
Ceramide and sphingosine-1-phosphate in cancer, two faces of the sphinx
Introduction
Extensive studies have cemented sphingolipids as reservoirs of critical bioactive molecules and undeniably propelled the study of these lipids in biology and disease. Previously considered purely structural lipids, sphingolipids, particularly ceramide and sphingosine-1-phosphate (S1P), are now established critical regulators of myriad biological processes. Sphingosine, which serves as the backbone structure of many sphingolipid species, was originally named by the German chemist, J. L. W. Thudichum in the 1880s, in reference to the many mysteries the study of this lipid presented. In Greek mythology, the sphinx guarded the entrance to the city of Thebes and only granted entry to travelers who answered its riddle. Today, the field of sphingolipid research is one that is equally complex and promising. Currently, sphingolipids are being studied as potential targets in cancer research due to their role in regulating cellular and pathological processes such as cell growth and survival, apoptosis, inflammation, vascular integrity and angiogenesis. The complexity is accentuated by the metabolic interconnected nature of these bioactive lipids. The levels of sphingolipids are regulated by multiple enzymes either through synthesis or breakdown reactions. This review will describe the fundamental interconnectivity of metabolic sphingolipids, will review and highlight recent data pertaining to the subcellular compartmentalization of sphingolipids, and will provide evidence of the opposite roles that ceramide and S1P play in various cellular processes relevant to cancer biology.
Sphingolipid biosynthetic pathways
Regulation of ceramide synthesis and breakdown
The central hub of the sphingolipid pathway is the bioactive lipid ceramide, which can be generated through different pathways (Figure 1) (1,2). The de novo generation of sphingolipids starts with the condensation of serine and palmitoyl CoA to generate 3-ketodihydrosphingosine in a reaction catalyzed by a family of enzymes, the serine palmitoyl transferases (SPTs) (3). SPTs also have the ability to utilize alanine and glycine instead of serine in a pathway that eventually forms deoxysphingolipids, a terminal class of lipids that is unable to exit the sphingolipid pathway due to the absence of enzymes that metabolize it (4,5). Following formation of 3-ketodihydrosphingosine, dihydrosphingosine is generated via a reduction reaction. The latter is subsequently N-acylated to dihydroceramide by ceramide synthases (CerS) (1,2). There are six mammalian CerS characterized to date. These differ by their fatty acyl chain length specificity and tissue distribution. As such, this generates ceramide species differing in the chain length of their fatty acid (6,7) which are then acted on by dihydroceramide reductase to insert a double bond at the 4,5 position of the sphingosine backbone to form ceramide (8).
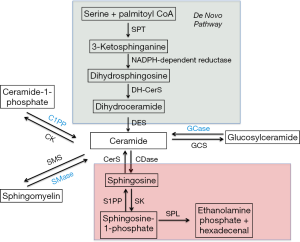
Subsequently, ceramide can have multiple fates. Addition of a phosphocholine headgroup by sphingomyelin synthases (SMS) results in the formation of sphingomyelin (SM), a major component of cell membranes (9). Ceramide can also be glycosylated by glycosyl or galactosyl CerS to form more complex glycosphingolipids (2), or phosphorylated by ceramide kinase to form ceramide-1-phosphate (C1P) (10). Conversely, ceramide can be generated back from these complex sphingolipids via lipid hydrolases. Thus, an intricate balance of ceramide synthesis and breakdown is present in the cell and is regulated by multiple mechanisms as will be discussed in subsequent sections.
Ceramide and sphingosine-1-phosphate (S1P) axis
Another fate of ceramide metabolism is the generation of sphingosine via a deacetylation reaction catalyzed by ceramidases. Five of these enzymes have been cloned and are classified based on their pH optima of activity into acid, neutral and alkaline (3 alkaline ceramidases exist) (11-13). It should be noted that some ceramidases have reverse activities and can convert sphingosine back to ceramide (14,15). Moreover sphingosine may be reacylated by one of the 6 CerS and this pathway is termed the “salvage pathway” as it rescues ceramide back and prevents its exit from the sphingolipid pathway (1,2). Additionally, sphingosine can be targeted by sphingosine kinase isoenzymes (SK1 or SK2) for phosphorylation on its hydroxyl head group to yield S1P (16). S1P can be dephosphorylated by the function of S1P phosphatases to generate sphingosine (17,18). Finally, a nonreversible step is the breakdown of S1P by a lyase to generate hexadecenal and ethanolamine phosphate, constituting the point of exit of the sphingolipid metabolic pathway (19).
Metabolic interconnectivity regulated by subcellular compartmentalization
Endoplasmic reticulum (ER)
The de novo production of ceramide constitutes the only point of entry into the sphingolipid pathway. The main reactions occur in the ER and once ceramide is produced, it is transferred to the Golgi apparatus where it can form SM by the addition of a phosphocholine head group, glycosphingolipids through the addition of sugar head groups, or C1P through phosphorylation (1,2). SM synthesis in the Golgi is thought to occur through sphingomyelin synthase 1 (SMS1) and sphingomyelin synthase 2 (SMS2) (20,21). It is believed that the ER to Golgi transport occurs in a non-vesicular fashion through the ceramide transfer protein (CERT) for SM and likely for C1P production and via vesicular transport for the generation of glucosylceramide, the precursor of complex glycosphingolipids (Figure 2) (22,23). After synthesis in the Golgi, complex sphingolipids are distributed in a vesicular fashion to different cellular compartments (24).
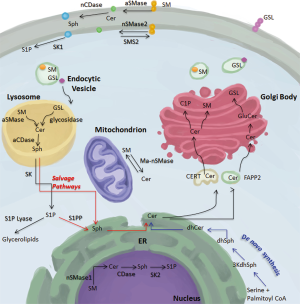
Plasma membrane
In the plasma membrane, SM is localized primarily on the outer leaflet where it is hydrolyzed by acid sphingomyelinase (aSMase) (25). However, a small pool of sphingomyelin is localized in the inner leaflet of the plasma membrane and is used as a substrate for the action of neutral sphingomyelinase-2 (nSMase2) to form ceramide (26). The reverse reaction of conversion of ceramide to sphingomyelin can occur at the plasma membrane and is catalyzed by SMS2 (27). Furthermore, neutral ceramidase (nCDase) is also present in the plasma membrane, albeit on the outer leaflet (28). It is unclear whether the substrate of nCDase comes from ceramide targeted to the plasma membrane in vesicular fashion or from the flipping of nSMase2-generated ceramide from the inner leaflet of the plasma membrane. Sphingosine kinase 1 (SK1) is also present in the PM and localizes there in response to different stimuli to produce S1P (29-32).
Lysosome
The bulk of sphingomyelin degradation occurs in the lysosome through the action of the lysosomal form of aSMase. Defective activity of this enzyme is the genetic basis for Niemann-Pick disease that is characterized by failure of growth and psychomotor regression in children as well as progressive worsening of respiratory functions (33). Ceramide generated in the lysosome can serve as a substrate for the generation of sphingosine through acid ceramidase (aCDase). The deficiency in the activity of this enzyme results in the lipid storage disorder Farber lipogranulomatosis (Farber disease). Affected patients suffer from progressive joint deformation and neurological complications, which typically result in early death (15,34). To date, there is no characterized presence of any of the two SK in the lysosome. As such, it is assumed that lysosomal sphingolipids must be transported into other organelles to exit the sphingolipid pathway.
Nucleus
SM presence in the nucleus has been reported and mapped to different subnuclear localizations (35). The intricacy of how SM gets to the nucleus is still unclear. Two distinct possibilities arise: the first is a vesicular transport from the Golgi and the second is its nuclear generation from ceramide that is transported from the ER. The second possibility is more probable as SMS activity was detected in nuclear enriched fractions (36). SM can be degraded in the nucleus by neutral sphingomyelinase-1 (nSMase1), which has been shown to localize to the nucleus (37). Furthermore, sphingosine can be generated through the action of nuclear ceramidases as well as S1P through sphingosine kinase two (SK2) (38-40).
Mitochondria
Mitochondrial sphingolipids have also been detected. Ceramide synthase activity has been detected in partially purified mitochondrial-enriched fractions suggesting the potential for either de novo synthesis of ceramide or salvage generation to occur in the mitochondria (41). Furthermore, the generation of ceramide from sphingosine and palmitoyl-CoA regulated by nCDase has been identified in liver mitochondria (42). Lastly, a recently cloned mitochondria-associated neutral sphingomyelinase was found to localize to the outer mitochondrial membrane (43,44).
Taken together, these results point to a very specific compartmentalization of sphingolipid metabolism. Functionally, as we continue to gain the molecular tools to study the enzymes, it would be of utmost importance to assign specific functions to organelle-specific sphingolipids and to be able to manipulate those organelle-specific pools. This could have major implications for understanding signaling processes, and using sphingolipids as potential targets for disease treatments.
Opposing roles of ceramide and S1P in cellular biology
Apoptosis vs. cell survival
Cellular amounts of ceramide and S1P are important regulators of cell survival. Extensive literature has described the effect of ceramide on cell death. The first reports described an effect of exogenous C2-ceramide treatments on induction of DNA fragmentation and programed cell death in leukemia cells (45). This was very selective to ceramide as dihydroceramide was unable to induce the same biology (46). Furthermore, exogenous addition of SMase to fibrosarcoma cells was demonstrated to induce DNA damage and apoptosis (47). At the time, that was the first report of an endogenously generated ceramide mediating an apoptotic function. Subsequently many stimuli were shown to induce ceramide generation and apoptosis and these included TNF-α, Fas ligand and ionizing radiation (1). Most of these effects were thought to occur through the hydrolysis of SM either by neutral or acid sphingomyelinase activities. However, the first reports of the involvement of de novo ceramide synthesis in mediating apoptosis came from studies on daunorubicin. These studies demonstrated a role for daunorubicin-generated ceramide in mediating cell death that was inhibited by the ceramide synthase inhibitor, Fumonisin B1 (48). Following these initial studies, many of the subsequent literature elaborated on different stimuli that induce ceramide generation and cell death in multiple cell lines. Interestingly, ceramide levels can also be regulated by inhibition of ceramide breakdown including inhibition of sphingosine kinase or S1P lyase (49,50).
The mechanism by which ceramide induces cell death involves activation of both the intrinsic and the extrinsic pathways of apoptosis. The intrinsic pathway is characterized by mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release. Interestingly, MOMP directly correlates with the level of ceramide in the outer mitochondrial membrane (51). However, some studies suggest that ceramide is not sufficient to induce MOMP but rather its synergism and activation of BAX is required for apoptosis (52-54). Ceramide can also induce MOMP through caspase-2 and caspase-8 activation. This can occur following glycogen synthase kinase-3β (GSK3β) activation in response to induction of cathepsins, downregulation of AKT or activation of protein phosphatase 2A (PP2A) (55-58). Finally, ceramide has also been shown to induce the mitochondrial translocation of PKC-δ which induces cytochrome c release and caspase-9 activation (59).
The extrinsic pathway of apoptosis is mediated mainly through receptor activation of endogenous caspases and cell death. Many receptors engage the extrinsic pathway and these include TNF receptors and the TNF-related apoptotic ligand (TRAIL) receptors. It is widely believed that these receptors generate ceramide at the plasma membrane and localize to ceramide-enriched membrane platforms (60,61). Much of the understanding of ceramide function comes from cancer cell resistance to the extrinsic pathway of apoptosis and the modulation of that resistance by sphingolipids and ceramide generation. For instance, resistance to TRAIL-induced cell death is overcome by expression of ceramide synthase 6 (CerS6) (62). Furthermore, TNF receptor 1 promotes the formation of ceramide at the plasma membrane through nSMase2 activation. Failure of that activation is associated with resistance to TNF-induced apoptosis of breast tumors (62). A last possible way of ceramide activating the extrinsic pathway is through downregulation of the FLICE protein, an endogenous inhibitor of caspases-8. This has been shown to occur in glioblastoma and prostate cancer (63,64).
The role of S1P as an anti-apoptotic regulator was first described in 1996, when it was demonstrated that S1P negatively regulates ceramide-mediated apoptosis (65). Early studies implicated SK1-derived S1P in cell survival, since overexpression of SK1 in NIH 3T3 fibroblasts and HEK293 cells was demonstrated to prevent apoptosis induced by ceramide and serum depravation (66). Numerous studies have since investigated the regulatory role of S1P in cell survival, establishing this bioactive lipid as a regulator of cancer cell proliferation, with elevated levels of SK1 and S1P present in various tumor tissues and cancers (67,68). S1P is a pleiotropic and mitogenic bioactive sphingolipid that is present in both tissues and circulation. S1P can regulate pro-survival cellular responses in an autocrine or paracrine manner (inside-out signaling), by activating a class of G-protein coupled receptors, S1P receptor 1-5 (S1PR1-5). Additionally, S1P can regulate cellular responses by S1PR-independent mechanisms (69), as it has been shown to promote growth and survival in S1PR-deficient mouse embryonic fibroblast (70).
S1P inhibits the intrinsic apoptotic machinery by regulating the release of cytochrome c and Smac/DIABLO from mitochondria, blocking the activation of executioner caspases and regulating Bax oligomerization (49,71,72). SK1 can also regulate the extrinsic apoptotic pathway by activating phosphatidylinositol-3 kinase (PI3K)/AKT and nuclear factor (NF)-κB (NFκB) signaling downstream of TNF receptor activation (73,74). In fact, inhibition of SK1 in apoptotic resistant cells enhances sensitivity to TNF-mediated cell death (75).
SK1/S1P play an important role in regulating chemotherapeutic responses, as inhibition of SK1 enhances sensitivity to apoptotic chemotherapeutic agents (76). Negative regulation of S1P, either through SK1 or S1P lyase, prevents drug resistance in tumor cells treated with the chemotherapeutic agent cisplatin (77). In triple-negative breast cancer cell lines, SK1 is overexpressed and this phenotype correlates with poor prognosis and resistance to doxorubicin therapy. Attenuation of SK1 expression sensitizes cells to chemotherapeutic drugs by regulating oncogenic signaling via ERK1/2 and AKT, thus representing a potential target for combinatorial therapy (78-80). In in vivo prostate cancer models, the efficacy of docetaxel and camptothecin treatment is mediated by inhibiting SK1, which results in decreased tumor cell growth (65,81). Furthermore, the generation of SK1 inhibitors has spurred the focus on SK1 as a potential drug target (82,83). Recent studies demonstrate that SKI-II inhibits growth of acute myeloid leukemia cells via a caspase-dependent mechanism and suppresses leukemic xenograft tumor growth in severe combined immunodeficient (SCID) mice (84). The S1P receptor antagonist FTY720 (Fingolimod), synergizes with ciplastin to reduce survival of melanoma cell lines by inhibiting the PI3K/AKT/mTOR pathway (85). The role of S1P in regulating oncogenic signaling via the PI3K pathway has also been shown in non-small cell lung cancer (NSCLC) cells (86).
The particular contribution of SK2 in cell survival is not as well established. Inhibition of SK2 using small-molecule inhibitors reduces the growth of prostate cancer cells by downregulating Myc and the androgen receptor (AR) (87). Using RNAi or the SK2-specific inhibitor ABC294640, another group demonstrated that SK2 regulates apoptosis in multiple myeloma (88). Other studies indicate only partial overlapping roles for the two SK isoenzymes in regulating cell survival, and suggest an optimal role for SK2 as a cancer therapeutic target (89).
Cell cycle regulation
The initial characterization of ceramide in cell growth arrest came from studies in the Hannun laboratory on serum-starved Molt-4 leukemia cells. It was noticed that, following serum withdrawal, cells arrested in G0/G1 phase with accumulation of ceramide from sphingomyelin hydrolysis. Conversely, exogenous addition of C6 ceramide recapitulated the same phenotype (90). Subsequent studies suggested that this effect was possibly mediated through the retinoblastoma (Rb) gene product (91). Studies on the involvement of ceramide in G0/G1 arrest demonstrated also the involvement of nSMase2 in mediating confluence induced growth arrest (92). This was attributed to the dephosphorylation of β-catenin in a protein phosphatase 1-γ (PP1C-γ) dependent manner, suggesting a signaling mechanism mediating this effect (93). Recent studies have identified dihydroceramide-mediated regulation of G0/G1 arrest induced by cell confluence in neuroblastoma cells (94).
More importantly, some studies on ceramide function in growth arrest concentrated on a potential role of ceramide in regulating cell cycle checkpoints. This is of particular interest as cell cycle regulators represent one of the major areas where focus is intense to develop novel chemotherapeutics to treat malignant tumors. The most developed of these studies suggest the major function of ceramide to occur at the G1/S transition through two signaling avenues. The first involved the activation of p21 and the dephosphorylation of Rb (95) and this can occur both in a p53-dependent and independent manner (96). The second involves the inhibition of the G1 cyclin-dependent kinase CDK2 (97). Interestingly, recent studies suggested a third mechanism by which ceramide can control the G1/S transition. In response to all trans retinoic acid (ATRA), nSMase2 is activated and appears to mediate a G1 arrest that is dependent on the dephosphorylation of S6 kinase with no effect on either Rb or p21 (98). Ceramide has also been implicated in the G2/M cell cycle checkpoint. Increase of ceramide levels in NIH 3T3 cells mediated by threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP), a glucosylceramide synthase inhibitor, demonstrated decreased activity of cyclin dependent kinase CDK1 and cell cycle arrest at the G2/M checkpoint (99). Likewise, inhibition of ceramide generation via the knockdown of β-glucosidase, also demonstrated regulation of G2/M arrest induced by the chemotherapeutic agent paclitaxel (100). Increase in ceramide levels has also been reported to occur right after the G2/M progression to regulate Rb dephosphorylation (101). More recent studies suggest that in rhabdomyosarcoma cells, ceramide induces a G2/M arrest that is concomitant with increased p21 and downregulation of cyclin D (102). The other suggested that ceramide suppresses survivin which controls the G2/M transition (103). As such, the distinct mechanisms of how ceramide controls the G2/M checkpoint, as well as its application to endogenous situations of ceramide upregulation, require further investigation. To note, there has not been to date studies that pointed to roles of ceramide in either the S phase checkpoint or in the mitotic spindle checkpoint.
Cell cycle progression is also regulated by S1P signaling as a mechanism to promote cell proliferation (104,105). Early reports identified the involvement of SK1 in cell cycle regulation activated in response to ganglioside GM1 mitogenic signaling (106). These authors found that inhibition of SK1 mediated by D,L-threo-dihydrosphingosine, results in a concomitant decrease in CDK2, and important regulator of G1 cell cycle progression. Following, SK nuclear activity was detected and found to correlate with cell cycle transition from G1 to the S phase (107). While both SK isoforms have been associated with cell cycle progression in glioblastoma cell lines (108), other conflicting studies have characterized SK2 as the only nuclear SK isoform, and correlated SK2 activity with G1/S cell cycle arrest (39). SK1-mediated regulation of G1 to S phase cell cycle progression has been investigated by loss of function studies utilizing RNAi in MCF7 cells (49) and in genetic studies of intestinal tumor epithelial cells (109). Mechanistically, these studies show that SK1 regulates the G1/S transition by increasing the expression of CDK4 and c-myc. Additional studies in breast cancer cells show that SK1 downregulates CDK1 and CHK1, which modulate spindle checkpoint function and cytokinesis (110). Pharmacological inhibition of SK1 using SKI-II and SKI-178 have also demonstrated regulation of CDK1 in natural killer-lymphocyte leukemia cells and associate this role with a G2/M cell cycle arrest (111). Conversely, inhibition of S1P receptor signaling with FTY720 in MDA-MB-361 breast cancer cells induces cell cycle arrest at the G0/G1 phase and a decrease in cells at S and G2/M phase. FTY720 treatment in these cells increases the anti-proliferative effect induced by single dose ionizing radiation (112).
Moreover, studies from the Obeid lab identified SK1 as a target of p53, a tumor suppressor and cell cycle regulator (113). While SK1 is elevated in p53 deficient mice, a double knock out model showed increase of cell cycle inhibitors p21 and p16 and decreased tumor formation. Though these studies have characterized S1P as an important regulator of cell cycle progression, the targets and biochemical pathways involved may be cell specific.
Senescence
Cellular senescence is a process by which cells lose the ability to proliferate. It is different from quiescence in that it is thought to be irreversible and associated with aging. The first observation implicating ceramide in senescence regulation came from the Obeid laboratory; ceramide was found to increase in senescent human diploid fibroblast due to neutral sphingomyelinase activity (114,115). This was also associated with dephosphorylation of Rb and inhibition of AP-1 activation (114,116). Subsequent studies showed induction of β-galactosidase by exogenous ceramide addition in these fibroblasts (117), and in human umbilical vain endothelial cells (118). Furthermore, exogenous treatment of ceramide in these cell lines was also found to dephosphorylate Rb, commonly seen with ceramide-induced cellular senescence. Recent evidence suggests that metformin, a drug used in the treatment of diabetes, can reverse ceramide-induced senescence in C2C12 myoblasts (119). However, it is unclear if the action of metformin is in the same pathway or in a parallel pathway to ceramide. While most of these studies implicated a neutral-sphingomyelinase generated ceramide in the induction of senescence, a recent study suggest that the lack of CERT, and thereby the lack of transfer of ceramide from the ER to the Golgi resulted in premature senescence in mouse embryonic fibroblasts (120).
Despite limited studies focusing on the role of S1P in regulating senescence, is not surprising that the metabolic interconnectivity of these lipids supports a role for SK/S1P in this response. Deletion of SK1 in p53 null mice results in induction of senescence and decreased thymus tumor burden (113). Recent studies have shown that SK2-generated S1P inhibits senescence by promoting telomerase stability. S1P regulates senescence and cell proliferation by binding the catalytic subunit of the telomerase reverse transcriptase (TERT) and thus stabilizing the enzyme during DNA replication (121).
Autophagy
Autophagy is a cellular protective mechanism aimed at the degradation of unused cellular metabolites and organelles to preserve cellular energy. However, cancer cells use this mechanism to promote their survival and as such, understanding the processes that regulate this phenomenon can lead to its efficacious targeting. Ceramide has long been implicated in autophagy (122-125). Many mechanisms have been proposed to elucidate the role of ceramide in regulating autophagy (126,127), the most convincing of which show regulation of the mTOR pathway and nutrient uptake (128). Some studies have implicated ceramide in the generation of autophagic vacuoles by upregulating Beclin-1 and inhibiting protein kinase B (122); others implicate ceramide in regulating the dissociation of Beclin-1 and Bcl-2 to promote autophagy (129). Interestingly enough, there seems to be a consensus that regulation of autophagy seems to occur through the long-chain dihydroceramides (122,130-132). The drug fenretinide has been used extensively in these studies due to its dual action as an activator of SPTs as well as an inhibitor of dihydroceramide desaturase, which results in the accumulation of dihydroceramide. Briefly, fenretinide was shown to induce autophagy in breast, pancreatic and cervical cancer cell lines (133-135).
S1P has also been implicated as a regulator of pro-survival autophagy (136,137). In PC-3 prostate cancer cells, exogenous S1P and dihydro-S1P treatment induced autophagy upon serum starvation (138). Similarly, increased expression of SK1 and depletion of the S1P phosphatase phosphohydrolase-1 promotes autophagy as determined by the formation of LC3-positive autophagosomes (139). In these studies, autophagy was specifically induced by SK1, since both dimethylsphingosine and overexpression of a catalytically inactive SK1 mutant abrogated autophagy induction. Interestingly, S1P-induced pro-survival autophagy is characterized by inhibition of mTOR but independent of AKT signaling (140). The role of dihydro-S1P in promoting autophagy is controversial (139). Loss of function studies demonstrated the need for S1PR5 in S1P-induced ER stress and autophagy (141), although the receptor specificity may be cell specific (125). Other studies showed that in a subpopulation of T-cell acute lymphoblastic leukemia (T-ALL), the use of SK1 and SK2 inhibitors induced the unfolded protein response (UPR) leading to ER stress, and autophagic cell death (142).
Cell motility and invasion
The cellular balance of sphingolipids metabolites, ceramide and S1P, plays an important role in regulating adhesion, migration and invasion, which are important precedents of cancer metastasis (143). The opposing roles of ceramide and S1P have been studied in vivo and in vitro cancer models. Increased expression of CerS2 and ceramide levels correlate with poorly invasive phenotypes of breast cancer cell lines. Mechanistically, CerS2 overexpression correlates with activation of metalloproteinases (MMP) and degradation of extracellular matrix, resulting in decreased invasion (144). CerS2 expression also correlates with less metastasis in human bladder cancer cell lines (145). The overexpression and increased metabolic function of aCDase in prostate cancer cells, results in decreased levels of long chain ceramide species and increased adhesion and migration on the ECM. Furthermore, acute treatment with C6 ceramide nanoliposomes suppresses tumor migration in a PKCζ-dependent manner to promote stress fiber depolymerization and focal adhesion disassembly (146). In addition, inhibition of aSMase—and thus decreased generation of ceramide—in Hela and MDA-MB-231 cells, reduced invasion in vitro (147). At the same time, other studies have identified a role for aSMase-derived ceramide in adhesion and metastasis. aSMase-deficient mice show reduced hematogenous melanoma metastasis. Melanoma cells triggered secretion of aSMase from platelet cells, resulting in increased ceramide and clustering of α5β1 integrin in melanoma cells to promote adhesion (148,149).
The role of SK1 in cell migration and invasion is an area of intense research focus (150). Migration regulated by extracellular S1P is dependent on the expression of specific S1PR, with S1PR1 and S1PR3 generally promoting migration and S1PR2 inhibiting it with some exceptions. The migratory role associated with different S1PRs is regulated by downstream coupling to different Gi proteins and consequently, activation of distinct downstream signaling pathways (136). For instance, coupling of S1PR2 to G12/13 results in Rho activation and inhibition of Rac and cell migration (151). Moreover, the role of S1PR in migration is also regulated by tissue distribution; for example, in Jurkat and U937 immune cells, S1PR3 but not S1PR1, is required for S1P-mediated migration and adhesion to the endothelial monolayer (152,153). While in melanoma and glioblastoma cells, expression of S1PR2 is predominant, thus S1P signaling results in reduced migration (136,154,155). The role of SIP in migration is further regulated by the extensive crosstalk with growth factor signaling (136). In MCF7 and glioblastoma cells, EGFR signaling promotes the translocation and activation of SK1 to the membrane (79,156). And is possible that this signal is amplified in a loop, since in other cells S1P has been shown to stimulate the expression of EGFR (157). Furthermore, through different S1PRs, S1P also mediates EGFR and VEGFR2 transactivation to regulate migration (158,159).
In addition to its pro-migratory role, S1P also regulates tumor cell invasion to promote metastasis (160,161), making this potent bioactive lipid, a “swiss army knife” for cancer cells. While S1P can also promote the secretion and activation of proteases and metalloproteinases to degrade the ECM, it can also transcriptionally regulate PAR and PAI-1, which are key modulators of invasion (162-164). More recently, our group described a mechanism by which EGF and S1P induce cell adhesion and invasion (165). By regulating ERM proteins, sphingolipids regulate cellular cytoskeleton dynamics and invasion (166,167). Furthermore, it is probable that different pools of S1P target different processes in cancer cells, as some studies have implicated that inhibition of SK2 upregulates SK1 protein, activity levels, and increases intracellular S1P (89). On the other hand, SK1 inhibition does not regulate SK2, but decreases intracellular S1P and increases ceramide. In this study, inhibition of SK2 resulted in a more dramatic regulation of invasion, supporting a non-overlapping role for SK1 and SK2 in cancer cells (89). Clearly, the multifaceted role of S1P in migration and invasion requires additional focus and investigation in order to understand the complex regulation of these processes as they represent important targets for cancer therapeutics.
Conclusions
Our current model of sphingolipid signaling has been defined by a vast number of studies that have provided deeper understanding of the mechanism of enzyme/lipid regulation as well as associated cellular targets. What is becoming clear is that the various levels of complexity associated with bioactive sphingolipid metabolites continue to riddle and challenge us to consider novel mechanistic avenues. Recent advances in molecular and analytical tools have been instrumental in uncovering intricacies and new paradigms. While ceramide and S1P have been traditionally considered to play pro-death and pro-growth roles, respectively, newer roles are continuing to surface and perpetuating the enigmatic facet of these lipids. For instance, SK2-derived S1P has been implicated in both pro- and anti-apoptotic functions (68). In addition, is becoming clear that not all ceramide species “are created equal” and that different chain length ceramides may play different roles in promoting or suppressing tumors (68). We expect that advances in the field will allow us to clearly characterize these new models.
Acknowledgments
We thank Ms. Katerina Sadov for assistance in design and illustration of the figures.
Funding: This work was supported by Veterans Affairs Merit Award (LM Obeid), NIH Grants R01 GM097741 (LM Obeid), R37 GM43825 (YA Hannun), R01 CA172517 (YA Hannun) and PO1CA097132 (LM Obeid and YA Hannun).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hsinyu Lee and Markus H. Gräler) for the series “Lysophospholipids on Immunity and Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.10.01). The series “Lysophospholipids on Immunity and Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 2004;4:604-16. [PubMed]
- Hannun YA, Obeid LM. Many ceramides. J Biol Chem 2011;286:27855-62. [PubMed]
- Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta 2003;1632:16-30. [PubMed]
- Gable K, Gupta SD, Han G, et al. A disease-causing mutation in the active site of serine palmitoyltransferase causes catalytic promiscuity. J Biol Chem 2010;285:22846-52. [PubMed]
- Penno A, Reilly MM, Houlden H, et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J Biol Chem 2010;285:11178-87. [PubMed]
- Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem 2006;281:25001-5. [PubMed]
- Stiban J, Tidhar R, Futerman AH. Ceramide synthases: roles in cell physiology and signaling. Adv Exp Med Biol 2010;688:60-71. [PubMed]
- Geeraert L, Mannaerts GP, van Veldhoven PP. Conversion of dihydroceramide into ceramide: involvement of a desaturase. Biochem J 1997;327:125-32. [PubMed]
- Taniguchi M, Okazaki T. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim Biophys Acta 2014;1841:692-703.
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008;9:139-50. [PubMed]
- Bai A, Szulc ZM, Bielawski J, et al. Targeting (cellular) lysosomal acid ceramidase by B13: design, synthesis and evaluation of novel DMG-B13 ester prodrugs. Bioorg Med Chem 2014;22:6933-44. [PubMed]
- Zeidan YH, Jenkins RW, Korman JB, et al. Molecular targeting of acid ceramidase: implications to cancer therapy. Curr Drug Targets 2008;9:653-61. [PubMed]
- Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta 2008;1781:424-34.
- Okino N, He X, Gatt S, et al. The reverse activity of human acid ceramidase. J Biol Chem 2003;278:29948-53. [PubMed]
- Park JH, Schuchman EH. Acid ceramidase and human disease. Biochim Biophys Acta 2006;1758:2133-8.
- Gandy KA, Obeid LM. Regulation of the sphingosine kinase/sphingosine 1-phosphate pathway. Handb Exp Pharmacol 2013;(216):275-303.
- Johnson KR, Johnson KY, Becker KP, et al. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. J Biol Chem 2003;278:34541-7. [PubMed]
- Gao XY, Li L, Wang XH, et al. Inhibition of sphingosine-1-phosphate phosphatase 1 promotes cancer cells migration in gastric cancer: Clinical implications. Oncol Rep 2015;34:1977-87. [PubMed]
- Pulkoski-Gross MJ, Donaldson JC, Obeid LM. Sphingosine-1-phosphate metabolism: A structural perspective. Crit Rev Biochem Mol Biol 2015;50:298-313. [PubMed]
- Jeckel D, Karrenbauer A, Birk R, et al. Sphingomyelin is synthesized in the cis Golgi. FEBS Lett 1990;261:155-7. [PubMed]
- Villani M, Subathra M, Im YB, et al. Sphingomyelin synthases regulate production of diacylglycerol at the Golgi. Biochem J 2008;414:31-41. [PubMed]
- Hanada K, Kumagai K, Yasuda S, et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature 2003;426:803-9. [PubMed]
- Yamaji T, Hanada K. Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins. Traffic 2015;16:101-22. [PubMed]
- van 't Hof W, van Meer G. Generation of lipid polarity in intestinal epithelial (Caco-2) cells: sphingolipid synthesis in the Golgi complex and sorting before vesicular traffic to the plasma membrane. J Cell Biol 1990;111:977-86. [PubMed]
- Jenkins RW, Canals D, Hannun YA. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal 2009;21:836-46. [PubMed]
- Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal 2007;19:229-37. [PubMed]
- Tani M, Kuge O. Sphingomyelin synthase 2 is palmitoylated at the COOH-terminal tail, which is involved in its localization in plasma membranes. Biochem Biophys Res Commun 2009;381:328-32. [PubMed]
- Tani M, Igarashi Y, Ito M. Involvement of neutral ceramidase in ceramide metabolism at the plasma membrane and in extracellular milieu. J Biol Chem 2005;280:36592-600. [PubMed]
- Johnson KR, Becker KP, Facchinetti MM, et al. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA). J Biol Chem 2002;277:35257-62. [PubMed]
- Sutherland CM, Moretti PA, Hewitt NM, et al. The calmodulin-binding site of sphingosine kinase and its role in agonist-dependent translocation of sphingosine kinase 1 to the plasma membrane. J Biol Chem 2006;281:11693-701. [PubMed]
- ter Braak M, Danneberg K, Lichte K, et al. Galpha(q)-mediated plasma membrane translocation of sphingosine kinase-1 and cross-activation of S1P receptors. Biochim Biophys Acta 2009;1791:357-70.
- Jarman KE, Moretti PA, Zebol JR, et al. Translocation of sphingosine kinase 1 to the plasma membrane is mediated by calcium- and integrin-binding protein 1. J Biol Chem 2010;285:483-92. [PubMed]
- Takahashi T, Suchi M, Desnick RJ, et al. Identification and expression of five mutations in the human acid sphingomyelinase gene causing types A and B Niemann-Pick disease. Molecular evidence for genetic heterogeneity in the neuronopathic and non-neuronopathic forms. J Biol Chem 1992;267:12552-8. [PubMed]
- Li CM, Park JH, He X, et al. The human acid ceramidase gene (ASAH): structure, chromosomal location, mutation analysis, and expression. Genomics 1999;62:223-31. [PubMed]
- Scassellati C, Albi E, Cmarko D, et al. Intranuclear sphingomyelin is associated with transcriptionally active chromatin and plays a role in nuclear integrity. Biol Cell 2010;102:361-75. [PubMed]
- Albi E, Magni MV. Sphingomyelin synthase in rat liver nuclear membrane and chromatin. FEBS Lett 1999;460:369-72. [PubMed]
- Mizutani Y, Tamiya-Koizumi K, Nakamura N, et al. Nuclear localization of neutral sphingomyelinase 1: biochemical and immunocytochemical analyses. J Cell Sci 2001;114:3727-36. [PubMed]
- Shiraishi T, Imai S, Uda Y. The presence of ceramidase activity in liver nuclear membrane. Biol Pharm Bull 2003;26:775-9. [PubMed]
- Igarashi N, Okada T, Hayashi S, et al. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem 2003;278:46832-9. [PubMed]
- Lucki NC, Li D, Bandyopadhyay S, et al. Acid ceramidase (ASAH1) represses steroidogenic factor 1-dependent gene transcription in H295R human adrenocortical cells by binding to the receptor. Mol Cell Biol 2012;32:4419-31. [PubMed]
- Shimeno H, Soeda S, Sakamoto M, et al. Partial purification and characterization of sphingosine N-acyltransferase (ceramide synthase) from bovine liver mitochondrion-rich fraction. Lipids 1998;33:601-5. [PubMed]
- Novgorodov SA, Wu BX, Gudz TI, et al. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J Biol Chem 2011;286:25352-62. [PubMed]
- Wu BX, Rajagopalan V, Roddy PL, et al. Identification and characterization of murine mitochondria-associated neutral sphingomyelinase (MA-nSMase), the mammalian sphingomyelin phosphodiesterase 5. J Biol Chem 2010;285:17993-8002. [PubMed]
- Rajagopalan V, Canals D, Luberto C, et al. Critical determinants of mitochondria-associated neutral sphingomyelinase (MA-nSMase) for mitochondrial localization. Biochim Biophys Acta 2015;1850:628-39.
- Obeid LM, Linardic CM, Karolak LA, et al. Programmed cell death induced by ceramide. Science 1993;259:1769-71. [PubMed]
- Bielawska A, Crane HM, Liotta D, et al. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J Biol Chem 1993;268:26226-32. [PubMed]
- Jarvis WD, Kolesnick RN, Fornari FA, et al. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci U S A 1994;91:73-7. [PubMed]
- Bose R, Verheij M, Haimovitz-Friedman A, et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell 1995;82:405-14. [PubMed]
- Taha TA, Kitatani K, El-Alwani M, et al. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J 2006;20:482-4. [PubMed]
- Reiss U, Oskouian B, Zhou J, et al. Sphingosine-phosphate lyase enhances stress-induced ceramide generation and apoptosis. J Biol Chem 2004;279:1281-90. [PubMed]
- Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion 2006;6:118-25. [PubMed]
- von Haefen C, Wieder T, Gillissen B, et al. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene 2002;21:4009-19. [PubMed]
- Martínez-Abundis E, Correa F, Pavón N, et al. Bax distribution into mitochondrial detergent-resistant microdomains is related to ceramide and cholesterol content in postischemic hearts. FEBS J 2009;276:5579-88. [PubMed]
- Belaud-Rotureau MA, Leducq N, Macouillard Poulletier de Gannes F, et al. Early transitory rise in intracellular pH leads to Bax conformation change during ceramide-induced apoptosis. Apoptosis 2000;5:551-60. [PubMed]
- Lin CF, Chen CL, Chiang CW, et al. GSK-3beta acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J Cell Sci 2007;120:2935-43. [PubMed]
- Heinrich M, Wickel M, Schneider-Brachert W, et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J 1999;18:5252-63. [PubMed]
- Darios F, Lambeng N, Troadec JD, et al. Ceramide increases mitochondrial free calcium levels via caspase 8 and Bid: role in initiation of cell death. J Neurochem 2003;84:643-54. [PubMed]
- Yuan H, Williams SD, Adachi S, et al. Cytochrome c dissociation and release from mitochondria by truncated Bid and ceramide. Mitochondrion 2003;2:237-44. [PubMed]
- Sumitomo M, Ohba M, Asakuma J, et al. Protein kinase Cdelta amplifies ceramide formation via mitochondrial signaling in prostate cancer cells. J Clin Invest 2002;109:827-36. [PubMed]
- Grassme H, Jekle A, Riehle A, et al. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem 2001;276:20589-96. [PubMed]
- Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett 2010;584:1728-40. [PubMed]
- White-Gilbertson S, Mullen T, Senkal C, et al. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene 2009;28:1132-41. [PubMed]
- Yoon G, Kim KO, Lee J, et al. Ceramide increases Fas-mediated apoptosis in glioblastoma cells through FLIP down-regulation. J Neurooncol 2002;60:135-41. [PubMed]
- Nam SY, Amoscato AA, Lee YJ. Low glucose-enhanced TRAIL cytotoxicity is mediated through the ceramide-Akt-FLIP pathway. Oncogene 2002;21:337-46. [PubMed]
- Cuvillier O, Pirianov G, Kleuser B, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996;381:800-3. [PubMed]
- Olivera A, Kohama T, Edsall L, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol 1999;147:545-58. [PubMed]
- Johnson KR, Johnson KY, Crellin HG, et al. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem 2005;53:1159-66. [PubMed]
- Ponnusamy S, Meyers-Needham M, Senkal CE, et al. Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol 2010;6:1603-24. [PubMed]
- Degagné E, Saba JD. S1pping fire: Sphingosine-1-phosphate signaling as an emerging target in inflammatory bowel disease and colitis-associated cancer. Clin Exp Gastroenterol 2014;7:205-14. [PubMed]
- Olivera S, Rodriguez-Ithurralde D, Henley JM. Acetylcholinesterase promotes neurite elongation, synapse formation, and surface expression of AMPA receptors in hippocampal neurones. Mol Cell Neurosci 2003;23:96-106. [PubMed]
- Cuvillier O, Levade T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood 2001;98:2828-36. [PubMed]
- Edsall LC, Cuvillier O, Twitty S, et al. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J Neurochem 2001;76:1573-84. [PubMed]
- Osawa Y, Banno Y, Nagaki M, et al. TNF-alpha-induced sphingosine 1-phosphate inhibits apoptosis through a phosphatidylinositol 3-kinase/Akt pathway in human hepatocytes. J Immunol 2001;167:173-80. [PubMed]
- Radeff-Huang J, Seasholtz TM, Chang JW, et al. Tumor necrosis factor-alpha-stimulated cell proliferation is mediated through sphingosine kinase-dependent Akt activation and cyclin D expression. J Biol Chem 2007;282:863-70. [PubMed]
- Xia P, Wang L, Gamble JR, et al. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J Biol Chem 1999;274:34499-505. [PubMed]
- Van Brocklyn JR, Williams JB. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: oxidative stress and the seesaw of cell survival and death. Comp Biochem Physiol B Biochem Mol Biol 2012;163:26-36. [PubMed]
- Min J, Stegner AL, Alexander H, et al. Overexpression of sphingosine-1-phosphate lyase or inhibition of sphingosine kinase in Dictyostelium discoideum results in a selective increase in sensitivity to platinum-based chemotherapy drugs. Eukaryot Cell 2004;3:795-805. [PubMed]
- Nava VE, Hobson JP, Murthy S, et al. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res 2002;281:115-27. [PubMed]
- Sarkar S, Maceyka M, Hait NC, et al. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett 2005;579:5313-7. [PubMed]
- Datta A, Loo SY, Huang B, et al. SPHK1 regulates proliferation and survival responses in triple-negative breast cancer. Oncotarget 2014;5:5920-33. [PubMed]
- Pchejetski D, Böhler T, Stebbing J, et al. Therapeutic potential of targeting sphingosine kinase 1 in prostate cancer. Nat Rev Urol 2011;8:569-678. [PubMed]
- Alshaker H, Sauer L, Monteil D, et al. Therapeutic potential of targeting SK1 in human cancers. Adv Cancer Res 2013;117:143-200. [PubMed]
- Gault CR, Obeid LM. Still benched on its way to the bedside: sphingosine kinase 1 as an emerging target in cancer chemotherapy. Crit Rev Biochem Mol Biol 2011;46:342-51. [PubMed]
- Yang L, Weng W, Sun ZX, et al. SphK1 inhibitor II (SKI-II) inhibits acute myelogenous leukemia cell growth in vitro and in vivo. Biochem Biophys Res Commun 2015;460:903-8. [PubMed]
- Ishitsuka A, Fujine E, Mizutani Y, et al. FTY720 and cisplatin synergistically induce the death of cisplatin-resistant melanoma cells through the downregulation of the PI3K pathway and the decrease in epidermal growth factor receptor expression. Int J Mol Med 2014;34:1169-74. [PubMed]
- Song L, Xiong H, Li J, et al. Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-κB pathway in human non-small cell lung cancer. Clin Cancer Res 2011;17:1839-49. [PubMed]
- Schrecengost RS, Keller SN, Schiewer MJ, et al. Downregulation of Critical Oncogenes by the Selective SK2 Inhibitor ABC294640 Hinders Prostate Cancer Progression. Mol Cancer Res 2015; [Epub ahead of print]. [PubMed]
- Venkata JK, An N, Stuart R, et al. Inhibition of sphingosine kinase 2 downregulates the expression of c-Myc and Mcl-1 and induces apoptosis in multiple myeloma. Blood 2014;124:1915-25. [PubMed]
- Gao P, Smith CD. Ablation of sphingosine kinase-2 inhibits tumor cell proliferation and migration. Mol Cancer Res 2011;9:1509-19. [PubMed]
- Jayadev S, Liu B, Bielawska AE, et al. Role for ceramide in cell cycle arrest. J Biol Chem 1995;270:2047-52. [PubMed]
- Dbaibo GS, Pushkareva MY, Jayadev S, et al. Retinoblastoma gene product as a downstream target for a ceramide-dependent pathway of growth arrest. Proc Natl Acad Sci U S A 1995;92:1347-51. [PubMed]
- Marchesini N, Osta W, Bielawski J, et al. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem 2004;279:25101-11. [PubMed]
- Marchesini N, Jones JA, Hannun YA. Confluence induced threonine41/serine45 phospho-beta-catenin dephosphorylation via ceramide-mediated activation of PP1cgamma. Biochim Biophys Acta 2007;1771:1418-28.
- Spassieva SD, Rahmaniyan M, Bielawski J, et al. Cell density-dependent reduction of dihydroceramide desaturase activity in neuroblastoma cells. J Lipid Res 2012;53:918-28. [PubMed]
- Alesse E, Zazzeroni F, Angelucci A, et al. The growth arrest and downregulation of c-myc transcription induced by ceramide are related events dependent on p21 induction, Rb underphosphorylation and E2F sequestering. Cell Death Differ 1998;5:381-9. [PubMed]
- Kim WH, Kang KH, Kim MY, et al. Induction of p53-independent p21 during ceramide-induced G1 arrest in human hepatocarcinoma cells. Biochem Cell Biol 2000;78:127-35. [PubMed]
- Lee JY, Bielawska AE, Obeid LM. Regulation of cyclin-dependent kinase 2 activity by ceramide. Exp Cell Res 2000;261:303-11. [PubMed]
- Jenkins RW, Clarke CJ, Canals D, et al. Regulation of CC ligand 5/RANTES by acid sphingomyelinase and acid ceramidase. J Biol Chem 2011;286:13292-303. [PubMed]
- Rani CS, Abe A, Chang Y, et al. Cell cycle arrest induced by an inhibitor of glucosylceramide synthase. Correlation with cyclin-dependent kinases. J Biol Chem 1995;270:2859-67. [PubMed]
- Swanton C, Marani M, Pardo O, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell 2007;11:498-512. [PubMed]
- Lee JY, Leonhardt LG, Obeid LM. Cell-cycle-dependent changes in ceramide levels preceding retinoblastoma protein dephosphorylation in G2/M. Biochem J 1998;334:457-61. [PubMed]
- Phillips DC, Hunt JT, Moneypenny CG, et al. Ceramide-induced G2 arrest in rhabdomyosarcoma (RMS) cells requires p21Cip1/Waf1 induction and is prevented by MDM2 overexpression. Cell Death Differ 2007;14:1780-91. [PubMed]
- Lechler P, Renkawitz T, Campean V, et al. The antiapoptotic gene survivin is highly expressed in human chondrosarcoma and promotes drug resistance in chondrosarcoma cells in vitro. BMC Cancer 2011;11:120. [PubMed]
- Loh KC, Baldwin D, Saba JD. Sphingolipid signaling and hematopoietic malignancies: to the rheostat and beyond. Anticancer Agents Med Chem 2011;11:782-93. [PubMed]
- Gangoiti P, Granado MH, Alonso A, et al. Implication of ceramide, ceramide 1-phosphate and sphingosine 1-phosphate in tumorigenesis. Transl Oncogenomics 2008;3:81-98. [PubMed]
- Wang F, Buckley NE, Olivera A, et al. Involvement of sphingolipids metabolites in cellular proliferation modulated by ganglioside GM1. Glycoconj J 1996;13:937-45. [PubMed]
- Kleuser B, Maceyka M, Milstien S, et al. Stimulation of nuclear sphingosine kinase activity by platelet-derived growth factor. FEBS Lett 2001;503:85-90. [PubMed]
- Van Brocklyn JR, Jackson CA, Pearl DK, et al. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol 2005;64:695-705. [PubMed]
- Kohno M, Momoi M, Oo ML, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol 2006;26:7211-23. [PubMed]
- Kotelevets N, Fabbro D, Huwiler A, et al. Targeting sphingosine kinase 1 in carcinoma cells decreases proliferation and survival by compromising PKC activity and cytokinesis. PLoS One 2012;7:e39209 [PubMed]
- LeBlanc FR, Liu X, Hengst J, et al. Sphingosine kinase inhibitors decrease viability and induce cell death in natural killer-large granular lymphocyte leukemia. Cancer Biol Ther 2015; [Epub ahead of print]. [PubMed]
- Marvaso G, Barone A, Amodio N, et al. Sphingosine analog fingolimod (FTY720) increases radiation sensitivity of human breast cancer cells in vitro. Cancer Biol Ther 2014;15:797-805. [PubMed]
- Heffernan-Stroud LA, Helke KL, Jenkins RW, et al. Defining a role for sphingosine kinase 1 in p53-dependent tumors. Oncogene 2012;31:1166-75. [PubMed]
- Venable ME, Lee JY, Smyth MJ, et al. Role of ceramide in cellular senescence. J Biol Chem 1995;270:30701-8. [PubMed]
- Venable ME, Webb-Froehlich LM, Sloan EF, et al. Shift in sphingolipid metabolism leads to an accumulation of ceramide in senescence. Mech Ageing Dev 2006;127:473-80. [PubMed]
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science 1996;274:1855-9. [PubMed]
- Mouton RE, Venable ME. Ceramide induces expression of the senescence histochemical marker, beta-galactosidase, in human fibroblasts. Mech Ageing Dev 2000;113:169-81. [PubMed]
- Venable ME, Yin X. Ceramide induces endothelial cell senescence. Cell Biochem Funct 2009;27:547-51. [PubMed]
- Jadhav KS, Dungan CM, Williamson DL. Metformin limits ceramide-induced senescence in C2C12 myoblasts. Mech Ageing Dev 2013;134:548-59. [PubMed]
- Rao RP, Scheffer L, Srideshikan SM, et al. Ceramide transfer protein deficiency compromises organelle function and leads to senescence in primary cells. PLoS One 2014;9:e92142 [PubMed]
- Panneer Selvam S, De Palma RM, Oaks JJ, et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci Signal 2015;8:ra58. [PubMed]
- Scarlatti F, Bauvy C, Ventruti A, et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem 2004;279:18384-91. [PubMed]
- Guenther GG, Peralta ER, Rosales KR, et al. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci U S A 2008;105:17402-7. [PubMed]
- Pozuelo-Rubio M. Regulation of autophagic activity by 14-3-3ζ proteins associated with class III phosphatidylinositol-3-kinase. Cell Death Differ 2011;18:479-92. [PubMed]
- Taniguchi M, Kitatani K, Kondo T, et al. Regulation of autophagy and its associated cell death by "sphingolipid rheostat": reciprocal role of ceramide and sphingosine 1-phosphate in the mammalian target of rapamycin pathway. J Biol Chem 2012;287:39898-910. [PubMed]
- Salazar M, Carracedo A, Salanueva IJ, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest 2009;119:1359-72. [PubMed]
- Park MA, Zhang G, Martin AP, et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther 2008;7:1648-62. [PubMed]
- Li Y, Li S, Qin X, et al. The pleiotropic roles of sphingolipid signaling in autophagy. Cell Death Dis 2014;5:e1245 [PubMed]
- Pattingre S, Bauvy C, Carpentier S, et al. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem 2009;284:2719-28. [PubMed]
- Zheng W, Kollmeyer J, Symolon H, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta 2006;1758:1864-84.
- Scarlatti F, Sala G, Ricci C, et al. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett 2007;253:124-30. [PubMed]
- Scarlatti F, Sala G, Somenzi G, et al. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. FASEB J 2003;17:2339-41. [PubMed]
- Messner MC, Cabot MC. Cytotoxic responses to N-(4-hydroxyphenyl)retinamide in human pancreatic cancer cells. Cancer Chemother Pharmacol 2011;68:477-87. [PubMed]
- Fazi B, Bursch W, Fimia GM, et al. Fenretinide induces autophagic cell death in caspase-defective breast cancer cells. Autophagy 2008;4:435-41. [PubMed]
- Liu XW, Su Y, Zhu H, et al. HIF-1α-dependent autophagy protects HeLa cells from fenretinide (4-HPR)-induced apoptosis in hypoxia. Pharmacol Res 2010;62:416-25. [PubMed]
- Yester JW, Tizazu E, Harikumar KB, et al. Extracellular and intracellular sphingosine-1-phosphate in cancer. Cancer Metastasis Rev 2011;30:577-97. [PubMed]
- Lavieu G, Scarlatti F, Sala G, et al. Is autophagy the key mechanism by which the sphingolipid rheostat controls the cell fate decision? Autophagy 2007;3:45-7. [PubMed]
- Chang CL, Ho MC, Lee PH, et al. S1P(5) is required for sphingosine 1-phosphate-induced autophagy in human prostate cancer PC-3 cells. Am J Physiol Cell Physiol 2009;297:C451-8. [PubMed]
- Lépine S, Allegood JC, Park M, et al. Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy. Cell Death Differ 2011;18:350-61. [PubMed]
- Lavieu G, Scarlatti F, Sala G, et al. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem 2006;281:8518-27. [PubMed]
- Huang YL, Chang CL, Tang CH, et al. Extrinsic sphingosine 1-phosphate activates S1P5 and induces autophagy through generating endoplasmic reticulum stress in human prostate cancer PC-3 cells. Cell Signal 2014;26:611-8. [PubMed]
- Evangelisti C, Evangelisti C, Teti G, et al. Assessment of the effect of sphingosine kinase inhibitors on apoptosis,unfolded protein response and autophagy of T-cell acute lymphoblastic leukemia cells; indications for novel therapeutics. Oncotarget 2014;5:7886-901. [PubMed]
- Brocklyn JR. Regulation of cancer cell migration and invasion by sphingosine-1-phosphate. World J Biol Chem 2010;1:307-12. [PubMed]
- Fan SH, Wang YY, Lu J, et al. CERS2 suppresses tumor cell invasion and is associated with decreased V-ATPase and MMP-2/MMP-9 activities in breast cancer. J Cell Biochem 2015;116:502-13. [PubMed]
- Zhao Q, Wang H, Yang M, et al. Expression of a tumor-associated gene, LASS2, in the human bladder carcinoma cell lines BIU-87, T24, EJ and EJ-M3. Exp Ther Med 2013;5:942-6. [PubMed]
- Zhang P, Fu C, Hu Y, et al. C6-ceramide nanoliposome suppresses tumor metastasis by eliciting PI3K and PKCζ tumor-suppressive activities and regulating integrin affinity modulation. Sci Rep 2015;5:9275. [PubMed]
- Perry DM, Newcomb B, Adada M, et al. Defining a role for acid sphingomyelinase in the p38/interleukin-6 pathway. J Biol Chem 2014;289:22401-12. [PubMed]
- Carpinteiro A, Becker KA, Japtok L, et al. Regulation of hematogenous tumor metastasis by acid sphingomyelinase. EMBO Mol Med 2015;7:714-34. [PubMed]
- Hannun YA, Newcomb B. A new twist to the emerging functions of ceramides in cancer: novel role for platelet acid sphingomyelinase in cancer metastasis. EMBO Mol Med 2015;7:692-4. [PubMed]
- Pitson SM, Powell JA, Bonder CS. Regulation of sphingosine kinase in hematological malignancies and other cancers. Anticancer Agents Med Chem 2011;11:799-809. [PubMed]
- Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta 2004;1682:48-55.
- Imeri F, Blanchard O, Jenni A, et al. FTY720 and two novel butterfly derivatives exert a general anti-inflammatory potential by reducing immune cell adhesion to endothelial cells through activation of S1P3 and phosphoinositide 3-kinase. Naunyn Schmiedebergs Arch Pharmacol 2015; [Epub ahead of print]. [PubMed]
- Wang H, Cai KY, Li W, et al. Sphingosine-1-phosphate induces the migration and angiogenesis of EPCs through the Akt signaling pathway via sphingosine-1-phosphate receptor 3/platelet-derived growth factor receptor-β. Cell Mol Biol Lett 2015;20:597-611. [PubMed]
- Lepley D, Paik JH, Hla T, et al. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res 2005;65:3788-95. [PubMed]
- Arikawa K, Takuwa N, Yamaguchi H, et al. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity. J Biol Chem 2003;278:32841-51. [PubMed]
- Paugh BS, Paugh SW, Bryan L, et al. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCdelta, and sphingosine kinase 1 in glioblastoma cells. FASEB J 2008;22:455-65. [PubMed]
- Hsieh HL, Sun CC, Wu CB, et al. Sphingosine 1-phosphate induces EGFR expression via Akt/NF-kappaB and ERK/AP-1 pathways in rat vascular smooth muscle cells. J Cell Biochem 2008;103:1732-46. [PubMed]
- Balthasar S, Bergelin N, Löf C, et al. Interactions between sphingosine-1-phosphate and vascular endothelial growth factor signalling in ML-1 follicular thyroid carcinoma cells. Endocr Relat Cancer 2008;15:521-34. [PubMed]
- Shida D, Kitayama J, Yamaguchi H, et al. Sphingosine 1-phosphate transactivates c-Met as well as epidermal growth factor receptor (EGFR) in human gastric cancer cells. FEBS Lett 2004;577:333-8. [PubMed]
- Van Brocklyn JR, Young N, Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett 2003;199:53-60. [PubMed]
- Stam JC, Michiels F, van der Kammen RA, et al. Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO J 1998;17:4066-74. [PubMed]
- Mahajan-Thakur S, Sostmann BD, Fender AC, et al. Sphingosine-1-phosphate induces thrombin receptor PAR-4 expression to enhance cell migration and COX-2 formation in human monocytes. J Leukoc Biol 2014;96:611-8. [PubMed]
- Lee MH, Hammad SM, Semler AJ, et al. HDL3, but not HDL2, stimulates plasminogen activator inhibitor-1 release from adipocytes: the role of sphingosine-1-phosphate. J Lipid Res 2010;51:2619-28. [PubMed]
- Bryan L, Paugh BS, Kapitonov D, et al. Sphingosine-1-phosphate and interleukin-1 independently regulate plasminogen activator inhibitor-1 and urokinase-type plasminogen activator receptor expression in glioblastoma cells: implications for invasiveness. Mol Cancer Res 2008;6:1469-77. [PubMed]
- Orr Gandy KA, Adada M, Canals D, et al. Epidermal growth factor-induced cellular invasion requires sphingosine-1-phosphate/sphingosine-1-phosphate 2 receptor-mediated ezrin activation. FASEB J 2013;27:3155-66. [PubMed]
- Adada M, Canals D, Hannun YA, et al. Sphingolipid regulation of ezrin, radixin, and moesin proteins family: implications for cell dynamics. Biochim Biophys Acta 2014;1841:727-37.
- Adada MM, Canals D, Jeong N, et al. Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion. FASEB J 2015; [Epub ahead of print]. [PubMed]


