Research Article Open Access
The Sensory Impact of Nicotine on Noradrenergic and Dopaminergic Neurons of the Nicotine Reward - Addiction Neurocircuitry
Jed E Rose1, Ozra Dehkordi2,3*, Kebreten F Manaye3, Richard M Millis4, Salman Ameri Cianaki2 and Annapurni Jayam-Trouth2
1Department of Psychiatry, Duke University Medical Centre, Durham, N.C. 27705, United States
2Department of Neurology, Howard University Hospital, Washington, D.C. 20060, United States
3Department of Physiology & Biophysics, Howard University College of Medicine, Washington, D.C. 20059, United States
4Department of Medical Physiology, American University of Antigua College of Medicine, St. John’s, Antigua and Barbuda
- Corresponding Author:
- Dehkordi Ozra
Associate Professor, Department of Neurology
Howard University Hospital, Washington, United States
Tel: +1 202 865 1978
Fax: +1 202 865 1977
E-mail: odehkordi@howard.edu
Received date: Feb 16, 2016; Accepted date: April 01, 2016; Published date: April 07, 2016
Citation: Rose JE, Dehkordi O, Manaye KF, Millis RM, Cianaki SA, et al. (2016) The Sensory Impact of Nicotine on Noradrenergic and Dopaminergic Neurons of the Nicotine Reward - Addiction Neurocircuitry. J Addict Res Ther 7:274. doi:10.4172/2155-6105.1000274
Copyright: © 2016 Rose JE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
The sensory experience of smoking is a key component of nicotine addiction known to result, in part, from stimulation of nicotinic acetylcholine receptors (nAChRs) at peripheral sensory nerve endings. Such stimulation of nAChRs is followed by activation of neurons at multiple sites in the mesocorticolimbic reward pathways. However, the neurochemical profiles of CNS cells that mediate the peripheral sensory impact of nicotine remain unknown. In the present study in mice, we first used c-Fos immunohistochemistry to identify CNS cells stimulated by nicotine (NIC, 40 μg/kg, IP) and by a peripherally-acting analog of nicotine, nicotine pyrrolidine methiodide (NIC-PM, 30 μg/kg, IP). Sequential double-labelling was then performed to determine whether noradrenergic and dopaminergic neurons of the nicotine reward-addiction circuitry were primary targets of NIC and NIC-PM. Double-labelling of NIC and/or NIC-PM activated c-Fos immunoreactive cells with tyrosine hydroxylase (TH) showed no apparent c-Fos expression by the dopaminergic cells of the ventral tegmental area (VTA). With the exception of sparse numbers of TH immunoreactive D11 cells, dopamine-containing neurons in other areas of the reward-addiction circuitry, namely periaqueductal gray, and dorsal raphe, were also devoid of c-Fos immune-reactivity. Noradrenergic neurons of locus coeruleus (LC), known to innervate VTA, were activated by both NIC and NIC-PM. These results demonstrate that noradrenergic neurons of LC are among the first structures that are stimulated by single acute IP injection of NIC and NIC-PM. Dopaminergic neurons of VTA and other CNS sites, did not respond to acute IP administration of NIC or NIC-PM by induction of c-Fos.
Keywords
Nicotine; Nicotine pyrrolidine methiodide; Tyrosine hydroxylase; c-Fos; Addiction; Ventral tegmental area; Locus coeruleus
Introduction
Addictive properties of nicotine in tobacco smoke stem from activation of neurons comprising the brain’s mesocorticolimbic reward pathways. These neurons are activated either directly or indirectly through nicotinic acetylcholine receptors (nAChRs), widely distributed in the central and peripheral nervous systems [1-4]. The direct effects of nicotine on CNS neurons are critical for its reward and addiction properties [5-9]. Additionally, the sensory impacts of nicotine, mediated by exteroceptive and interoceptive sensory nerve endings in the gustatory, respiratory and circulatory systems, are important components of the nicotine reward-addiction circuitry [10-17]. The importance of the peripheral sensory impact of nicotine in its reward and addiction properties is documented in recent animal studies from our laboratory [17]. We demonstrated that nicotine pyrrolidine methiodide (NIC-PM), a peripherally-acting analog of nicotine, that does not cross the blood brain barrier, activates virtually all the CNS sites that are direct targets of nicotine. However, the neurochemical profiles of cells that mediate the sensory impact of nicotine in the CNS are not known.
Of numerous neurotransmitters that mediate different aspects of nicotine addiction, dopaminergic neurons of the midbrain ventral tegmental area (VTA) are particularly important because of their critical role in the reinforcing effects of nicotine [18-19]. Dopaminergic neurons of VTA send projections to two principal targets, the ventral striatum (nucleus accumbens and olfactory tubercle) and prefrontal cortex (PFC). These projections control reward-related behaviours by affecting reinforcement, learning and memory [20]. In addition to VTA, dopaminergic neurons located in periaqueductal gray (PAG), dorsal raphe nucleus (DR) and hypothalamus are known to project to areas implicated in drug addiction [21], suggesting that these neurons could also participate in nicotine addiction. The cascade of molecular events that leads to activation of dopaminergic neurons following systemic exposure to nicotine is not fully known and the neurochemical profile of CNS neurons targeted directly by nicotine and/or its peripheral sensory impact is not clear. We previously demonstrated that nicotine (NIC) and NIC-PM activate neurons at multiple CNS sites including areas overlapping VTA, PAG, DR, hypothalamus and locus coeruleus (LC), known to contain dopaminergic and noradrenergic neurons [17]. The present study was, therefore, designed to determine whether dopaminergic and noradrenergic neurons of the reward-addiction neurocircuitry are the immediate targets of acute intraperitoneal administration of NIC and/or NIC-PM.
Materials and Methods
Subjects
Experiments were performed in adult, wild-type CD-1 mice weighing 20-25 g. All procedures including the anaesthesia and surgery were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Howard University. All efforts were made to minimize the number of animals used and their suffering.
Experimental protocol
Animals (N = 15) were housed at room temperature (22-24°C) with water and food freely available. To reduce the nonspecific effects of handling and experimental environment, animals were handled daily and exposed to the same conditions as during the actual experiments. Following an adaptation period of 3-4 d, the mice were treated by IP administration of physiological saline (control; PS), nicotine hydrogen tartrate salt (NIC, Sigma Aldrich, St. Louis, MO) and/or NIC-PM (Toronto Research Chemicals, Toronto, Ontario, Canada), a peripherally-acting nicotine analog that does not cross the blood-brain barrier [22-24]. In the present study, NIC was used at a dose of 40 μg/kg. The dose of NIC used in the present study is within the range reported as optimal for maintaining IV self-administration of nicotine in rats [25,26] and comparable to doses of nicotine delivered by smoking 1-2 cigarettes [27]. This dose is also within the range reported to induce c-Fos activations at multiple brain regions [17]. The NIC-PM dose in the present study (30 μg/kg) is approximately equimolar to the NIC dose (40 μg/kg). Both NIC and NIC-PM were dissolved in PS (vehicle) and injected IP in volumes of 0.2 ml/injection. Two hours after IP injection of PS, NIC and/or NIC-PM, the mice were anesthetized with 5% isoflurane and perfused transcardially with saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB) at pH 7.4. After perfusion, the brains were postfixed in 4% paraformaldehyde for 1 h and then cryoprotected in a 30% sucrose solution for a minimum of 2 d. Transverse sections of the brain were cut at 40 μm using a Bright OTF Cryostat (Hacker Instruments and Industries) and stored in 0.5% sodium azide in 0.1 M PB (pH 7.4).
Immunohistochemistry
Immunohistochemical procedures were performed using free floating sections as follows: Briefly, 1-in-5 series of brain sections extending from bregma –5.63 mm to bregma 2.33 mm [28] were rinsed 3 times in 0.1 M phosphate-buffered saline (PBS) at pH 7.4. Nonspecific binding was blocked by incubating the tissues overnight in loading buffer containing 2% normal donkey serum (NDS, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and 0.3% Triton X-100. Tissues were then washed and processed for sequential double labelling of NIC-, NIC-PM- and/or PS-induced c-Fos expressing cells with tyrosine hydroxylase (TH) immunoreactive (IR) cells according to the following protocols.
Tissues were washed and incubated with a PBS cocktail consisting of 0.3% Triton X-100, rabbit anti-c-Fos (1:5000 of Cat # PC38, Millipore Corporation, Temecula, CA) and mouse anti-TH (TH: 1:1000; Cat # T1299, Sigma-Aldrich) antibody at 4°C for 48 h. The sections were then washed and incubated in Alexa Fluor 594 donkey anti-rabbit secondary antibody (1:100; Jackson ImmunoResearch Laboratories Inc.) in 0.1 M PBS for 2½ h. After washing in PBS, sections were incubated with Alexa-Fluor 488 donkey anti-mouse (1:100, Jackson) for 2½ h. Finally, the sections were rinsed in PBS and cover-slipped using Vecta Shield (Vector Laboratories) anti-fade mounting media.
Controls for each experiment were performed to determine whether the primary or the secondary antibodies produced false-positive results. The controls involved omission of the primary and/or secondary antisera to eliminate the corresponding specific labelling. Nonspecific activation of c-Fos was assessed by evaluating the CNS expression of c-Fos in animals receiving IP injection of physiological saline.
Data analysis
High resolution fluorescent images were acquired using Nikon (Nikon Instruments, Melville, NY) and Confocal (Olympus AX70, Olympus America) microscopes equipped with the adequate filter systems to observe the red and green fluorescence. Co-localization of the PS-, NIC or NIC-PM-induced c-Fos immunoreactive (IR) cells with TH-positive adrenergic and/or dopaminergic cells was detected by sequential capturing of the images, alternating between filters appropriate for each labelling and by analyzing the merged images of the exact same sites. Images from all the brain regions of interest were captured at 4X, 10X and 20X magnification and minor adjustments of brightness and contrast were made using Adobe Photoshop CS3.
Cell counting
A semi-quantitative estimate of the total number of NIC-, NIC-PMor PS- induced c-Fos-activated cells in LC that were TH IR was performed as follows: Three 40 μm sections from different rostrocaudal levels of LC were selected for each group (N = 4). We counted the total numbers of NIC-, NIC-PM- or PS-induced c-Fos IR cells, the total number of cells that exhibited TH immunoreactivity and the number of c-Fos IR cells in LC that co-expressed TH. One-way ANOVA followed by post-hoc t-test was employed to evaluate the effects of the PS, NIC and NIC-PM treatments on number of LC cells that were activated by c-Fos and co-expressed TH. The data were expressed as means ± standard deviations. The results were considered significant at P < 0.01.
Results
Neuroanatomical location of NIC and NIC-PM activated cells with respect to noradrenergic cells of LC.
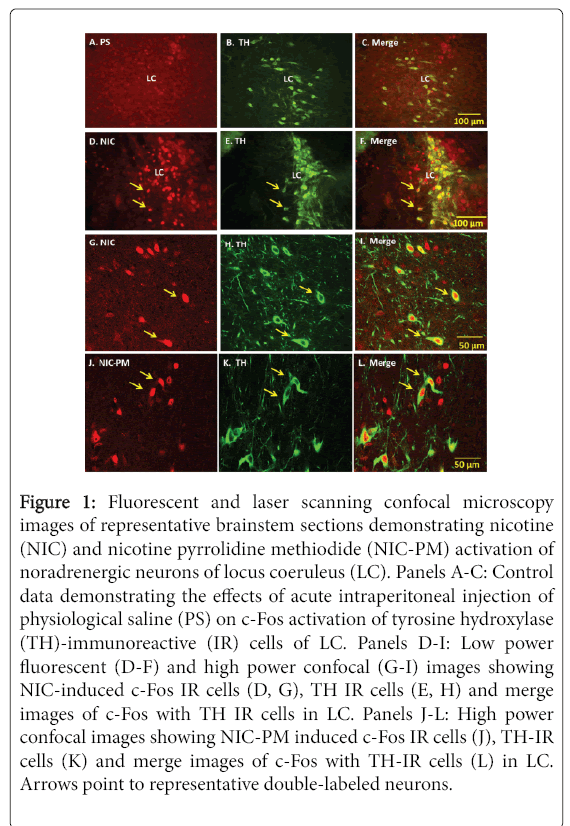
Consistent with our previous studies [17], NIC and NIC-PM both produced c-Fos activation of neurons at areas overlapping LC. Doublelabelling of c-Fos with TH demonstrated that a large proportion of the cells stimulated by NIC and NIC-PM were noradrenergic neurons of LC (Figures 1).
Figure 1: Fluorescent and laser scanning confocal microscopy images of representative brainstem sections demonstrating nicotine (NIC) and nicotine pyrrolidine methiodide (NIC-PM) activation of noradrenergic neurons of locus coeruleus (LC). Panels A-C: Control data demonstrating the effects of acute intraperitoneal injection of physiological saline (PS) on c-Fos activation of tyrosine hydroxylase (TH)-immunoreactive (IR) cells of LC. Panels D-I: Low power fluorescent (D-F) and high power confocal (G-I) images showing NIC-induced c-Fos IR cells (D, G), TH IR cells (E, H) and merge images of c-Fos with TH IR cells in LC. Panels J-L: High power confocal images showing NIC-PM induced c-Fos IR cells (J), TH-IR cells (K) and merge images of c-Fos with TH-IR cells (L) in LC. Arrows point to representative double-labeled neurons.
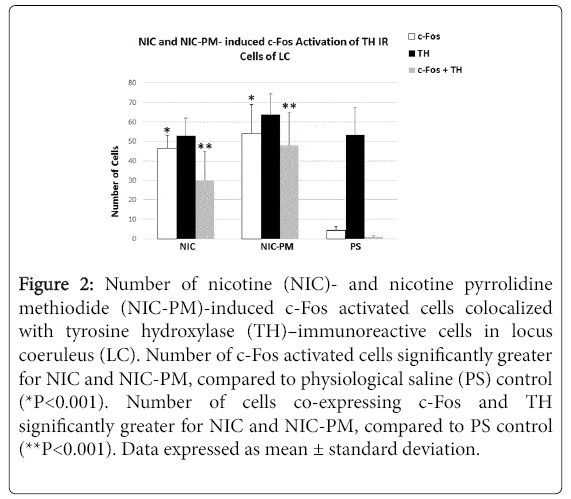
Semi-quantification of c-Fos immunoreactive (IR) cells in LC showed that the number of c-Fos activated cells was significantly greater in the NIC- and NIC-PM-treated animals compared to the physiological saline (PS) treated controls (46.50 ± 6.79 cells and 54.17 ± 14.84 cells respectively, compared to 4.41 ± 1.98 cells, P < 0.001).
The number of c-Fos activated cells was also greater in NIC-PM compared to NIC treated animals. Double-labelling of c-Fos activated cells with TH demonstrated a significantly greater number of cells that were both c-Fos- and TH-positive in the NIC- and NIC-PM-treated animals than in the PS controls (29.83 ± 15.07 and 47.87 ± 17.01 respectively, compared to 1.5 ± 0.76, P< 0.001, Figure 2).
Figure 2: Number of nicotine (NIC)- and nicotine pyrrolidine methiodide (NIC-PM)-induced c-Fos activated cells colocalized with tyrosine hydroxylase (TH)–immunoreactive cells in locus coeruleus (LC). Number of c-Fos activated cells significantly greater for NIC and NIC-PM, compared to physiological saline (PS) control (*P<0.001). Number of cells co-expressing c-Fos and TH significantly greater for NIC and NIC-PM, compared to PS control (**P<0.001). Data expressed as mean ± standard deviation.
Neuroanatomical location of NIC and NIC-PM activated cells with respect to dopaminergic cells of the nicotine reward-addiction circuitry
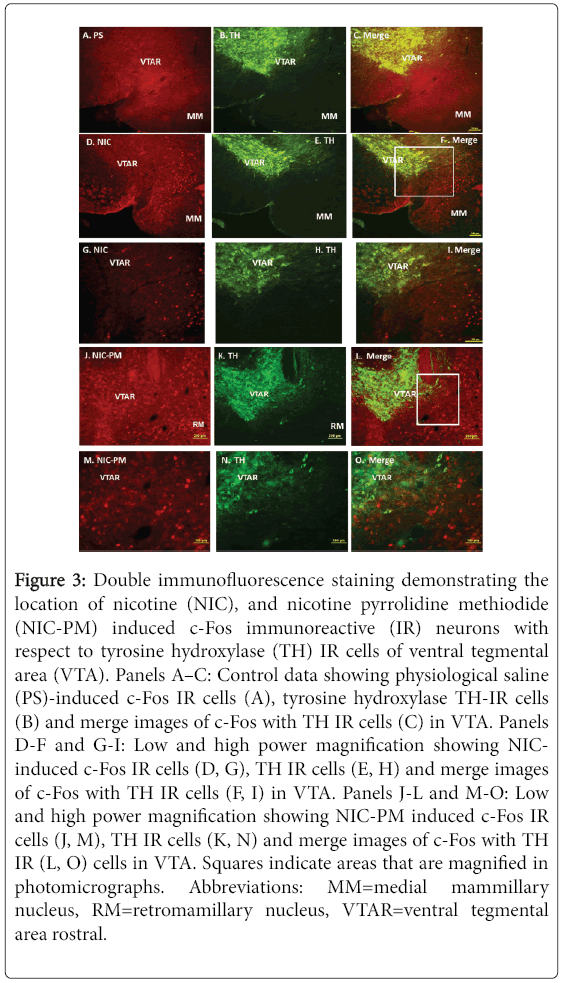
Figure 3 is a photomicrograph of representative midbrain regions illustrating the locations of NIC and NIC-PM c-Fos activated cells with respect to dopaminergic neurons of VTA. Both NIC and NIC-PM produced c-Fos activation of varying intensities in areas overlapping VTA. Double-labelling of c-Fos IR cells with TH demonstrated that the dopaminergic neurons in various subregions of VTA were not activated by NIC and NIC-PM.
Figure 3: Double immunofluorescence staining demonstrating the location of nicotine (NIC), and nicotine pyrrolidine methiodide (NIC-PM) induced c-Fos immunoreactive (IR) neurons with respect to tyrosine hydroxylase (TH) IR cells of ventral tegmental area (VTA). Panels A–C: Control data showing physiological saline (PS)-induced c-Fos IR cells (A), tyrosine hydroxylase TH-IR cells (B) and merge images of c-Fos with TH IR cells (C) in VTA. Panels D-F and G-I: Low and high power magnification showing NICinduced c-Fos IR cells (D, G), TH IR cells (E, H) and merge images of c-Fos with TH IR cells (F, I) in VTA. Panels J-L and M-O: Low and high power magnification showing NIC-PM induced c-Fos IR cells (J, M), TH IR cells (K, N) and merge images of c-Fos with TH IR (L, O) cells in VTA. Squares indicate areas that are magnified in photomicrographs. Abbreviations: MM=medial mammillary nucleus, RM=retromamillary nucleus, VTAR=ventral tegmental area rostral.
In the most caudal extent of VTA (bregma -3.87 mm to -3.51 mm), NIC and NIC-PM activated c-Fos IR cells were sparsely scattered among the dopaminergic cells of paranigral nucleus (PN), parainterfascicular nucleus (PIF) and parabrachial pigmented nucleus (PBP). c-Fos IR cells were also detected at sites medial and ventral to the dopaminergic cells in regions which correspond to interpeduncular nucleus rostral (IPR) and pontine nuclei (Pn). More rostrally in the anterior extension of VTA (bregma -3.15 mm to -3.07 mm), NIC and NIC-PM activated cells were found mainly ventral and medial to the dopaminergic cells of VTA rostral (VTAR) and PBP, at sites which overlapped interfascicular nucleus (IF), rostral linear nucleus (RLi), IPR and retromamillary nucleus (RM) (Figure 3).
c-Fos IR cells were also seen medial and dorsal to the dopaminergic cells of substantia nigra pars compacta (SNC). At the most rostral extension of VTA, in the midbrain-diencephalon border, c-Fos IR cells were found medial and ventral to the dopaminergic cells of VTAR and PBP, at sites that correspond to the posterior hypothalamus area (PHA).
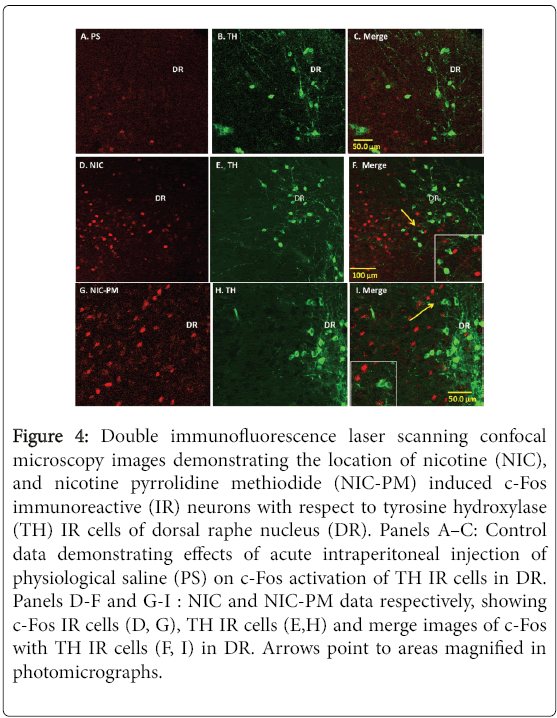
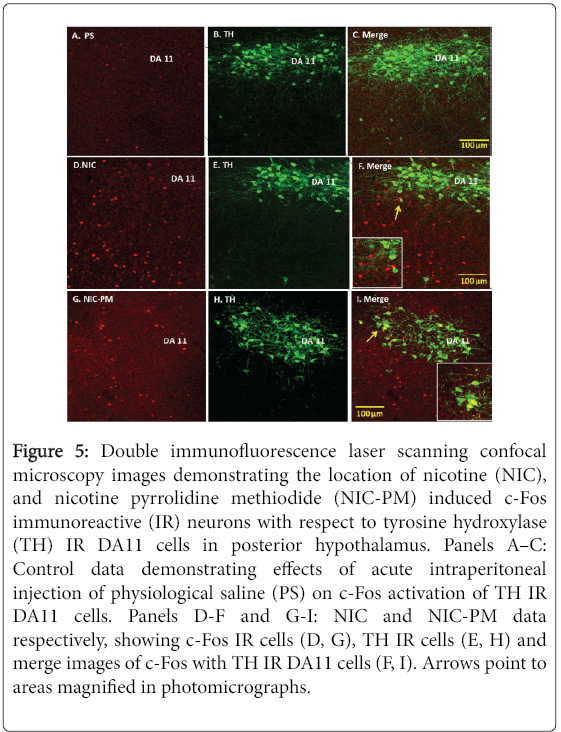
In addition to VTA, we also evaluated the effects of NIC and NICPM on activation of dopaminergic cells in the dorsal caudal extension of VTA, which includes the DR and PAG, as well as dopaminergic cells outside VTA which includes the DA11 cells of posterior hypothalamus and the DA12 cells of arcuate hypothalamic nucleus (Arc). With the exception of a sparse number of DA11 cells in posterior hypothalamus, the dopaminergic cells at these sites were not activated by NIC and/or NIC-PM (Figures 4 and 5). In PAG/DR areas, NIC and NIC-PM activated cells were detected bilaterally near the midline dopaminergic neurons (Figure 4, Panels D-F, G-I). c-Fos IR cells were also found interspersed with dopaminergic neurons of caudal linear nucleus of the raphe (CLI) and medial raphe nucleus (MnR). In posterior hypothalamic regions, DA11 dopaminergic cells were surrounded by NIC and NIC-PM activated cells (Figure 5, Panels D-F, G-I). In Arc, dopaminergic neurons were found intermingled with c-Fos IR cells.
Figure 4: Double immunofluorescence laser scanning confocal microscopy images demonstrating the location of nicotine (NIC), and nicotine pyrrolidine methiodide (NIC-PM) induced c-Fos immunoreactive (IR) neurons with respect to tyrosine hydroxylase (TH) IR cells of dorsal raphe nucleus (DR). Panels A–C: Control data demonstrating effects of acute intraperitoneal injection of physiological saline (PS) on c-Fos activation of TH IR cells in DR. Panels D-F and G-I : NIC and NIC-PM data respectively, showing c-Fos IR cells (D, G), TH IR cells (E,H) and merge images of c-Fos with TH IR cells (F, I) in DR. Arrows point to areas magnified in photomicrographs.
Figure 5: Double immunofluorescence laser scanning confocal microscopy images demonstrating the location of nicotine (NIC), and nicotine pyrrolidine methiodide (NIC-PM) induced c-Fos immunoreactive (IR) neurons with respect to tyrosine hydroxylase (TH) IR DA11 cells in posterior hypothalamus. Panels A–C: Control data demonstrating effects of acute intraperitoneal injection of physiological saline (PS) on c-Fos activation of TH IR DA11 cells. Panels D-F and G-I: NIC and NIC-PM data respectively, showing c-Fos IR cells (D, G), TH IR cells (E, H) and merge images of c-Fos with TH IR DA11 cells (F, I). Arrows point to areas magnified in photomicrographs.
Discussion
The present immunohistochemical studies demonstrate that noradrenergic cells of LC are one of the primary CNS targets for NIC and its peripherally-acting analog NIC-PM. Dopaminergic neurons at various subregions of VTA, as well as those located in PAG, DR, and different hypothalamic regions, did not show c-Fos activation by acute IP administration of NIC and NIC-PM. However, non-dopaminergic neurons within VTA responded to NIC and NIC-PM by induction of c-Fos in this experimental paradigm.
NIC and NIC-PM activation of noradrenergic cells
Activation of noradrenergic neurons in LC by NIC and its peripherally-acting analog NIC-PM suggests that noradrenergic neurons of LC are important components of the nicotine rewardaddiction circuitry. These data are consistent with previous reports demonstrating NIC-induced expression of c-fos mRNA and c-Fos protein in LC [29-33]. Activation of LC by NIC-PM further indicates that LC is also one of the primary sites mediating the peripheral sensory impact of NIC. However, NIC-PM appeared to be more potent than NIC in producing c-Fos activation of LC. The higher potency of NIC-PM compared to NIC may result from higher concentration of NIC-PM at local sensory neurons which may lead to lower volume of distribution, not diffusing as readily into tissues as does NIC. Other factors such as differences in the metabolism of NIC and NIC-PM may also have contributed to this observation.
Our findings that both NIC and NIC-PM induced c-Fos activation of the cells in LC are consistent with previous electrophysiological studies demonstrating that NIC activation of LC is the result of two separable actions; one related to the activation of peripheral nAChRs located on peripheral sensory nerve fibers and another related to the activation of nAChRs in LC and/or other CNS structures [34,35].
In addition to being a target for NIC, LC is believed to be a target for a number of other drugs of abuse including opioids and cocaine [36,37]. This nucleus, which is believed to function as a relay station for peripheral autonomic input to the brain, has widespread projections throughout the entire neuraxis [38]. LC is also involved in the control of alertness and exploratory responses to environmental stimuli [39]. Many structures of the mesolimbic reward pathways, including Acb, VTA, amygdala, and bed nucleus of stria terminalis (BNST) receive noradrenergic input [40-43]. Activation of the noradrenergic neurons in LC is reported to augment activity of the dopaminergic neurons in VTA [44]. Lesions of noradrenergic neurons in LC are also shown to attenuate dopamine release in Acb [45]. Furthermore, noradrenergic blocking agents are purported to be partially effective for modulating the effects of NIC addiction and withdrawal in experimental animals [46,47], and clonidine, an alpha-2 noradrenergic agonist, has shown efficacy in smoking cessation treatment [48]. Thus, results of the present study, demonstrating activation of the noradrenergic neurons in LC by NIC and NIC-PM, implies that LC is a putative site for mediating both the central and the peripheral sensory impacts of NIC.
Effects of NIC and NIC-PM on dopaminergic cells of VTA
The mesocorticolimbic dopaminergic system is known to play a critical role in modulating the rewarding effects of NIC and other drugs of abuse. However, the neurochemical mechanisms which lead to NIC activation of VTA dopaminergic neurons following peripheral administration of NIC have not been fully elucidated. Dopaminergic neurons express mRNAs coding for most neuronal nAChR subunits [49-51]. A combination of single cell PCR, immunohistochemical and electrophysiological studies in brain slices from rat and transgenic mice lacking nAChR subunits provide evidence for the presence of various nAChR subtypes on soma of dopaminergic cells in VTA, on local GABAergic interneurons and on presynaptic glutamatergic axon terminals that innervate dopaminergic neurons of VTA [49,52-57]. These findings suggest that NIC may influence the activity of dopaminergic neurons directly, by acting at somatodendritic or presynaptic nAChRs, or indirectly by modulation of inputs to VTA.
Several studies have used the c-fos gene and c-Fos protein as markers for identifying CNS neurons activated by NIC, including those in VTA. Ren and Sagar [58] reported that IV injection of NIC failed to produce c-fos gene expression in rat VTA and substantia nigra. Shram and associates [32] have demonstrated that acute subcutaneous administration of NIC activates c-fos mRNA in VTA of adolescent, but not of adult, rats. Results of the present study, in adult mice, demonstrates that NIC, acting through central and peripheral nAChRs, causes sparse to moderate c-Fos activation of nondopaminergic neurons in VTA.
These findings are consistent with those of Pang et al. [59] reporting that acute injection of NIC induces c-Fos expression in non-dopaminergic neurons of VTA. Our data are contrary to studies by Zhao-Shea et al. [57] reporting that IP injection of NIC selectively activates a subpopulation of dopaminergic neurons in the posterior VTA but not in the anterior or tail of VTA. These disparate findings may be explained by differences in the doses and routes of administration, the techniques for c-Fos detection and the acclimation of animals prior to the administration of NIC and NICPM [60,61].
The lack of c-Fos expression following acute injection of NIC and NIC-PM in dopaminergic cells of VTA may also be explained by differences in the thresholds for c-Fos activation in the dopaminergic versus the non-dopaminergic cells [61]. Other possibilities are that NIC and NIC-PM induction of c-Fos in the dopaminergic cells may require more time than allowed in this study. It is also possible that induction of c-Fos may not be a sensitive marker for activation of dopaminergic cells in VTA.
Electrophysiological studies have shown that systemic administration of nicotine increases firing activity of dopaminergic cells in VTA [62,63]. These studies have often identified the dopaminergic neurons by their location and/or unique electrophysiological and pharmacological properties. However, VTA is a heterogeneous structure and contains about 40% non-dopaminergic cells, mainly GABAergic and glutamatergic neurons [64]. This heterogeneity of cell types in VTA makes it necessary to precisely identify the neurons being recorded in order to rule out the possibility that a non-dopaminergic neuron might be misidentified as dopaminergic. Other studies evaluating the direct effect of NIC on TH gene expression in cultured dopaminergic neurons from midbrain have shown that although NIC increases TH gene transcription, it is short-lived and is not sufficient to induce TH mRNA or TH protein [65]. These results imply that the induction of TH mRNA and TH protein in midbrain by nicotine may not be solely due to direct interaction of nicotine with nAChRs on dopaminergic cells of VTA. The results of the present study demonstrate that a single IP injection of NIC causes c-Fos activation of noradrenergic cells of LC but not dopaminergic cells of the VTA. These findings are consistent with previous studies demonstrating that a single systemic injection of nicotine increased TH mRNA in LC but not in VTA [66]. Taken together, these findings suggest that NIC-induced c-Fos activation of dopaminergic neurons may require administration of NIC over time, involving trans-synaptic depolarization of dopaminergic neurons.
Conclusion
The main finding of this study is that noradrenergic cells of LC are one of the primary CNS targets mediating the peripheral sensory impact of nicotine. Although dopaminergic cells are important components of the reward-addiction neurocircuitry, they do not appear to respond to a single acute IP injection of NIC or NIC-PM by induction of c-Fos. In addition to LC, multiple brain regions are affected by NIC and NIC-PM [17,67]. Future studies focusing on characterizing the neurochemical profiles of neurons that are directly impacted by nicotine may help shed light on mechanisms which drive reward-addiction signaling within the dopaminergic mesocorticolimbic neurocircuitry.
Acknowledgment
This work was supported by a grant from Philip Morris USA; the company had no role in the design and execution of the study, data analysis or publication of results. The authors acknowledge use of the resources provided by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number G12MD007597.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: OD, JER. Acquisition of data: OD, SAC. Analysis and interpretation of data: OD, RMM. Drafting of the manuscript: OD, RMM. Critical revision of the manuscript for important intellectual content: OD, JER, RMM. Statistical analysis: OD, RMM. Obtained funding: OD, JER. Administrative, technical, and material support: KFM, AJT. Study supervision: OD.
References
- WonnacottS, Barik J, Dickinson J, Jones IW (2006) Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. J MolNeurosci 30: 137-140.
- Dani JA, Bertrand D (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev PharmacolToxicol 47: 699-729.
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89: 73-120.
- Zoli M, Pistillo F, Gotti C (2015) Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 96: 302-311.
- Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275: 1593-1599.
- Wise RA (2009) Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci 32: 517-524.
- Schultz W (2010) Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct 6: 24.
- Ikemoto S (2010) Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. NeurosciBiobehav Rev 35: 129-150.
- De Biasi M, Dani JA (2011) Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci 34: 105-130.
- Gu Q, Ni D, Lee LY (2008) Expression of neuronal nicotinic acetylcholine receptors in rat vagal pulmonary sensory neurons. RespirPhysiolNeurobiol 161: 87-91.
- Rose JE, Zinser MC, Tashkin DP, Newcomb R, Ertle A (1984) Subjective response to cigarette smoking following airway anesthetization. Addict Behav 9: 211-215.
- Rose JE, Tashkin DP, Ertle A, Zinser MC, Lafer R (1985) Sensory blockade of smoking satisfaction. PharmacolBiochemBehav 23: 289-293.
- Rose, JE, Westman EC, Behm FM, Johnson MP, Goldberg JS (1999) Blockade of smoking satisfaction using the peripheral nicotinic antagonist trimethaphan. PharmacolBiochemBehav 62: 165-172.
- Alimohammadi H, Silver WL (2000) Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. ChemSenses 25: 61-66.
- Dehkordi O, Rose JE, Balan KV, Kc P, Millis RM, et al. (2009) Neuroanatomical relationships of substance P-immunoreactive intrapulmonary C-fibers and nicotinic cholinergic receptors. J Neurosci Res 87: 1670-1678.
- Dehkordi O, Rose JE, Balan KV, Millis RM, Bhatti B, et al. (2010) Co-expression of nAChRs and molecules of the bitter taste transduction pathway by epithelial cells of intrapulmonary airways. Life Sci 86: 281-288.
- Dehkordi O, Rose JE, Asadi S, Manaye KF, Millis RM, et al. (2015) Neuroanatomical circuitry mediating the sensory impact of nicotine in the central nervous system. J Neurosci Res 93: 230-243.
- Corrigall WA, Coen KM (1991) Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 104: 171-176.
- Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010) Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68: 815-834.
- Pistillo F, Clementi F, Zoli M, Gotti C (2015) Nicotinic, glutamatergic and dopaminergic synaptic transmission and plasticity in the mesocorticolimbic system: focus on nicotine effects. ProgNeurobiol 124: 1-27.
- Li S, Shi Y, Kirouac GJ (2014) The hypothalamus and periaqueductal gray are the sources of dopamine fibers in the paraventricular nucleus of the thalamus in the rat. Front Neuroanat 8: 136.
- Gillis CN, Lewis JJ (1956) The pharmacology of nicotine monomethiodide. J Pharm Pharmacol 8: 46-54.
- Aceto MD, Awaya H, Martin BR, May EL (1983) Antinociceptive action of nicotine and its methiodide derivatives in mice and rats. Br J Pharmacol 79: 869-876.
- Lenoir M, Tang JS, Woods AS, Kiyatkin EA (2013) Rapid sensitization of physiological, neuronal, and locomotor effects of nicotine: critical role of peripheral drug actions. J Neurosci 33: 9937-9949.
- Cox BM, Goldstein A, Nelson WT (1984) Nicotine self-administration in rats. Br J Pharmacol 83: 49-55.
- Donny EC, Caggiula AR, Knopf S, Brown C (1995) Nicotine self-administration in rats. Psychopharmacology (Berl) 122: 390-394.
- Rose JE, Corrigall WA (1997) Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology (Berl) 130: 28-40.
- Paxinos G, Franklin K (2013) The Mouse Brain in Stereotaxic Coordinates (4th edn.) New York, Academic Press in the mesocorticolimbic system: Focus on nicotine effects ProgNeurobiol 124: 1-27.
- Matta SG, Foster CA, Sharp BM (1993) Nicotine stimulates the expression of cFos protein in the parvocellularparaventricular nucleus and brainstem catecholaminergic regions. Endocrinology 132: 2149-2156.
- Mitchell SN (1993) Role of the locus coeruleus in the noradrenergic response to a systemic administration of nicotine. Neuropharmacology 32: 937-949.
- Egan TM, North RA (1986) Actions of acetylcholine and nicotine on rat locus coeruleus neurons in vitro. Neuroscience 19: 565-571.
- Shram MJ, Funk D, Li Z, Lê AD (2007) Acute nicotine enhances c-fos mRNA expression differentially in reward-related substrates of adolescent and adult rat brain. NeurosciLett 418: 286-291.
- Yu G, Sharp BM (2012) Nicotine modulates multiple regions in the limbic stress network regulating activation of hypophysiotrophic neurons in hypothalamic paraventricular nucleus. J Neurochem 122: 628-640.
- Svensson TH, Engberg G (1980) Effect of nicotine on single cell activity in the noradrenergic nucleus locus coeruleus. ActaPhysiolScandSuppl 479: 31-34.
- Engberg G, Hajos M (1994) Nicotine-induced activation of locus coeruleus neurons--an analysis of peripheral versus central induction. NaunynSchmiedebergs Arch Pharmacol 349:443-446.
- Van Bockstaele EJ, Reyes BA, Valentino RJ (2010) The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Res 1314: 162-174.
- Liu LN, Zhu FP, Song MY, Kang XJ, Shang SJ, et al. (2014) Effect of cocaine on ion channels and glutamatergic EPSCs in noradrenergic locus coeruleus neurons. J MolNeurosci 53: 345-351.
- Svensson TH (1987) Peripheral, autonomic regulation of locus coeruleus noradrenergic neurons in brain: putative implications for psychiatry and psychopharmacology. Psychopharmacology (Berl) 92: 1-7.
- Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63: 844-914.
- Ungerstedt U (1971) Stereotaxic mapping of the monoamine pathways in the rat brain. ActaPhysiolScandSuppl 367: 1-48.
- AlheidGF, Heimer L (1988) New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantiainnominata. Neuroscience 27: 1-39.
- Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR (2004) Ultrastructural interactions between terminals expressing the norepinephrine transporter and dopamine neurons in the rat and monkey ventral tegmental area. Synapse 52: 233-244.
- Flavin SA, Winder DG (2013) Noradrenergic control of the bed nucleus of the striaterminalis in stress and reward. Neuropharmacology 70: 324-330.
- Grenhoff J, Nisell M, Ferré S, Aston-Jones G, Svensson TH (1993) Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect 93: 11-25.
- Lategan AJ, Marien MR, Colpaert FC (1990) Effects of locus coeruleus lesions on the release of endogenous dopamine in the rat nucleus accumbens and caudate nucleus as determined by intracerebralmicrodialysis. Brain Res 523: 134-138.
- Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, et al. (2010) Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35: 1751-1760.
- Semenova S, Markou A (2010) The alpha2 adrenergic receptor antagonist idazoxan, but not the serotonin-2A receptor antagonist M100907, partially attenuated reward deficits associated with nicotine, but not amphetamine, withdrawal in rats. EurNeuropsychopharmacol 20:731-746.
- Gourlay SG, Stead LF, Benowitz NL (2004) Clonidine for smoking cessation. Cochrane Database Syst Rev 3: CD000058.
- Klink R, de Kerchoved'Exaerde A, Zoli M, Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21: 1452-1463.
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM (2002) Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol 444: 260-274.
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA (2003) Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci 23: 3176-3185.
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, et al. (2002) Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci 22: 1208-1217.
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, et al. (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23: 7820-7829.
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, et al. (2003) The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviours. J Neurosci 23: 11045-11053.
- Jones IW, Wonnacott S (2004) Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci 24:1244-1252.
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, et al. (2010) Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci 30: 5311-5325.
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, et al. (2011) Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology 36: 1021-1032.
- Ren T1, Sagar SM (1992) Induction of c-fosimmunostaining in the rat brain after the systemic administration of nicotine. Brain Res Bull 29: 589-597.
- Pang Y, Kiba H, Jayaraman A (1993) Acute nicotine injections induce c-fos mostly in non-dopaminergic neurons of the midbrain of the rat. Brain Res Mol Brain Res 20: 162-170.
- Dong Y, Zhang T, Li W, Doyon WM, Dani JA (2010) Route of nicotine administration influences in vivo dopamine neuron activity: Habituation, needle injection, and cannula infusion. J MolNeurosci 40:164-171.
- Kovács KJ (1998) c-Fos as a transcription factor: a stressful (re)view from a functional map. NeurochemInt 33: 287-297.
- Grenhoff J, Aston-Jones G, Svensson TH (1986) Nicotinic effects on the firing pattern of midbrain dopamine neurons. ActaPhysiolScand 128: 351-358.
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, et al. (2006) Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50: 911-921.
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, et al. (2008) Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantianigra and retrorubral field in the rat. Neuroscience 152: 1024-1031.
- Radcliffe PM, Sterling CR, Tank AW (2009) Induction of tyrosine hydroxylase mRNA by nicotine in rat midbrain is inhibited by mifepristone. J Neurochem 109: 1272-1284.
- Mitchell SN, Smith KM, Joseph MH, Gray JA (1993) Increases in tyrosine hydroxylase messenger RNA in the locus coeruleus after a single dose of nicotine are followed by time-dependent increases in enzyme activity and noradrenaline release. Neuroscience 56: 989-997.
- Rose JE (2007) Multiple brain pathways and receptors underlying tobacco addiction. BiochemPharmacol 74: 1263-1270.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 11407
- [From(publication date):
April-2016 - May 21, 2024] - Breakdown by view type
- HTML page views : 10644
- PDF downloads : 763