colloidal nanoparticles, when activated by adsorption of chloride ions and when mixed with palladium as catalyzing metal or with titania as photoactive material. In this way, chemical reactions of adsorbed species can be observed in time as they proceed on the catalyst surface, allowing detecting reaction products and intermediates under in situ ambient conditions. In particular, Ag/titania colloidal nanocomposites are able to promote photodegradation processes of dangerous pollutants by effect of the catalytic activity of titania, which can be monitored by SERS investigation.
In the future, the application of the SERS technique in heterogeneous catalysis will be extended to different reactions, by introducing other catalyzing metals as nickel [17] or ruthenium [18] and other photoactive metal oxide like ZnO [19] in the formation of nanoparticles containing SERS-active metals like silver or gold.
Acknowledgements
The author gratefully thanks Dr. Barbara Pergolese, Dr. Emilia Giorgetti and Dr. Cristina Gellini for their contributions to the research described in this paper.
References
- Schlücker S (2011) Surface Enhanced Raman Spectroscopy. Wiley-VCH, Weinheim.
- Muniz-Miranda M, Sbrana G (1996) Evidence for Surface Ag+ Complex Formation by an Anion-Induced Effect in the SER Spectra of Phthalazine Adsorbed on Silver Sols. J Raman Spectrosc 27: 105-110.
- Muniz-Miranda M, Neto N, Sbrana G (1988) SER spectra of diazines adsorbed on silver sols: spectroscopic evidence of reduction products. J Mol Struct 174: 351-356.
- Pergolese B, Muniz-Miranda M, Sbrana G, Bigotto A (2006) Surface-enhanced Raman scattering investigations of 4-nitro(pyridine N-oxide) and 4,4'-azo.bis(pyridine N-oxide) adsorbed on silver colloidal nanoparticles. Faraday Discuss 132: 111-120.
- Muniz-Miranda M (2013) SERS investigation on the adsorption and photoreaction of 4-nitroanisole in Ag hydrosols. J Raman Spectrosc 44: 1416-1421.
- Gao P, Gosztola D, Weaver MJ (1988) Surface-enhanced raman spectroscopy as a probe of electroorganic reaction pathways. 1. Processes involving adsorbed nitrobenzene, azobenzene, and related species. J Phys Chem 92: 7122-7130.
- Pagliai M, Muniz-Miranda F, Schettino V, Muniz-Miranda M (2012) Competitive Solvation and Chemisorption in Silver Colloidal Suspensions. Progr Colloid Polym Sci 139: 39-44.
- Pergolese B, Bigotto A, Muniz-Miranda M, Sbrana G (2005) Gold/Palladium and Silver/Palladium Colloids as Novel Metallic Substrates for Surface-Enhanced Raman Scattering. Appl Spectrosc 59: 194-199.
- Muniz-Miranda M, Pergolese B, Bigotto A (2006) SERS monitoring of Pd-catalysed reduction processes of nitroarenes adsorbed on Ag/Pd colloidal nanoparticles. Chem Phys Lett 423: 35-38.
- Muniz-Miranda M, Innocenti M, Foresti ML (2006) Relation between the photoreaction of p-nitrobenzoic acid onto silver-coated filters and the surface roughness, as detected by SERS and AFM. Surf Sci 600: 2096-2102.
- Park H, Lee SB, Kim K, Kim MS (1990) Surface-enhanced Raman scattering of p-amino-benzoic acid at silver electrode. J Phys Chem 94: 7576-7580.
- Pergolese B, Muniz-Miranda M, Bigotto A (2007) Catalytic activity of Ag/Pd bimetallic nanoparticles immobilized on quartz surfaces. Chem Phys Lett 438: 290-293.
- Muniz-Miranda M, Pergolese B, Bigotto A (2008) Surface-Enhanced Raman Scattering and Density Functional Theory Study of 4-Nitrobenzonitrile Adsorbed on Ag and Ag/Pd Nanoparticles. J Phys Chem C 112: 6988-6992.
- Muniz-Miranda M, Gellini C, Canton P, Marsili P, Giorgetti E (2014) SERS and catalytically active Ag/Pd nanoparticles obtained by combining laser ablation and galvanic replacement. J Alloys Comps 615: S352-S356.
- Peng T, Zhao D, Dai K, Shi W, Hirao K (2005) Synthesis of titanium dioxide nanoparticles with mesoporous anatase wall and high photocatalytic activity. J Phys Chem B 109: 4947-4952.
- Muniz-Miranda M (2014) SERS monitoring of the catalytic reduction of 4-nitrophenol on Ag-doped titania nanoparticles. Appl Catalysis B Environ 146: 147-150.
- Rathore PS, Patidar R, Rathore S, Thakore S (2014) Nickel Nanoparticles as Efficient Catalyst for Electron Transfer Reactions. Catalysis Lett 144: 439-446.
- Niu M, Wang Y, Li W, Jiang J, Jin Z (2013) Highly efficient and recyclable ruthenium nanoparticle catalyst for semihydrogenation of alkynes. Catalysis Commun 38: 77-81.
- Pudukudy M, Yaakob Z (2015) Facile Synthesis of Quasi Spherical ZnO Nanoparticles with Excellent Photocatalytic Activity. J Cluster Sci 26: 1187-1201.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16258
- [From(publication date):
December-2015 - May 31, 2024] - Breakdown by view type
- HTML page views : 11745
- PDF downloads : 4513