Diagnosis of pneumocystis pneumonia using serum (1-3)-β-D-Glucan: a bivariate meta-analysis and systematic review
Introduction
Pneumocystis pneumonia (PCP) remains the frequent opportunistic infection in immunocompromised patients, including those with human immunodeficiency virus (HIV) infection, and is associated with serious mortality (1-3). Hospital survival due to HIV-related PCP ranges from 10% to 20% (4), whereas it reaches to 60% in HIV-unrelated patients (5). The high mortality rate results partly from difficulties in establishing an early diagnosis, because of nonspecific clinical features, concurrent use of prophylactic drugs, possible coinfection with various microorganisms (1,3). With the development of medicine, as a study reported, the survival rate of HIV-related PCP had approaches 90% in many treatment centers (5). Therefore, it is of importance to find out a feasible method which may allow an early diagnosis of PCP.
Since Pneumocystis jirovecii cannot be cultured, the diagnosis of PCP relies on the detection of cysts and/or trophozoites by colorimetric or immunofluorescent stains or by polymerase chain reaction (PCR) (3). In a meta-analysis of seven reports in which PCP was diagnosed by staining, the average sensitivity (Se) of sputum in HIV patients was only 56%, whereas that of bronchoalveolar lavage (BAL) fluid (BALF) was >95% (6). A bronchoscopy with BAL should be performed when we acquire negative results of sputum. However, the invasive procedure is not always feasible for individuals with severe diseases. Other diagnostic procedures, such as PCR, have greater Se but less specificity (Sp) due to the problem of false positive (1). Furthermore, good-quality respiratory samples, such as BAL, always require the invasive procedure.
(1-3)-β-D-Glucan (BG) is a common cell wall constituent of most pathogenic fungi, including P. jirovecii. The detection of serum-BG has gained widespread acceptance as a useful diagnostic tool for invasive fungal diseases (IFDs) (mainly refer to pulmonary aspergillosis and candidiasis in this context) (7). There have been some encouraging reports describing the diagnosis of PCP with serum-BG (8-13). In 2009, the Japanese guidelines even recommended that the positive serum-BG should be regarded as a marker of PCP for patients with rheumatoid arthritis (14). Karageorgopoulos et al. published a meta-analysis about the accuracy of BG for the diagnosis of PCP, reporting a high Se of BG (15). However, most of the patients were not HIV and they did not separately assessed the HIV-related or -unrelated patients. Onishi et al. performed another meta-analysis and reported similar results in terms of the Se and Sp between HIV and non-HIV patients (16). But they excluded the patients who were diagnosed with other IFDs, which would exaggerate the Sp and positive predictive value. Besides, both meta-analyses included several studies in which the patients were not selected consecutively or randomly and the accuracy would be affected by the subjective enrollment of the numbers of patients in the control group. However, some guidelines, which focused on the opportunistic infections in HIV-related or -unrelated patients, did not confirm “serum-BG” as microbiologic evidence for PCP (5,17,18). In recent years, several trials elucidating serum-BG in HIV patients have been published; it is more valuable to assess the overall accuracy of serum-BG for diagnosing PCP in immunocompromised patients with some performance indicators and associated 95% confidence interval (CI).
Methods
Study identification and selection
Two investigators (Wei-Jie Li and Ya-Ling Guo) searched MEDLINE and EMBASE databases for relevant articles published until May, 2015, following the PRISMA flow diagram (19). Search terms included: “pneumocystis pneumonia”, “pneumocytis carinii pneumonia”, “pneumonia, pneumocystis carinii”, “pneumocystis jirovecii pneumonia”, “pneumonia, pneumocystis jirovecii”, “PCP”, “pneumocystosis”, “(1-3)-β-D-Glucan”, “β-D-Glucan”, “BG”, “BDG” and “β-Glucan”, and the syntax for MEDLINE searches was as follows: (“pneumocystis pneumonia” OR “pneumocystis carinii pneumonia” OR “pneumonia, pneumocystis carinii” OR “pneumocystis jirovecii pneumonia” OR “pneumonia, pneumocystis jirovecii” OR “PCP” OR “pneumocystosis”) AND [“(1-3)-β-D-Glucan” OR “β-D-Glucan” OR “β-Glucan” OR “BG” OR “BDG”]. The searches were limited to English publications in humans. We screened the reference lists of included studies and related publications. The results were hand searched for eligible trials. We did not include abstracts or meeting’s proceedings. Results were arbitrated by a third investigator (Ke Wang).
Full-text publications were included if (I) they compared the serum-BG assay with the reference standard for diagnosing PCP in a relevant clinical population. A relevant clinical population was defined as a group of at-risk individual with pulmonary diseases and in an immunocompromised status; (II) they provided data of true positive, false positive, true negative and false negative; (III) they were concerned with definite PCP. Diagnosis of definite PCP was categorized as proven (positive conventional stains) or probable (underlying host factors, compatible clinical and radiological findings, along with positive PCR). Since the detection of Pneumocystis in individuals without signs and symptoms of clinical infection has been defined as colonization (20), PCR combined with clinical manifestations are effect in diagnosing PCP (3,14). The studies in which the subjects were not selected consecutively or randomly were excluded. The under-ten-patients studies were excluded to avoid selection bias.
Data extraction and quality assessment
Two investigators (Wei-Jie Li and Tang-Juan Liu) independently abstracted the following information: population characteristics, reference standard, assay characteristics, methodological quality and diagnostic data for two-by-two tables. Since the disease does not clinically result from immunologically normal individuals (1,3), the numbers of healthy volunteers in the control group were excluded. When the same population was analyzed in several publications, the results were accounted for only once. When HIV-related and -unrelated PCP patients were assessed in the same report, we e-mailed the author for further information and extracted data separately if available. If several cutoffs were reported in one study, we used the cutoff that offered the best test performance for PCP. Any disagreements were resolved by a third author (Jin-Liang Kong).
We assessed the methodological quality using the updated quality assessment of diagnostic accuracy studies [quality assessment of diagnostic accuracy studies (QUADAS)-2] tool (21), evaluating the bias and applicability of the patient selection, index test, reference standard and flow and timing.
Data synthesis
We constructed and analyzed two-by-two tables (PCP vs. non-PCP). By using a bivariate regression approach, we estimated the overall Se and Sp as the main outcome measures, and then constructed summary receiver operating characteristic (SROC) curves. This method has been extensively described elsewhere and has been recently recommended for meta-analysis of diagnostic tests (22,23). Based on random-effects models, this bivariate regression model accounts for potential between-study heterogeneity, incorporates the possible association between Se and Sp (22). By using pooled estimates for Se and Sp, we also calculated positive likelihood ratio (PLR) and negative likelihood ratio (NLR) (22,24). PLRs above 10 and NLRs below 0.1 have been noted as providing convincing diagnostic evidence, whereas those above 5 and below 0.2 give strong diagnostic evidence.
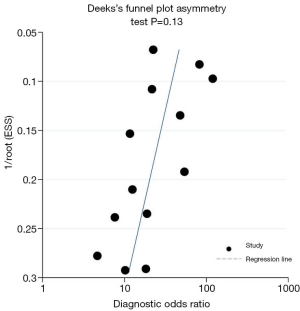
We assessed statistically significant heterogeneity using the I2 statistic, because I2 does not inherently depend on the number of studies in the meta-analysis (25). Potential between-study heterogeneity was explored by subgroup analysis (26). Covariates requiring that at least 80% of studies reported on a particular item were analyzed: HIV status (related vs. unrelated), study subjects (only PCP vs. IFDs including PCP), study design (prospective vs. retrospective) and reference standard (staining vs. PCR vs. autopsy) (26). We assessed the potential publication bias by using Deeks’s funnel plots (27).
All analyses were performed using STATA (version 10; Stata Corporation; College Station, TX, USA) with the program “MIDAS” (28). Except for Deeks’s funnel plot using P values of 0.10, other statistical tests were two-sided with P values of 0.05 denoting statistical significance.
Results
Eligible study characteristics and quality assessment
Of the 183 references identified, 12 studies met our criteria (29-40). Additionally, one Chinese trial was retrieved by screening the reference lists of the included studies (41). Overall, 13 studies were finally pooled in our meta-analysis. Figure 1 shows the literature search leading to selection of the 13 reports.
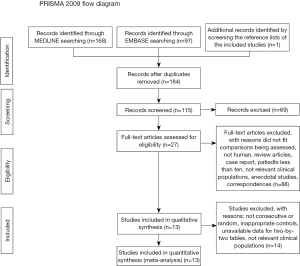
Table 1 listed the characteristics of eligible studies. Among the 13 studies, only three trials were prospective studies (37-39) and the rest were retrospective trials. Besides, three trials (30,31,36) evaluated the accuracy of BG in HIV-related patients. Five trials (34,35,37,38,40) exclusively consisted of HIV-unrelated patients and the other four trials (29,32,33,39) enrolled all of the immunocompromised patients including HIV. In one of the latter four trials (33), since the HIV patients were just small part of the overall subjects, we excluded the HIV patients based on the original data offered by the authors, so the rest of patients could be analyzed in the subgroup of non-HIV. One study (41) had not mentioned whether they had enrolled HIV patients. All or part of patients had accepted prophylactic treatment in 11 trials.
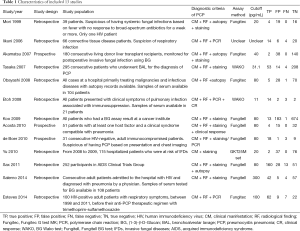
Full table
In terms of reference standard of PCP in each study, autopsy was performed in four trials (30,32,33,38). PCR was applied in four trials (35-37,40) and cytology by staining was performed in nine trials. All of trials accept one (41) had taken radiology characteristic into consideration when patients were diagnosed with PCP. Four kinds of assays were applied for the measure of BG. WAKO was used in two trials (29,40), Fungitell was used in five trials (30,31,34,37,39), Fungitec was used in four trials (32,33,36,38) and GKT25M set was used in only one trial (41).
Four domains of risk of bias and three domains of applicability were assessed for all studies. The results are shown in Table 2. Since the studies were properly designed and we excluded studies in which the subjects were not selected consecutively or randomly, the risk of bias and the concerns about applicability were acceptable. To be noted, all studies did not directly indicate whether the results of index test and the reference standard were independently interpreted, we cautiously rated the concerns about the applicability as “unclear”. Overall, the quality of the included trials was superior, guaranteeing the reliability of our meta-analysis.

Full table
Diagnostic accuracy of serum-BG
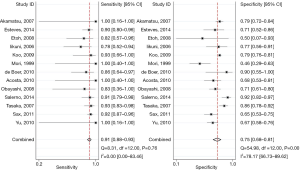
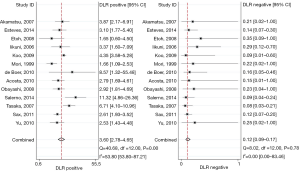
For the overall analysis, the summary estimates and 95% CI were as follows: Se, 0.91 (95% CI, 0.88−0.93), I2=0; Sp, 0.75 (95% CI, 0.68−0.81), I2=78.17, shown as the Figure 2; PLR, 3.60 (95% CI, 2.78−4.65), I2=53.80; NLR, 0.12 (95% CI, 0.09−0.17), I2=0 (Figure 3). I2 values indicated that significant heterogeneity for Sp and PLR.
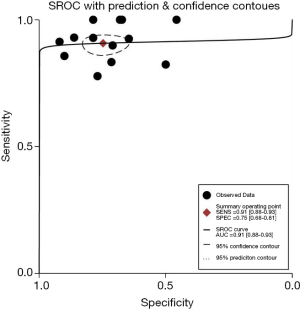
Hierarchical SROC curve is displayed in Figure 4. The SROC curve represented the relationship between Se and Sp across studies, recognizing that they might have used different thresholds. Because the bivariate approach estimates the strength and the shape of the correlation between Se and Sp, we can draw a 95% confidence ellipse and a 95% prediction ellipse. The area under the SROC curve was 0.91 (range, 0.88−0.93), indicating that the serum-BG assay had a good discrimination ability.
Investigations of heterogeneity, publication bias
For subgroup analysis by HIV-related and HIV-unrelated patients (Table 3), the Se in patients with HIV infection was 0.92 (95% CI, 0.88−0.95), while in those without HIV infection Se was only 0.85 (95% CI, 0.77−0.94). Significant heterogeneity between Se was presented (P=0.04), which was not in line with the former study (16). However, the Sp was 0.78 and 0.73 for HIV-related and HIV-unrelated patients, respectively, showing no significant heterogeneity between them (P=0.47).

Full table
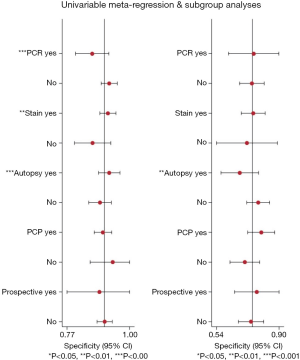
Figure 5 shows the results of the subgroup analyses by reference standard, study design and the study subjects. Regarding the subgroup analysis based on the reference standard of staining, autopsy or PCR, the Se shown significant heterogeneity between groups (P<0.05), while Se seemed not to be influenced by the investigated disease, whether it was PCP only or other IFDs including PCP (P=0.17). Besides, there was no significant heterogeneity between the prospective studies and retrospective studies in terms of Se (P=0.13). However, the heterogeneity of Sp was not significant; accept for the subgroup by autopsy. The Sp would be worse if we performed autopsy as one of the criteria for the diagnosis of PCP (P=0.00). The publication bias shown as Figure 6 was found in Deeks’s funnel plot (P=0.13).
Discussion
PCP is a life-threatening opportunistic infection that occurs exclusively in patients whose immune systems have been compromised due to disease and/or immunosuppressive treatments. Compared with HIV patients, HIV-unrelated patients develop PCP abruptly and progress to acute fulminating pneumonia with acute respiratory failure. To evaluate the overall accuracy for diagnosing PCP between HIV and non-HIV patients, we performed this meta-analysis with bivariate regression approach.
Thirteen publications comparing serum-BG with reference standard were ultimately included. In our meta-analysis, we drew a conclusion that serum-BG assay was moderately useful for the diagnosis of PCP in individuals especially with HIV infection. An exploration of the reasons for heterogeneity is an important goal of meta-analysis (25,26). Part of variability is due to chance, because some studies have small sample sizes (33,35,37,39,40). The remaining heterogeneity may be due to differences in study populations, assay characteristics and reference standards.
In some situations, variations may occur between studies due to differences in cutoff values. For example, the turbidimetric BG test Wako, the colorimetric Fungitec G test MK (Fungitec) and the Fungitell BG test (Fungitell) have been applied to most clinical studies. Many differences are identified in these kits, such as pretreatment, BG standard, and detection range (32,33,42). In fact, a value of 11 pg/mL measured by the Wako assay is equivalent to a 113.7 pg/mL measurement by the Fungitec method, and a reading of 60 pg/mL by the Fungitell method corresponds to a measurement of 20 pg/mL by the Fungitec assay (32,42). According to our results, we found the partial heterogeneity due to threshold effect. Since these variations failed to account fully for the heterogeneity, searching other differences was very important in our meta-analysis.
The status of HIV infection was found to have great influence on the positivity rates of stained specimens. The decreased of positivity rates might be explained by lower burdens of Pneumocystis in HIV-unrelated PCP individuals (1). As to serum-BG, some studies found that the BG value reflected Pneumocystis burden (43,44). According to subgroup analysis, the Se of serum-BG was greater in HIV individuals, with 0.92 and 0.85 for patients with and without HIV infection, respectively (P=0.04). As a result, it may be interpreted that the superior Se of BG in HIV patients was related with the higher burden of Pneumocystis. Therefore, if we get a negative result, serum-BG is valuable to be used as a screening test for PCP in HIV patients. Furthermore, extra economic burden and unnecessary prophylactic treatment can be avoided. For non-HIV patients, the results should be cautiously interpreted in parallel with clinical characteristic, radiological findings and other diagnostic evidence. This was not in line with the former trial which reported that there was no heterogeneity between different HIV statuses (16).
However, there were slightly difference in terms of the Sp with a moderate result (P=0.47). Although the nonsignificant difference was probably related to small numbers of patients and/or studies included, along with the high degree of variation in BG levels among individuals, we were not surprised for the poor Sp without remarked heterogeneity between different HIV statuses. Because BG was not a specific biomarker of Pneumocystis and it would be positive in various pathogenic fungi (45). Furthermore, as some studies reported, the elevated false positive was associated with the application of cellulose membranes during hemodialysis, certain treatment including antimicrobial agents or immunological preparations and even some diseases such as renal failure and severe mucositis (46-48). As a result, for patients whose BG was positive, more clinical and laboratorial information such as PCR, BAL, computed tomography (CT) scan or autopsy would be needed to validate the diagnosis of PCP.
Given that the reference standard varied, we undertook a subgroup analysis by the diagnostic method. When staining or autopsy was used to define proven PCP, the Se of BG assay was 0.92 and 0.93, respectively. When lower degrees of proof for PCP, including PCR method, were used as the reference standard, the Se decreased to 0.86. Since many investigators have detected the carriage of Pneumocystis by staining and/or PCR methods (20), Pneumocystis colonization could be misclassified to PCP. However, compared with PCR techniques, the staining assays and autopsy are generally not adequate for detection of colonization (20). Although we applied the combination of clinical manifestations and PCR to differentiate PCP from colonization, it was unavoidable that Pneumocystis colonization with other pulmonary disease may still exist (49). Recently, the serum-BG values of individuals colonized with Pneumocystis were found to be significantly lower than those of patients with PCP (49,50), indicating that serum-BG assay is effective in discriminate PCP from colonization. Therefore, PCR may increase the number of false-negativity, decreasing the Se of BG assay. Furthermore, to exclude Pneumocystis colonization, we recommend the diagnostic criteria of PCP proposed by Tokuda et al. (44). According to their opinions, the diagnosis of PCP is based on the combination of clinical and radiological findings, positive staining and/or PCR, along with positive serum-BG results.
Since serum-BG values ≥500 pg/mL (Fungitell kit) were observed in most PCP individuals, a higher cutoff value has been recommended for differential diagnosis from IFDs (9). In Koo’s reports, BG values ≥500 pg/mL (Fungitell kit) were found in 12 of 16 PCP patients, while those found in 22 of 98 patients with IFDs (8,34). However, our data are too limited to confirm an optimal cutoff value for discrimination between PCP and IFDs. On the other hand, the typical findings of CT and galactomannan assay between IFDs and PCP are quite different (7). For IFDs, the features of CT are the presence of dense, well-circumscribed lesions(s) with or without a halo sign, air-crescent sign and/or cavity (7), while most PCP patients present with bilateral diffuse infiltrate (1,3,5,14). Thus, discrimination might be achieved when different tests are used in combination, like CT, BG and galactomannan assay. In fact, the recent published guidelines also recommended a similar diagnostic flow sheet from CT to BG for PCP (14). Since CT is routinely used for evaluating the pulmonary diseases of at-risk patients in most hospitals, we suggest that a diagnosis strategy with CT-based BG detection for PCP could be used safely. Further prospective studies are needed with a larger sample size to confirm this diagnosis strategy.
Our study had several important limitations. First, only two trials included patients without prophylaxis (36,37). However, in the rest studies, not all of the patients accepted prophylactic treatments, which blocked us to take a further heterogeneity analysis. But it was not humanistic and rational to prevent the at-risk individual especially with a suspicious clinical manifestation of the lethal PCP from the prophylaxis, though we all knew it would definitely affect the accuracy of BG assay. Second, in the subgroup analysis by the HIV status, three trials consisted of various immunocompromised patients had to be excluded because the detail of the HIV and non-HIV patients were not provided and we could not classify them into any of the subgroup. We failed to obtain the data from these authors, potentially resulting in biased results and less precise estimates of pooled diagnostic accuracy. Third, the publication bias was present. A major reason is that studies with nonsignificant results are less likely to be published than studies with significant findings (51). However, it is not easy to investigate and analyze publication bias for diagnostic tests (27,52). Furthermore, the included trials were not large enough and we could not acquire sufficient data to explore the potential interference between various factors that created the significant heterogeneity.
In conclusion, the current meta-analysis suggests that serum-BG assay can be used as a screening adjunct with high Se and moderate Sp in patients with HIV. When it is applied to patients with non-HIV, the results should be interpreted in parallel with clinical and radiological findings. Given to the limitations, more well-designed, prospective studies with larger sample size are needed to confirm the diagnosis value of BG detection.
Acknowledgements
Funding: This work was supported by General Project of the Guangxi Education Department [201010LX039 to K.W].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004;350:2487-98. [PubMed]
- Kaplan JE, Benson C, Holmes KK, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009;58:1-207; quiz CE1-4.
- Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA 2009;301:2578-85. [PubMed]
- Curtis JR, Yarnold PR, Schwartz DN, et al. Improvements in outcomes of acute respiratory failure for patients with human immunodeficiency virus-related Pneumocystis carinii pneumonia. Am J Respir Crit Care Med 2000;162:393-8. [PubMed]
- Sepkowitz KA. Opportunistic infections in patients with and patients without Acquired Immunodeficiency Syndrome. Clin Infect Dis 2002;34:1098-107. [PubMed]
- Cruciani M, Marcati P, Malena M, et al. Meta-analysis of diagnostic procedures for Pneumocystis carinii pneumonia in HIV-1-infected patients. Eur Respir J 2002;20:982-9. [PubMed]
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. [PubMed]
- Marty FM, Koo S, Bryar J, et al. (1->3)beta-D-glucan assay positivity in patients with Pneumocystis (carinii) jiroveci pneumonia. Ann Intern Med 2007;147:70-2. [PubMed]
- Desmet S, Van Wijngaerden E, Maertens J, et al. Serum (1-3)-beta-D-glucan as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J Clin Microbiol 2009;47:3871-4. [PubMed]
- Watanabe T, Yasuoka A, Tanuma J, et al. Serum (1-->3) beta-D-glucan as a noninvasive adjunct marker for the diagnosis of Pneumocystis pneumonia in patients with AIDS. Clin Infect Dis 2009;49:1128-31. [PubMed]
- Pisculli ML, Sax PE. Use of a serum beta-glucan assay for diagnosis of HIV-related Pneumocystis jiroveci pneumonia in patients with negative microscopic examination results. Clin Infect Dis 2008;46:1928-30. [PubMed]
- Cuétara MS, Alhambra A, Chaves F, et al. Use of a serum (1-->3)-beta-D-glucan assay for diagnosis and follow-up of Pneumocystis jiroveci pneumonia. Clin Infect Dis 2008;47:1364-6. [PubMed]
- Fujii T, Nakamura T, Iwamoto A. Pneumocystis pneumonia in patients with HIV infection: clinical manifestations, laboratory findings, and radiological features. J Infect Chemother 2007;13:1-7. [PubMed]
- Koike R, Harigai M, Atsumi T, et al. Japan College of Rheumatology 2009 guidelines for the use of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, in rheumatoid arthritis. Mod Rheumatol 2009;19:351-7. [PubMed]
- Karageorgopoulos DE, Qu JM, Korbila IP, et al. Accuracy of β-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect 2013;19:39-49. [PubMed]
- Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-β-D-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol 2012;50:7-15. [PubMed]
- Martin SI, Fishman JA, AST Infectious Diseases Community of Practice. Pneumocystis pneumonia in solid organ transplant recipients. Am J Transplant 2009;9 Suppl 4:S227-33. [PubMed]
- Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014;44:1350-63. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, W64.
- Morris A, Wei K, Afshar K, et al. Epidemiology and clinical significance of pneumocystis colonization. J Infect Dis 2008;197:10-7. [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [PubMed]
- Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. [PubMed]
- Guo YL, Chen YQ, Wang K, et al. Accuracy of BAL galactomannan in diagnosing invasive aspergillosis: a bivariate metaanalysis and systematic review. Chest 2010;138:817-24. [PubMed]
- Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994;271:703-7. [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [PubMed]
- Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 2002;21:1525-37. [PubMed]
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. [PubMed]
- Dwamena BA. Midas: a program for meta-analytical integration of diagnostic accuracy studies in Stata. University of Michigan Medical School; 2007. Available online: http://fmwww.bc.edu/repec/bocode/m/midas.pdf
- Tasaka S, Hasegawa N, Kobayashi S, et al. Serum indicators for the diagnosis of pneumocystis pneumonia. Chest 2007;131:1173-80. [PubMed]
- Sax PE, Komarow L, Finkelman MA, et al. Blood (1->3)-beta-D-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis 2011;53:197-202. [PubMed]
- Salerno D, Mushatt D, Myers L, et al. Serum and bal beta-D-glucan for the diagnosis of Pneumocystis pneumonia in HIV positive patients. Respir Med 2014;108:1688-95. [PubMed]
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1-->3)-beta-D-glucan assay for the diagnosis of invasive fungal infections--a study based on autopsy cases from 6 years. Clin Infect Dis 2008;46:1864-70. [PubMed]
- Mori T, Matsumura M. Clinical evaluation of diagnostic methods using plasma and/or serum for three mycoses: aspergillosis, candidosis, and pneumocystosis. Nihon Ishinkin Gakkai Zasshi 1999;40:223-30. [PubMed]
- Koo S, Bryar JM, Page JH, et al. Diagnostic performance of the (1-->3)-beta-D-glucan assay for invasive fungal disease. Clin Infect Dis 2009;49:1650-9. [PubMed]
- Iikuni N, Kitahama M, Ohta S, et al. Evaluation of Pneumocystis pneumonia infection risk factors in patients with connective tissue disease. Mod Rheumatol 2006;16:282-8. [PubMed]
- Esteves F, Lee CH, de Sousa B, et al. (1-3)-beta-D-glucan in association with lactate dehydrogenase as biomarkers of Pneumocystis pneumonia (PcP) in HIV-infected patients. Eur J Clin Microbiol Infect Dis 2014;33:1173-80. [PubMed]
- de Boer MG, Gelinck LB, van Zelst BD, et al. β-D-glucan and S-adenosylmethionine serum levels for the diagnosis of Pneumocystis pneumonia in HIV-negative patients: a prospective study. J Infect 2011;62:93-100. [PubMed]
- Akamatsu N, Sugawara Y, Kaneko J, et al. Preemptive treatment of fungal infection based on plasma (1 --> 3)beta-D-glucan levels after liver transplantation. Infection 2007;35:346-51. [PubMed]
- Acosta J, Catalan M, del Palacio-Peréz-Medel A, et al. A prospective comparison of galactomannan in bronchoalveolar lavage fluid for the diagnosis of pulmonary invasive aspergillosis in medical patients under intensive care: comparison with the diagnostic performance of galactomannan and of (1→ 3)-β-d-glucan chromogenic assay in serum samples. Clin Microbiol Infect 2011;17:1053-60. [PubMed]
- Etoh K. Evaluation of a real-time PCR assay for the diagnosis of Pneumocystis pneumonia. Kurume Med J 2008;55:55-62. [PubMed]
- Yu J, Li RY, Gao LJ, et al. Utility of galactomannan enzyme immunoassay and (1,3)beta-D-glucan assay in invasive fungal infection. Zhonghua Yi Xue Za Zhi 2010;90:371-4. [PubMed]
- Odabasi Z, Mattiuzzi G, Estey E, et al. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis 2004;39:199-205. [PubMed]
- Nakamura H, Tateyama M, Tasato D, et al. Clinical utility of serum beta-D-glucan and KL-6 levels in Pneumocystis jirovecii pneumonia. Intern Med 2009;48:195-202. [PubMed]
- Tokuda H, Sakai F, Yamada H, et al. Clinical and radiological features of Pneumocystis pneumonia in patients with rheumatoid arthritis, in comparison with methotrexate pneumonitis and Pneumocystis pneumonia in acquired immunodeficiency syndrome: a multicenter study. Intern Med 2008;47:915-23. [PubMed]
- Racil Z, Kocmanova I, Lengerova M, et al. Difficulties in using 1,3-{beta}-D-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies--high frequency of false-positive results and their analysis. J Med Microbiol 2010;59:1016-22. [PubMed]
- Digby J, Kalbfleisch J, Glenn A, et al. Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients. Clin Diagn Lab Immunol 2003;10:882-5. [PubMed]
- Ellis M, Al-Ramadi B, Finkelman M, et al. Assessment of the clinical utility of serial beta-D-glucan concentrations in patients with persistent neutropenic fever. J Med Microbiol 2008;57:287-95. [PubMed]
- Marty FM, Koo S. Role of (1-->3)-beta-D-glucan in the diagnosis of invasive aspergillosis. Med Mycol 2009;47 Suppl 1:S233-40. [PubMed]
- Shimizu Y, Sunaga N, Dobashi K, et al. Serum markers in interstitial pneumonia with and without Pneumocystis jirovecii colonization: a prospective study. BMC Infect Dis 2009;9:47. [PubMed]
- Mori S, Cho I, Ichiyasu H, et al. Asymptomatic carriage of Pneumocystis jiroveci in elderly patients with rheumatoid arthritis in Japan: a possible association between colonization and development of Pneumocystis jiroveci pneumonia during low-dose MTX therapy. Mod Rheumatol 2008;18:240-6. [PubMed]
- Song F, Eastwood AJ, Gilbody S, et al. Publication and related biases. Health Technol Assess 2000;4:1-115. [PubMed]
- Leeflang MM, Deeks JJ, Gatsonis C, et al. Systematic reviews of diagnostic test accuracy. Ann Intern Med 2008;149:889-97. [PubMed]