Corticosteroid therapy against treatment-related pulmonary toxicities in patients with lung cancer
Introduction
Lung cancer has been a leading cause of cancer-related death worldwide including South Korea (1). To date, chemotherapy, molecular-targeted therapy and radiation therapy (RT) have been known to be effective for the treatment of inoperable lung cancer. Still, however, much treatment-related pulmonary toxicity remains fatal (2,3). Currently, new anticancer agents and novel radiation techniques are becoming available for the treatment of lung cancer. This is also accompanied by increased risks of developing pulmonary toxicities in patients with lung cancer (4). It has been reported that the degree of the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib, is relatively higher compared with chemotherapy in patients with EGFR mutations (5,6). It remains problematic, however, EGFR-TKIs can cause interstitial lung disease (ILD); overall, the incidence of erlotinib-induced and gefinitib-induced ILD has been reported to reach 0.8% and 1%, respectively. Moreover, the incidence of gefitinib-induced ILD is estimated at 2% in Japan and 0.3% in the United States (7-13). Furthermore, the mortality of gefitinib-induced ILD exceeds 30% (14). The incidence of symptomatic RT-induced lung injury is estimated at 20% in patients with lung cancer who were treated with RT. In patients with severe RT-induced lung injury, the survival period is relatively shorter (2).
To date, there are no established treatment guidelines for patients with pulmonary toxicities due to anticancer therapies. In these patients, however, clinicians have attempted to discontinue the use of the causative agents and to administer systemic corticosteroids on empirical basis (3,4). It has also been reported that corticosteroid therapy is effective against the episodes of acute exacerbation of chronic obstructive pulmonary disease (AE COPD) and ILD; both entities may be concurrently present in patients with lung cancer (15-18). Nevertheless, there is a paucity of data regarding the optimal timing, dosage and duration of corticosteroid therapy in the treatment of patients with lung cancer (19).
Given the above background, we conducted this study to evaluate the clinical characteristics of patients with lung cancer who presented with treatment-related pulmonary toxicities and to analyze the dosage pattern of corticosteroid therapy against them.
Materials and methods
Study population
To collect the baseline data from the patients with lung cancer who developed treatment-related pulmonary toxicities, we initially selected those who were prescribed corticosteroids at Lung and Esophageal Cancer Clinic of Chonnam National University Hwasun Hospital between January 1, 2008 and December 31, 2012.
We excluded the patients without lung cancer and patients who received corticosteroid therapy to control extra-pulmonary conditions like increased intracranial pressure or spinal cord compression, septic shock, adrenal insufficiency, rheumatoid arthritis or systemic sclerosis (Figure 1). The current study was approved by the Institutional Review Board (IRB) of Chonnam National University Hwasun Hospital (IRB approval number: 2013-118). Informed consent was waived due to the retrospective nature of the current study.
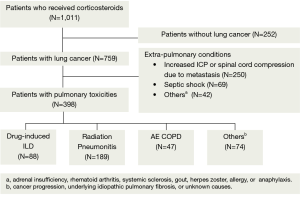
Classification of pulmonary toxicities
Two chest radiologists comprehensibly reviewed all X-ray and computed tomography (CT) scans, thus attempting to determine the imaging characteristics of pulmonary toxicities. Depending on radiological diagnosis, we classified treatment-related pulmonary toxicities into drug-induced ILD (DILD), radiation pneumonitis, AE COPD and others. In addition, drugs were referred to as those that are used for both conventional chemotherapy and molecular-targeted therapy. In our series, we made a diagnosis of DILD after ruling out other possible diagnoses, such as infection, cancer progression, and acute exacerbation of underlying idiopathic pulmonary fibrosis or unknown conditions. We performed laboratory tests such as C-reactive protein, procalcitonin, serologic tests, sputum and blood cultures to rule out the patients with infection.
Demographics and clinical variables
Baseline demographics and clinical data include age, sex, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), histology, cancer stage and pulmonary function test before corticosteroid treatment. Histology was classified as small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC was divided into three categories: squamous cell cancer (SQC), adenocarcinoma (ADC) and others. Cancer stage was presented as non-metastatic or metastatic.
Corticosteroid treatment
We also collected the data about corticosteroid regimen. Corticosteroid treatments include three regimens: pulse, high-dose and low-dose therapy. Pulse was ≥500 mg/day methylprednisolone for 3 days followed by high-dose steroid, high-dose was ≥0.5 mg/kg/day prednisolone and low-dose was <0.5 mg/kg/day prednisolone.
Imaging characteristics of DILD
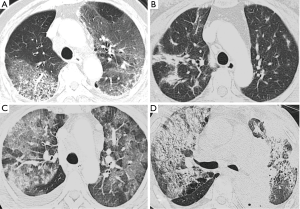
Chest CT findings of DILD were classified into non-specific interstitial pneumonia (NSIP) pattern, bronchiolitis obliterans organizing pneumonia (BOOP) pattern, diffuse alveolar damage (DAD) pattern and mixed pattern (Figure 2). We examined the number of the involved pulmonary lobes in a total of five lobes.
Outcome measures
The prescribed dosage pattern of corticosteroid was served as the primary outcome measure in the current study, for which we evaluated the patient response to the corticosteroid therapy based on two categories: ‘recovered’ and ‘fatal’. Thus, we defined ‘fatal’ cases as in-hospital death or death outside hospital and ‘recovered’ ones as better outcomes at discharge or tapered/discontinued use of corticosteroids in an outpatient setting.
We also performed a subgroup analysis of the patients presenting with DILD, for which we measured the 1-month and 2-month mortality after initiating the corticosteroid therapy.
Statistical analysis
We performed univariate analysis with the χ2 test to analyze categorical variables. We also performed one-way analysis of variance (ANOVA) to analyze continuous variables. We performed stratified multivariate logistic regression analysis with a backward stepwise procedure to identify the factors that are associated with the fatality of treatment-related pulmonary toxicities. All the tests were two-sided, and a P value of 0.05 was considered statistically significant. Statistical analysis was done using the SPSS version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of patients
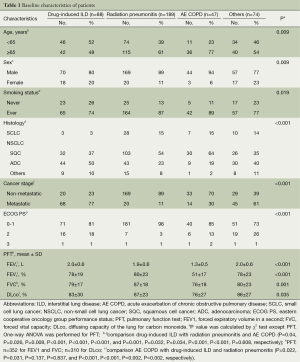
We enrolled a total of 398 patients (n=398) in the current study, whose median age was 67 years. Our clinical series of the patients include men (n=340, 85%), thus showing a male predilection. We divided our patients into four groups, depending on the classification of pulmonary toxicities, and these include 88 cases (22%) of DILD, 189 cases (47%) of radiation pneumonitis, 47 cases (12%) of AE COPD and 74 cases (19%) of other conditions (Table 1).
Full table
In the patients with DILD, age of ≤65 years, female sex, never smokers, ADC histology and metastatic stage were more prevalent. In the patients with acute exacerbation of COPD, the pulmonary function was poor. In the patients with DILD, possible causative agents include gefitinib (n=32), erlotinib (n=27), docetaxel (n=9), gemcitabine (n=5), paclitaxel (n=3), etoposide (n=3), pemetrexed (n=2), afatinib (n=2), belotecan (n=2), vinorelbine (n=2), dacomitinib (n=1) and tegafur-uracil (n=1). In our series, there were 14 patients who underwent platinum combination therapy.
Outcome measures
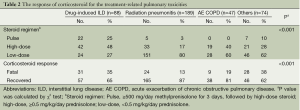
As shown in Table 2, the patients with DILD were mainly treated with pulse (25%) or high-dose (48%) therapy. In addition, the patients with radiation pneumonitis were routinely treated with low-dose steroid therapy (80%). The prescribed rate of pulse or high-dose steroid was measured as 73% in DILD, 20% in radiation pneumonitis, 40% in AE COPD and 38% in others (P<0.001). We evaluated the response of corticosteroid therapy by measuring the rate of fatality and recovery in the patients who were treated with corticosteroids. This showed that the overall rate of fatality was 23% (92/398); the rate of fatality was 35% in the patients with DILD, 13% in those with radiation pneumonitis, 19% in those with AE COPD and 38% in those with other conditions (P<0.001). However, if we compared the number of patients who received pulse or high dose steroid treatment with the number of patients had fatal outcome among the four groups, actually the mortality rate of DILD (31/64, 48.4%) was lower than the radiation pneumonitis (24/38, 63.2%), and others (28/28, 100%) groups.
Full table
Odds ratio (OR) for the fatality
On stratified multivariate logistic regression analysis, only the steroid regimen was independently correlated with the fatality of treatment-related pulmonary toxicities (Table 3). In addition, such variables as the age, sex, smoking status, histology, cancer stage and ECOG PS had no significant correlation with the fatality of treatment-related pulmonary toxicities. Furthermore, the degree of the fatality of treatment-related pulmonary toxicities was significantly higher in the patients receiving higher-dose regimen (pulse and high-dose therapy) as compared with those doing low-dose one {DILD [OR 8.41, 95% confidence interval (CI), 1.81-39.04]}, radiation pneumonitis (OR 24.44, 95% CI, 7.77-76.90) and others (OR 21.00, 95% CI, 5.63-78.30). But this was not seen in the patients with acute exacerbation of COPD. In the patients with acute exacerbation of COPD, we could not estimate OR for the fatality of treatment-related pulmonary toxicities because of a smaller sample size.

Full table
Imaging characteristics and mortality of DILD
The mode and median of the number of involved lobes was 3 and 4, respectively. The 2-month mortality due to treatment-related pulmonary toxicities was higher in the patients where the number of involved lobes was 4-5 as compared with those where it was 1-3. But this was not statistically significant (P=0.090). In our series, the NSIP pattern was the most prevalent; it was seen at a frequency of 51% (45/88). In addition, there were 33 cases (38%) of the BOOP pattern and seven cases (8%) of the DAD one (Table 4). But there were only three cases of the mixed pattern; these include one case of the NSIP and BOOP pattern, one case of the BOOP and DAD one and one case of the DAD pattern and radiation pneumonitis. Furthermore, there was a significant correlation between the CT findings and the mortality due to treatment-related pulmonary toxicities. In other words, the 2-month mortality due to treatment-related pulmonary toxicities was significantly higher in the patients with the DAD or mixed pattern (100% or 67%, respectively) as compared with those with the NSIP or BOOP one (62% or 42%, respectively) (P=0.032).
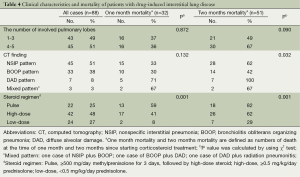
Full table
On univariate analysis, the steroid regimen had a significant correlation with the mortality due to treatment-related pulmonary toxicities. In addition, the 2-month mortality due to treatment-related pulmonary toxicities was higher in the patients who received the pulse or high-dose therapy as compared with those who did the low-dose one (82% or 62% vs. 29%, P=0.001).
Discussion
With the recent increased use of new anti-neoplastic agents, molecular-targeted drugs and radiation in patients with lung cancer, there has been an increase in the occurrence drug- or radiation-induced pulmonary toxicities. In these patients, we empirically use corticosteroids for disease modulation and symptomatic improvement (4,20,21). Our study documented that the prescribed rate of higher-dose regimen (pulse or high-dose therapy) was higher in DILD (73%) than in radiation pneumonitis (20%) or AE COPD (40%) group. As long as more intensive and higher dose steroid is administered for patients with severer conditions, patients treated with higher dose steroid would show the worse outcome. So our results may reflect the natural course of each disease. However, if we compared the mortality only in patients who received pulse or high dose steroid therapy, the mortality rates of DILD (48.4%) and AE COPD (47.4%) were lower than those of radiation pneumonitis (63.2%) and others (100%) groups. Further prospective studies are therefore warranted to conclude the prognosis and propose the treatment guidelines for the optimal regimen and schedule. Thus, efforts should be made to minimize risk and to maximize benefit of anti-cancer treatment in this series.
In a current clinical setting, drug-induced pulmonary toxicity poses diagnostic challenges for clinicians. It would therefore be mandatory to determine the time course of the disease and the timing of causative drug medication as well as to rule out other possible diagnoses, including infections in particular. In addition, it would also be mandatory to evaluate the degree of the treatment response to the discontinued causative drugs and corticosteroids (4,20). Furthermore, previous studies have shown that radiological findings are a key diagnostic clue (10,22-24). It has been reported that the clinical and radiologic findings due to causative drugs are suggestive of the underlying histopathologic processes (23). Rossi et al. classified the radiologic manifestations as DAD, NSIP, BOOP, eosinophilic pneumonia, obliterative bronchiolitis, pulmonary hemorrhage, edema, hypertension, or veno-occlusive disease (23). And knowledge of the drugs most frequently involved, together with an understanding of the typical histopathologic and radiologic manifestations of toxicity are necessary for institution of appropriate treatment (23). In this study, we examined whether CT findings would be a prognostic factor in the patients with DILD. We analyzed chest radiographic images and this led to the clinical and radiologic diagnosis of NSIP, BOOP, DAD, and mixed pattern. Our results showed that the 2-month mortality due to treatment-related pulmonary toxicities was significantly higher in the patients with the DAD as compared with those with the BOOP or NSIP one on univariate analysis. This finding of DILD is not different from various ILD in which NSIP or BOOP have better response to steroid treatment and better outcome than DAD.
There are several limitations of the current study as shown below: (I) we conducted the current study under the retrospective design in the patients with moderate to severe symptoms who were in need of in-hospital treatment and corticosteroid therapy. Our results cannot be applied to the patients with mild pulmonary toxicities who are not in need of corticosteroid therapy; (II) we classified the patients with pulmonary toxicities into four groups depending on the clinical and radiological diagnoses rather than biopsy and microbiological examinations. But we performed serologic tests and sputum and blood cultures to rule out the patients with infection; (III) we considered the fatality of treatment-related pulmonary toxicities to evaluate the efficacy of corticosteroids against them. But this may be insufficient for the assessment of the treatment response to corticosteroids, as previously shown in a report that a longitudinal data of carbon monoxide diffusing capacity would be a more objective indicator (25); (IV) we failed to identify the significant difference in the degree of treatment-related pulmonary toxicities between the causative drugs because we used many drugs in the treatment of our cases; (V) we failed to consider the accumulated toxicities from the previous several anti-cancer therapies. In other words, we failed to consider radiation recall pneumonitis that may occur during the follow-up chemotherapy after prior radiation therapy. Nevertheless, our study is of significance in that we have evaluated clinical characteristics of treatment-related pulmonary toxicities and the prescribed dosage pattern of corticosteroid therapy through a large-scaled clinical and radiological review of patients with moderate to severe respiratory symptoms.
Conclusions
In conclusion, our results showed that the natural course of DILD had more unfavorable outcome requiring higher dose steroid therapy as compared with those with radiation pneumonitis or AE COPD. And this study suggested that the patients with the BOOP and NSIP pattern on initial chest CT scans of DILD had more favorable outcomes.
Acknowledgements
Funding: This work was supported by a clinical research grant from Chonnam National University Hwasun Hospital 2013.
Disclosure: The authors declare no conflict of interest.
References
- In KH, Kwon YS, Oh IJ, et al. Lung cancer patients who are asymptomatic at diagnosis show favorable prognosis: a korean Lung Cancer Registry Study. Lung Cancer 2009;64:232-7. [PubMed]
- Miller KL, Shafman TD, Marks LB. A practical approach to pulmonary risk assessment in the radiotherapy of lung cancer. Semin Radiat Oncol 2004;14:298-307. [PubMed]
- Müller NL, White DA, Jiang H, et al. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer 2004;91 Suppl 2:S24-30. [PubMed]
- Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest 2008;133:528-38. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Cohen MH, Johnson JR, Chen YF, et al. FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist 2005;10:461-6. [PubMed]
- Cohen MH, Williams GA, Sridhara R, et al. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist 2003;8:303-6. [PubMed]
- Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2006;24:2549-56. [PubMed]
- Endo M, Johkoh T, Kimura K, et al. Imaging of gefitinib-related interstitial lung disease: multi-institutional analysis by the West Japan Thoracic Oncology Group. Lung Cancer 2006;52:135-40. [PubMed]
- Lind JS, Smit EF, Grunberg K, et al. Fatal interstitial lung disease after erlotinib for non-small cell lung cancer. J Thorac Oncol 2008;3:1050-3. [PubMed]
- Makris D, Scherpereel A, Copin MC, et al. Fatal interstitial lung disease associated with oral erlotinib therapy for lung cancer. BMC Cancer 2007;7:150. [PubMed]
- Nagaria NC, Cogswell J, Choe JK, et al. Side effects and good effects from new chemotherapeutic agents.Case 1. Gefitinib-induced interstital fibrosis. J Clin Oncol 2005;23:2423-4. [PubMed]
- Barber NA, Ganti AK. Pulmonary toxicities from targeted therapies: a review. Target Oncol 2011;6:235-43. [PubMed]
- Fabbri LM, Hurd SS, GOLD Scientific Committee. Global strategy for the Diagnosis, Management and Prevention of COPD: 2003 update. Eur Respir J 2003;22:1-2. [PubMed]
- Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356-63. [PubMed]
- Wang S, Wong ML, Hamilton N, et al. Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans. J Clin Oncol 2012;30:1447-55. [PubMed]
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol 2006;54:723-7. [PubMed]
- Inoue A, Kunitoh H, Sekine I, et al. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys 2001;49:649-55. [PubMed]
- Kim TO, Oh IJ, Kang HW, et al. Temozolomide-associated bronchiolitis obliterans organizing pneumonia successfully treated with high-dose corticosteroid. J Korean Med Sci 2012;27:450-3. [PubMed]
- Yoneda KY, Shelton DK, Beckett LA, et al. Independent review of interstitial lung disease associated with death in TRIBUTE (paclitaxel and carboplatin with or without concurrent erlotinib) in advanced non-small cell lung cancer. J Thorac Oncol 2007;2:537-43. [PubMed]
- Souza CA, Muller NL, Johkoh T, et al. Drug-induced eosinophilic pneumonia: high-resolution CT findings in 14 patients. AJR Am J Roentgenol 2006;186:368-73. [PubMed]
- Rossi SE, Erasmus JJ, McAdams HP, et al. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics 2000;20:1245-59. [PubMed]
- Torrisi JM, Schwartz LH, Gollub MJ, et al. CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology 2011;258:41-56. [PubMed]
- Kalaycioglu M, Kavuru M, Tuason L, et al. Empiric prednisone therapy for pulmonary toxic reaction after high-dose chemotherapy containing carmustine (BCNU). Chest 1995;107:482-7. [PubMed]


