Size of solitary pulmonary nodule was the risk factor of malignancy
Introduction
Solitary pulmonary nodule (SPN) is a single mass in the lung less than or equal to 3 cm in diameter, without concomitant pneumonia and atelectasis of involved lung segments and lobes (1-3). Diagnoses of benign and malignant SPN has been concerned and become a challenge for radiological studies (4-7). Some SPNs are indicated pathologically in the early stages of lung cancers (8,9). Therefore, it is utmost important to utilize preoperative radiography in the characterization of SPN (10,11).
Computed tomography (CT) is the preferred radiological approach to examine SPNs. CT scans can clearly show the size, internal features, edge changes, changes of adjacent structures, and enhancement of pulmonary nodules and could provide comprehensive radiological evidences for the differential diagnosis between benign and malignant diseases (12,13). However, since most radiological signs were present in both benign and malignant lesions, it is necessary to balance the weight of various radiological signs in the identification of pulmonary nodules, in order to further understand the role of CT in the diagnosis and differential diagnosis of pulmonary nodules.
Known as one of the most common methods for medical statistical analysis, logistic regression model could be constructed to predict benign and malignant probability of pulmonary nodules as well as relevant features based on various CT signs. In literature described previously, logistic regression model was established to predict the CT diagnosis of SPN (14,15). However, no stratification in the size of pulmonary nodules was used. In this study, the size of pulmonary nodules was stratified to analyze the relevant risk factors of malignant pulmonary nodules, in order to improve the knowledge of CT signs for malignant pulmonary nodules in various sizes.
Patients and methods
General data
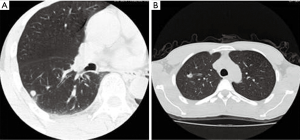
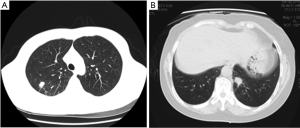
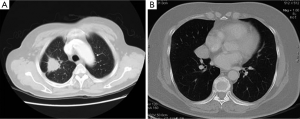
Totally 379 cases of SPNs, including 120 benign lesions and 259 malignant lesions were collected and confirmed by clinical pathology or biopsy. There were 38 cases of pulmonary nodules with the size ≤10 mm (20 benign lesions and 18 malignant lesions; Figure 1), 178 cases of pulmonary nodules with the size of 11-20 mm (51 benign lesions and 127 malignant lesions; Figure 2) and 163 cases of pulmonary nodules with the size >20 mm (49 benign lesions and 114 malignant lesions; Figure 3). There were 211 men and 168 women aged 21 to 87 years (median: 53 year). CT scanning conditions: helical scan; slice thickness of 2-5 mm, the interlamination of 2-5 mm, scanning from pulmonary apex to the basis.
Radiological examinations
CT images were examined by two radiologists with relevant experience for more than five years, and variables examined include the size of lesions, edge sharpness, glitches, air cavity density (including vacuole sign and inflatable bronchioles), calcification, pleural indentation, satellite lesions, vascular aggregation and extent of enhancement. In case of inconsistent results, the final decision was to be made after discussion.
Statistical analysis
SPSS 17.0 software was employed for statistical analysis, and comparison of different age groups was conducted by t-test. Comparisons of gender, size and enumeration data were performed by chi-square test. For variables with statistical significance, logistic regression method was employed to make a differential diagnosis between benign and malignant pulmonary nodules. Forward stepwise regression analysis was used based on the maximum likelihood estimate for Logistic regression method, and a P≤0.05 was considered as the criteria for variable inclusion. Dependent variables include benign and malignant pulmonary nodules (number of malignant nodules, 1; number of benign nodules, 0), with gender (male, 1; female, 0), age (based on actual age), size (≤10 mm, 0; 11-20 mm, 1; >20 mm, 2), lobulation sign (presence, 1; absence, 0), edge profile (clear, 1; ambiguous, 0), pleural indentation (presence, 1; absence, 0), satellite lesions (presence, 1; absence, 0) and vascular aggregation (presence, 1; absence, 0).
Results
Analysis of relevant risk factors
There were 379 cases of plain scans, with the age of 59.77±11.09 years for the group of malignant lesion and 51.17±13.79 years for the group of benign lesion. According to the results of t-test, there was statistically significant difference in the age between the two groups (Table 1).
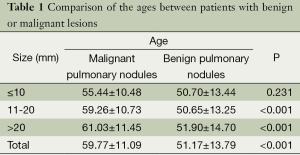
Full table
Pulmonary nodules with various sizes
The size of nodules had a significant effect on diagnosis. Changes of risk factors could be monitored during the course of size changes from small to large lesions, in order to better understand the significance of various signs for the diagnosis and differential diagnosis of pulmonary nodules.
The group of ≤10 mm pulmonary nodules
There were 38 cases in the group of ≤10 mm pulmonary nodules. According to chi-square test, there were statistically significant difference in air cavity density and satellite lesions between benign and malignant pulmonary nodules, but no statistically significant differences were observed for the gender, size of nodules, lobulation, glitches, edge, calcification, pleural indentation and vascular aggregation (Table 2).
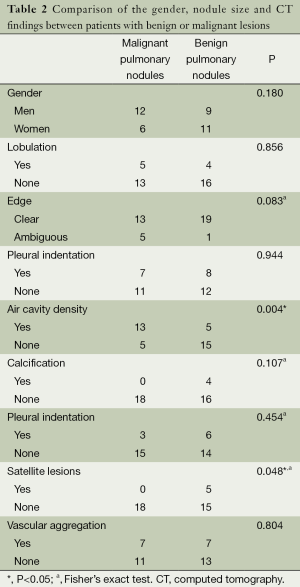
Full table
The variables with statistical significance in Tables including air cavity density, satellite lesions, and age were input into the regression model, yielding the results of regression analysis (Table 3).

Full table
The regression equation was defined as logit P=–0.875+2.342× (air cavity density)–21.699× satellite lesions. Hosmer-Lemeshow fitted index test showed χ2 <0.001, ν =1, P=1.000, suggesting the statistical significance of model fitting. As demonstrated by the results, the hazard of malignant lesion is as higher as 10.4-fold for the presence of air cavity density in comparison to its absence. According to the prediction results of 38 cases with benign and malignant pulmonary nodules, the sensitivity, specificity and accuracy were determined to be 72.2%, 85.0% and 78.9% respectively.
The group of 11-20 mm pulmonary nodules with plain scans
There were 178 cases with 11-20 mm pulmonary nodules in this group. According to chi-square test, there were statistically significant difference in lobulation, glitches, air cavity density, calcification, satellite lesions and vascular aggregation between benign and malignant pulmonary nodules, but no statistically significant differences were observed for gender, edge, calcification or not and pleural indentation (Table 4). The variables of statistically significant difference from chi-square test of Table 4 and age were input into the regression model to afford the results of regression analysis (Table 5).
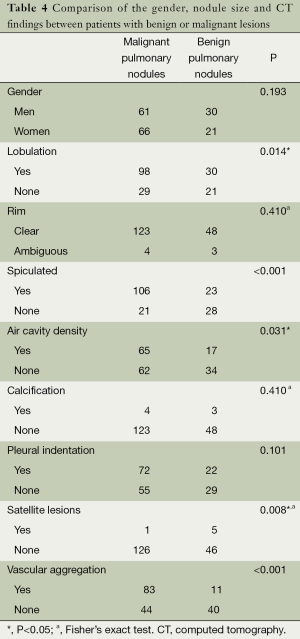
Full table
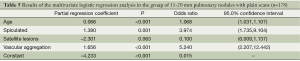
Full table
The regression equation was defined as logit P=–4.233+0.066× age +1.380× glitches–2.301× (satellite lesions) +1.656× (vascular aggregation). Hosmer-Lemeshow fitted index test showed χ2 =12.316, ν =8, P=0.138, suggesting the statistical significance of model fitting. As demonstrated by the results, the hazard of malignant lesion was increased by 6.8% in proportion to the increase of one year, and as higher as 3.97-fold for the presence of glitches in comparison to its absence; and as higher as 5.24-fold for the presence of vascular aggregation in comparison to its absence. However, the presence of satellite lesions seemed to be protective from malignant pulmonary nodules. According to the differential diagnosis prediction between benign and malignant pulmonary nodules, the sensitivity, specificity and accuracy were determined to be 91.3%, 56.9% and 81.5%.
The group of >20 mm pulmonary nodules
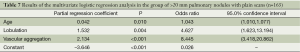
There were 163 cases with >20 mm pulmonary nodules in this group. According to chi-square test, there were no statistically significant difference in gender and edge sharpness, but statistically significant differences were observed for lobulation, glitches, pleural indentation, air cavity density, calcification, satellite lesions and vascular aggregation (Table 6). The variables of statistically significant difference from chi-square test of Table 6 and age were input into the regression model to afford the results of regression analysis (Table 7).
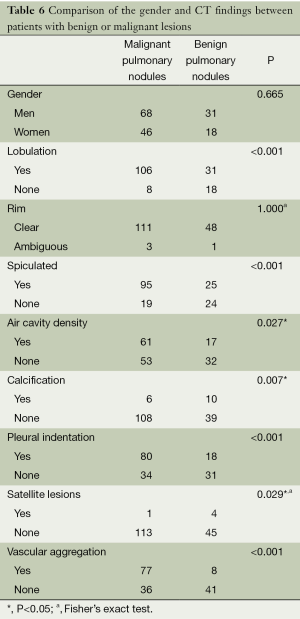
Full table
Full table
The regression equation was defined as logit P=–3.646+0.042× age +1.532× glitches +2.134× (vascular aggregation). Hosmer-Lemeshow fitted index test showed χ2 =8.216, ν =8, P=0.413, suggesting the statistical significance of model fitting. As demonstrated by the results, the hazard of malignant lesion was increased by 4.3% in proportion to the increase of one year, and as higher as 4.63-fold for the presence of lobulation in comparison to its absence; and as higher as 8.45-fold for the presence of vascular aggregation in comparison to its absence. According to the differential diagnosis prediction between benign and malignant pulmonary nodules in these 163 patients, the sensitivity, specificity and accuracy were determined to be 88.6%, 57.1% and 79.1%.
Discussion
CT method is the most effective method for current diagnosis of pulmonary nodule (16), and the general data and CT signs were analyzed in this study. There were no statistically significant difference in gender and edge sharpness, but statistically significant differences were observed for lobulation, glitches, air cavity density, pleural indentation, calcification, satellite lesions, vascular aggregation and extent of enhancement.
In this study, a close correlation was demonstrated between the incidence of lung cancer and subject age. In the diagnosis model for the group of patients with CT more than 10 mm, the variable of age is a dependent risk factor for malignant pulmonary nodules (17). The incidence of was approximate 2.5-fold in 2009 in comparison to in 1985 for lung cancer in the District Jinsan, Shanghai, representing a continuous tend of higher incidence of this disease (18). However, with age disposition excluded, the growth of normalized incidence of lung caner was insignificant, suggesting the age-dependent risk of malignant lesions.
Based on investigation of 1,000 pulmonary nodules, it was found that, for <10 mm pulmonary nodules, benign lesions represent 67.5%; for 10-20 mm pulmonary nodules, benign and malignant nodules share the equal probability; for >20 mm pulmonary nodules, malignant nodules represent 85% (19). In this study, similar findings were observed: for <10 mm pulmonary nodules, malignant nodules represent 47.4%, for >10 mm pulmonary nodules, malignant nodules represent 70.1%, suggesting a close correlation between the size versus benign and malignant characteristics. Smaller nodules are associated with benign lesions, while larger nodules tend to be malignant (20). Therefore, in clinical practice, small nodules also have considerable probability of malignant transformation and should be closely monitored or receive active surgical intervention.
Air cavity density was identified to be a risk factor for <10 mm pulmonary nodules; and aging, glitches and vascular aggregation were risk factors for 11-20 mm pulmonary nodules. For the >20 mm pulmonary nodules, the risk factors were aging, lobulation, and vascular aggregation. The presence of satellite lesions seemed to be protective from malignant pulmonary nodules. In the prediction of malignancies, the <10 mm pulmonary nodules had lower sensitivity and higher specificity, whereas the >10 mm pulmonary nodules had higher sensitivity and remarkably lower specificity.
Air cavity density was defined as vacuole sign and inflatable bronchiole sign. For vacuole sign, there were point-like translucent low-density shadows with the size of 1-2 mm in pulmonary nodules. Inflatable bronchiole sign was defined as the direct involvement of bronchiole in nodules or bronchiole shadows in pulmonary nodules, which are frequently detected in adenocarcinoma and are of importance for the diagnosis of small nodules (19,21). Intralesional fibrosis is the pathological basis for vascular aggregation. Nodules are concomitant with aggregation of few small vascular involvement and invasion towards nodules. Vascular surrounding and interruptions are frequently indicative of malignancy. Therefore, vascular aggregation is one of important signs for the diagnosis of malignant SPNs.
Glitch is a risk factor for 11-20 mm pulmonary nodules, while lobulation is a risk factor for >20 mm pulmonary nodules, which suggested glitch might be developed earlier than lobulation and relatively large size of nodules might be observed for lobulation. Glitch formation is a result of direct invasion of tumors into adjacent bronchial vascular sheath or local expansion of the lymphatic nodules or fibrous lines radiating into the surrounding lung fields which were induced by desmoplastic response in nodules (22). Therefore, glitch formation could be detected in the early stages of pulmonary glitch. Lobulation is associated with the size of tumors (23). Round or round-like appearance of lobulation could be observed in case of small tumors, and lobulation genesis might be induced by 1-1.5 cm tumor. In response to the increase of tumor size, lobulation might become continuously obvious and deepened.
Satellite lesions are point-like or line-like high-density shadow scattered around pulmonary nodules, with proliferation, calcification and fibrosis lesions as their major presentations. The probability of benign lesion is increased if there were satellite lesions around the lesion. Although calcification might also occur in case of lung cancer, a >5 cm calcification is more common and the primary calcification of lung cancer is relatively rare (24). For <3 cm pulmonary nodules, calcification findings are more likely to be correlated to benign lesions. Moreover, there was a statistically significant difference in the probability of pleural indentation sign between benign and malignant pulmonary nodules. However, it has not become a risk factor for the diagnosis of malignant pulmonary nodules. As possible reasons, the images of 2-5 mm in thickness were selected for these patients and some patients did not undergo high-resolution CT scanning. Moreover, some benign pulmonary nodules (e.g., tuberculosis mass) might also develop signs of pleural indentation.
Edge sharpness has been identified not to be a risk or protection factor for the diagnosis of pulmonary nodules. The facts of the sharp edges of most pulmonary nodules, ambiguous edges of some pulmonary nodules and unclear edges of malignant pulmonary nodules might be the results of invasive growth of tumors. Moreover, nodules are frequently associated surrounding inflammatory reactions. For inflammatory nodules, ambiguous changes might occur in the edges of nodules. Therefore, there was no clinical significance of edge sharpness of pulmonary nodules in the differential diagnosis between malignant and benign lesions.
Conclusions
In conclusion, according to CT-based diagnosis of SPNs, the relevant factors of age, size, glitches, lobulation, vascular aggregation, air cavity density, calcification and satellite lesions should be considered; meanwhile, during the course of development from small to large nodules, air cavity density could be firstly detected in early stages, followed by glitches and vascular aggregation. Lobulation is associated with relatively large lesions. These findings deepened the understandings and knowledge of radiological signs of pulmonary nodules in different sizes.
Acknowledgements
This study was supported by National Natural Science Fund project (81202284), Guangdong Provincial Natural Science Fund project (S2011040004735), Project for Outstanding Young Innovative Talents in Colleges and Universities of Guangdong Province (LYM11106), and Special Research Fund for Basic Scientific Research Projects in Central Universities (21612305/21612101). Guangzhou Municipal Science and Technology Fund project (2014J4100119).
Disclosure: The authors declare no conflict of interest.
References
- Sim YT, Poon FW. Imaging of solitary pulmonary nodule-a clinical review. Quant Imaging Med Surg 2013;3:316-26. [PubMed]
- Khan AN, Al-Jahdali H. Value of delayed 18F-FDG PET in the diagnosis of solitary pulmonary nodule. J Thorac Dis 2013;5:373-4. [PubMed]
- Tofts RP, Lee PM, Sung AW. Interventional pulmonology approaches in the diagnosis and treatment of early stage non small cell lung cancer. Transl Lung Cancer Res 2013;2:316-31.
- Henzler T, Shi J, Jafarov H, et al. Functional CT imaging techniques for the assessment of angiogenesis in lung cancer. Transl Lung Cancer Res 2012;1:78-83.
- Shao W, Wang W, Yin W, et al. Nonintubated thoracoscopic lobectomy plus lymph node dissection following segmentectomy for central type pulmonary masses. Chin J Cancer Res 2013;25:124-7. [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Zhan P, Qian Q, Wan B, et al. Prognostic value of TTF-1 expression in patients with non-small cell lung cancer: a meta-analysis. Transl Cancer Res 2013;2:25-32.
- Bar J, Urban D, Borshtein R, et al. EGFR mutation in lung cancer: tumor heterogeneity and the impact of chemotherapy. Chin Clin Oncol 2013;2:2.
- Shimizu K, Okita R, Nakata M. Clinical significance of the tumor microenvironment in non-small cell lung cancer. Ann Transl Med 2013;1:20.
- Albert RH, Russell JJ. Evaluation of the solitary pulmonary nodule. Am Fam Physician 2009;80:827-31. [PubMed]
- Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience. Radiology 2005;235:259-65. [PubMed]
- Hashimoto M, Miyauchi T, Heianna J, et al. Accurate diagnosis of peripheral small cell lung cancer with computed tomography. Tohoku J Exp Med 2009;217:217-21. [PubMed]
- Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med 2012;185:363-72. [PubMed]
- Li Y, Wang J. A mathematical model for predicting malignancy of solitary pulmonary nodules. World J Surg 2012;36:830-5. [PubMed]
- Gould MK, Ananth L, Barnett PG, et al. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131:383-8. [PubMed]
- Zerhouni EA, Stitik FP, Siegelman SS, et al. CT of the pulmonary nodule: a cooperative study. Radiology 1986;160:319-27. [PubMed]
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Chen W, Zheng R, Zhang S, et al. Report of cancer incidence and mortality in China, 2010. Ann Transl Med 2014. [Epub ahead of print].
- Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [PubMed]
- Stoller JK, Ahmad M, Rice TW. Solitary pulmonary nodule. Cleve Clin J Med 1988;55:68-74. [PubMed]
- Libby DM, Smith JP, Altorki NK, et al. Managing the small pulmonary nodule discovered by CT. Chest 2004;125:1522-9. [PubMed]
- Aberle DR, Brown K. Lung cancer screening with CT. Clin Chest Med 2008;29:1-14. [PubMed]
- Lee JW, Goo JM, Lee HJ, et al. The potential contribution of a computer-aided detection system for lung nodule detection in multidetector row computed tomography. Invest Radiol 2004;39:649-55. [PubMed]
- Mahoney MC, Shipley RT, Corcoran HL, et al. CT demonstration of calcification in carcinoma of the lung. AJR Am J Roentgenol 1990;154:255-8. [PubMed]


