Interplay between gait and neuropsychiatric symptoms in Parkinson’s Disease
Accepted: 5 May 2022
HTML: 3
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
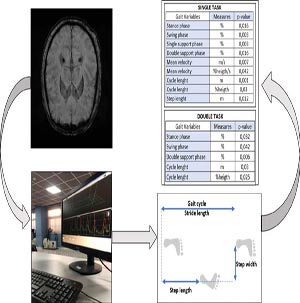
Parkinson’s Disease (PD) is a neurodegenerative disease which involves both motor and non-motor symptoms. Non-motor mental symptoms are very common among patients with PD since the earliest stage. In this context, gait analysis allows to detect quantitative gait variables to distinguish patients affected by non-motor mental symptoms from patients without these symptoms. A cohort of 68 PD subjects (divided in two groups) was acquired through gait analysis (single and double task) and spatial temporal parameters were analysed; first with a statistical analysis and then with a machine learning (ML) approach. Single-task variables showed that 9 out of 16 spatial temporal features were statistically significant for the univariate statistical analysis (p-value< 0.05). Indeed, a statistically significant difference was found in stance phase (p-value=0.032), swing phase (p-value=0.042) and cycle length (p-value=0.03) of the dual task. The ML results confirmed the statistical analysis, in particular, the Decision Tree classifier showed the highest accuracy (80.9%) and also the highest scores in terms of specificity and precision. Our findings indicate that patients with non-motor mental symptoms display a worse gait pattern, mainly dominated by increased slowness and dynamic instability.
Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2009;24(11):1641-1649. DOI: https://doi.org/10.1002/mds.22643
Hely MA, Morris JGL, Reid WGJ, Trafficante R. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord Off J Mov Disord Soc. 2005;20(2):190-199.. DOI: https://doi.org/10.1002/mds.20324
Amboni M, Barone P, Hausdorff JM. Cognitive contributions to gait and falls: evidence and implications. Mov Disord Off J Mov Disord Soc. 2013;28(11):1520-1533. DOI: https://doi.org/10.1002/mds.25674
Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord Off J Mov Disord Soc. 2008;23(6):837-844. DOI: https://doi.org/10.1002/mds.21956
Mollenhauer B, Rochester L, Chen-Plotkin A, Brooks D. What can biomarkers tell us about cognition in Parkinson’s disease? Mov Disord Off J Mov Disord Soc. 2014;29(5):622-633. DOI: https://doi.org/10.1002/mds.25846
Avanzino L, Lagravinese G, Abbruzzese G, Pelosin E. Relationships between gait and emotion in Parkinson’s disease: A narrative review. Gait Posture. 2018;65:57-64. DOI: https://doi.org/10.1016/j.gaitpost.2018.06.171
Aleksovski D, Miljkovic D, Bravi D, Antonini A. Disease progression in Parkinson subtypes: the PPMI dataset. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2018;39(11):1971-1976. DOI: https://doi.org/10.1007/s10072-018-3522-z
Amboni M, Ricciardi C, Cuoco S, et al. Mild Cognitive Impairment Subtypes Are Associated With Peculiar Gait Patterns in Parkinson’s Disease. Front Aging Neurosci. 2022;14:781480. DOI: https://doi.org/10.3389/fnagi.2022.781480
Rochester L, Yarnall AJ, Baker MR, et al. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain. 2012;135(9):2779-2788. DOI: https://doi.org/10.1093/brain/aws207
Celik Y, Stuart S, Woo WL, Godfrey A. Gait analysis in neurological populations: Progression in the use of wearables. Med Eng Phys. 2021;87:9-29. DOI: https://doi.org/10.1016/j.medengphy.2020.11.005
Picillo M, Ricciardi C, Tepedino MF, et al. Gait Analysis in Progressive Supranuclear Palsy Phenotypes. Front Neurol. 2021;12:674495. DOI: https://doi.org/10.3389/fneur.2021.674495
Landolfi A, Ricciardi C, Donisi L, et al. Machine Learning Approaches in Parkinson’s Disease. Curr Med Chem. 2021;28(32):6548-6568. DOI: https://doi.org/10.2174/0929867328999210111211420
Park H, Shin S, Youm C, Cheon SM, Lee M, Noh B. Classification of Parkinson’s disease with freezing of gait based on 360° turning analysis using 36 kinematic features. J Neuroengineering Rehabil. 2021;18(1):177. DOI: https://doi.org/10.1186/s12984-021-00975-4
Ricciardi C, Amboni M, De Santis C, et al. Machine learning can detect the presence of Mild cognitive impairment in patients affected by Parkinson’s Disease. In: 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA). 2020:1-6. DOI: https://doi.org/10.1109/MeMeA49120.2020.9137301
Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33-39. DOI: https://doi.org/10.1001/archneur.56.1.33
Amboni M, Barone P, Iuppariello L, et al. Gait patterns in parkinsonian patients with or without mild cognitive impairment. Mov Disord. 2012;27(12):1536-1543. DOI: https://doi.org/10.1002/mds.25165
Davis RB, Õunpuu S, Tyburski D, Gage JR. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10(5):575-587. DOI: https://doi.org/10.1016/0167-9457(91)90046-Z
Amboni M, Iuppariello L, Iavarone A, et al. Step length predicts executive dysfunction in Parkinson’s disease: a 3-year prospective study. J Neurol. 2018;265(10):2211-2220. DOI: https://doi.org/10.1007/s00415-018-8973-x
Cantoni V, Green R, Ricciardi C, et al. Comparing the Prognostic Value of Stress Myocardial Perfusion Imaging by Conventional and Cadmium-Zinc Telluride Single-Photon Emission Computed Tomography through a Machine Learning Approach. Comput Math Methods Med. 2021;2021:5288844. DOI: https://doi.org/10.1155/2021/5288844
Tougui I, Jilbab A, Mhamdi J. Heart disease classification using data mining tools and machine learning techniques. Health Technol. 2020;10. DOI: https://doi.org/10.1007/s12553-020-00438-1
Ricciardi C, Cuocolo R, Megna R, Cesarelli M, Petretta M. Machine learning analysis: general features, requirements and cardiovascular applications. Minerva Cardiol Angiol. Published online May 4, 2021. DOI: https://doi.org/10.23736/S2724-5683.21.05637-4
Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018;17(6):559-568. DOI: https://doi.org/10.1016/S1474-4422(18)30127-3
Plotnik M, Giladi N, Dagan Y, Hausdorff JM. Postural instability and fall risk in Parkinson’s disease: impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Exp Brain Res. 2011;210(3-4):529-538. DOI: https://doi.org/10.1007/s00221-011-2551-0
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2022.10463
https://doi.org/10.4081/ejtm.2022.10463



