Abstract
Sodium and potassium are known to be present as neutral elements in the exosphere of Europa. The question of the origin of these alkalis—endogenous or exogenous—remains open. They have been ascribed to exogenous contamination due to volcanism from nearby Io, or the accretion of meteorites and dust. However, these mechanisms fail to fit the observed sodium-to-potassium ratio. Sodium and potassium have also been considered to originate from Europa's putative subsurface ocean, generated by past rock-water leaching. The latter scenario implies a journey of the ions and atoms throughout the ice covering Europa. This raises questions about their stability into the bulk as well as on top of ice. These questions are addressed with first principle periodic solid-state density functional theory simulations describing the relative propensities of sodium, potassium, and calcium for being trapped in the bulk. The evolution of the ionic character of these atoms is followed by means of a topological analysis as they come up to the surface of the ice crust. We find that the metals, almost totally ionized in the ice bulk (net charge ∼+0.8) where they are stabilized by ∼1 eV or more, recover a quasi-neutrality (net charge ∼+0.2) when weakly adsorbed at the surface by ∼0.15 eV. Our results are consistent with the assumption that sodium and potassium observed in Europa exosphere come from the sputtering of materials issued from the underlying ocean and exposed by resurfacing events. They also suggest that calcium should be searched for by future missions.
Export citation and abstract BibTeX RIS
1. Introduction
Europa hides a liquid ocean in its interior, beneath an icy cover. This ocean may provide some habitats for life (Kargel et al. 2000; Prieto-Ballesteros et al. 2011; Noell et al. 2015). Alkali sodium and potassium are considered key ingredients in biochemistry. So, the fact that Na and K have been detected in the exosphere of this moon (Brown & Hill 1996; Brown 2001) raises the question of whether they might be potential proxies for the ocean's composition.
The origin of these alkalis, whether endogenous or exogenous, remains uncertain. Several mechanisms have been proposed to explain the sodium-to-potassium ratio of ∼25 ± 2 measured in Europa's extended atmosphere. It has been proposed that these trace elements form contaminants inherited either from the intense volcanism of Io or from meteoritic bombardment (Brown 2001). However, Europa's observed Na/K ratio is difficult to reconcile with the value of 10 ± 3 observed in Io's extended atmosphere, implying that this mechanism is at least not the dominant one (Brown 2001). Also, the Na/K ∼ 15 average ratio deduced from meteorites collected on Earth (Lodders 2003; Barrat et al. 2012) appears much too low for meteorite in-falls to constitute a credible solution. In contrast, an endogenous origin seems plausible. In this scenario, Na and K are issued from a subsurface ocean in which their presence originates from a long-time rock-water interaction in the history of Europa (Zolotov & Shock 2001; Johnson et al. 2002; Leblanc et al. 2002, 2005).
In the absence of in situ measurements of Europa's ocean, we had to consider data supposed to be relevant to the situation. Analyses of meteorite composition suggest that sodium, potassium, and calcium have essentially been incorporated into the refractory materials condensed in the protosolar nebula (Hutchinson 2004). Assuming that these elements have been trapped in solar proportions (Asplund et al. 2009) in Europa's building blocks, the Na/K and Ca/K bulk ratios should be ∼15.4 and ∼16.9, respectively. Otherwise, the quantities of ions transferred from rocks to the ocean are directly related to their respective solubilities into water. The relative molality in the aqueous phase can be evaluated from experimental studies of water-rock interaction in terrestrial environments (Zhang 2001). Those measurements have been carried out in Icelandic geothermal fields, showing that the molalities depend on the water to rock ratio, with an asymptotic behavior of the abundances with increasing dilution. The ionic molalities (mmole/l) of alkali Na+ and K+, and Earth alkali Ca++ derived from experiments conducted at 120°C (temperature relevant for Earth's geothermal fields) are 600, 20, and 120, respectively, which amounts to abundances ratios of Na+/K+ = 30 and Ca++/K+ = 6 (Zhang 2001). Similar orders of magnitudes correspond to the theoretical estimates of the composition of the Archean ocean (De Ronde et al. 1997).
As Europa's temperature decreased following its hot formation, the ocean froze from the top, resulting in the formation of an ice crust whose thickness progressively increased with time. In this process, foreign bodies present in liquid water are naturally embedded in the ice matrix; among them are metallic ions. The subsequent transfer of the metals to the top ice layers can occur via an ice convection (Pappalardo et al. 1998; Ashkenazy et al. 2018), often referred to as diapirism. Because convection is a physical process, the metals are pushed up to the surface accompanied by their close water environment. This scenario implies an upward migration of Na, K, and Ca through the different layers of ice, thus raising the question of their stabilities in the ice. It raises also the question of whether the metals transform from ions in the ocean water to neutrals when ejected.
Both questions are addressed in Section 2 by means of quantum chemistry tools. The relative propensities of Na, K, and Ca for being trapped in the ice are described by periodic solid-state calculations. The evolution of the electronic structure of each metal is followed with a topological analysis. The results are presented and discussed in Section 3. Section 4 is devoted to conclusions.
2. Computational Background
A methodology based on periodic density functional theory, often referred to as first principle calculations, has been employed throughout this work. Such a treatment has been previously used and has proved satisfactory for modeling bulk and surface ice structures (Casassa et al. 2005). With the same methods, the adsorption of organic molecules (Lattelais et al. 2011; Bertin et al. 2017), as well as the incorporation of atoms (Ellinger et al. 2015) and simple diatomics (Mousis et al. 2016, 2017) in cometary ices, have been successfully investigated. Within this framework the stabilization energies of these metallic entities are defined as
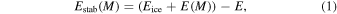
where Eice is the energy of the pristine crystalline or amorphous ice, E(M) is the energy of the metal atom M and E is the total energy of the [ice + M] system. In this expression, all energies are calculated in the periodic context of solid-state physics, all entities being optimized in isolation.
In practice, all calculations were performed with the Vienna ab initio simulation package (Kresse & Hafner 1994; Kresse & Furthmüller 1996; Kresse & Joubert 1999), a code specifically designed for studying solid-state electronic structures in a periodic formalism. The main characteristics of this code is to use a plane-wave expansion of the basis set associated to projector-augmented wave ultra-soft pseudo-potentials for the atomic cores. There is no basis set superposition error (BSSE) artifact in this formalism (Boys & Bernardi 1970). The functional form that we chose to use is the PBE exchange-correlation functional (Perdew et al. 1996). The contribution of long-range weak interactions due to the spatial extension of the alkali/Earth alkali highest s orbital was taken care of within the so-called D2 Grimme correction scheme (Grimme et al. 2010).
A set of representative environments of the different atoms within the bulk or on top of apolar solid water ice has been computed, using the periodic structure of water ice built by assembling up to six water bi-layers in the unit cell. The layers structures were taken from Ih crystallographic data. The simulation of the defects containing structures was performed by removing a few H2O molecules from selected positions in the bulk, followed by an optimized reconstruction of the solid. Based on computational experiments we had realized in related previous studies, a structure of six bi-layers of ice was retained for insertion and substitution calculations. The topmost and bottom bi-layers are frozen, allowing the four middle bi-layers to relax within the bulk and optimize the shape of the cavities to the embedded element or to the void resulting from a possibly empty cavity. For adsorption, only four bi-layers are taken into account. The two bottom bi-layers are frozen, leaving the top ones free to adapt to the adsorbates.
In all cases, the dimensions of the unit cell are fixed at 13.7 Å × 13.7 Å in the x and y directions of the basal plane, and a vacuum of 15 Å high in the z direction is maintained between two successive slabs.
These parameters have been chosen to avoid any spurious lateral and vertical interaction. We applied K-point grids of 3 × 3 × 1 for x, y, and z directions, respectively, allowing automatic Monkshort & Pack sampling to generate a satisfactory representation of the plane-wave basis set. A standard kinetic energy cut-off of 400 eV was employed for all calculations. More technical details concerning the optimization processes, the construction of the molecular slabs, and the definition of the unit cell structural parameters can be found in the aforementioned papers.
The variations of the electronic charge of the atoms according to the ice environment have been addressed by means of a Bader charge analysis known as the AIM (atom in molecule) method (Bader 1990). The implementation in periodic codes is that using an on-grid method to partition the charge density into Bader volumes (Henkelman et al. 2006; Tang et al. 2009).
3. Results
During the liquid-to-solid transformation of the ocean, the metals originally dispersed in the ocean water as positive ions (Zhang 2001) find themselves progressively embedded into a compact water ice matrix over time.
Three typical situations of trapping are to be considered possible along the convective diapirism: insertion, substitution, and adsorption (see Table 1 left to right). Together with the stabilization energies of the metals, we followed the evolution of their electronic structures, i.e., how they change from ionic to neutral species when approaching the surface. The results are summarized in Table 1 and, taking Na as example, illustrated by Figures 1–3, depending on the situations considered as follows.
- 1.In the insertion situation (Figure 1), the metal atoms have forced their ways into the bulk by themselves. Sodium, still largely ionized (net charge ∼+0.9), is stabilized by less than 0.1 eV, i.e., by less than 20% of the cohesion energy of the ice (∼0.5 eV; Fraser et al. 2001), showing that its direct insertion into the bulk is not favorable. Potassium appears as ionic as sodium but seems a little more at ease (stabilized by ∼0.2 eV), probably due to its larger polarizability. As to calcium, a doubly charge positive ion, it has gained 0.7 e−, resulting in a net charge of ∼+1.3, and is stabilized like potassium. Here we have evidence that, for all metals, insertion that would keep the ice lattice unchanged is rather improbable.
- 2.In the substitution case (Figure 2), the metal atoms may be seen as trapped in small cavities inside porous ice, replacing one or two water molecules in a pristine ice mesh. In these voids, they behave in a completely different way from the insertion case. Sodium, bearing only a partial charge, is stabilized by at least ∼1 eV, a value much stronger than the ice cohesion. The stabilization energy seems to increase slightly with the dimension of the cavity, while the charge is insensitive to this parameter; all three metals have about the same net charge, i.e., ∼+0.8 in the bulk. While approaching the surface but still part of the top layer, Na is still attached but has lost half of its charge. Potassium and calcium follow the same evolution, but calcium remains more strongly encapsulated in the bulk by ∼1.5–1.7 eV. When trapped within the top layer, Ca remains stabilized more than the two alkalis, keeping a net charge of ∼+0.7, close to that in the ice bulk.
- 3.In the adsorption case (Figure 3) there are two possibilities, depending on whether the metal is closer to an oxygen or hydrogen on the surface. In the first case, there is a clear decrease of the binding energies close to that of an H2O to the ice for Na and K, whereas Ca remains still attached by ∼1 eV. Meanwhile, the net charges all decrease to within +0.3 and +0.4 electron. In the second case, a drastic drop occurs in both the stabilization energies, to values between 0.1 and 0.2 eV, and the net charges to +0.2 electron. The calcium ion, though normally bearing a double charge, behaves just like the two others.
Figure 1. Apolar ice six bi-layers Ih unit cell used in calculations of inclusion energies in the bulk (13.7 × 13.7 Å2 basal surface).
Download figure:
Standard image High-resolution imageFigure 2. Apolar ice six bi-layers Ih unit cell used in calculations of substitution energies in typical situations: in a hole open by removal of 4 H2O in the bulk (left panel) and in the topmost layer (right panel) by removal of one H2O (13.7 × 13.7 Å2 basal surface).
Download figure:
Standard image High-resolution imageFigure 3. Apolar ice four bi-layers Ih unit cell used in calculations of adsorption energies on top of surface oxygens (left panel) and (right panel) hydrogen atoms (13.7 × 13.7 Å2 basal surface).
Download figure:
Standard image High-resolution imageTable 1. Evolution of Stabilization Energies and Atomic Charges (Energies in eV/Charges in Electron) of Na, K, and Ca Elements as a Function of Their Positions in the Ice
| Metal | Insertion | Substitution | Substitution | Substitution | Adsorption | Adsorption |
|---|---|---|---|---|---|---|
| I (bulk) | S2 (bulk) | S1 (bulk) | S1 (surface) | A (O) | A (H) | |

|

|

|

|
|||
| Na | 0.06/0.9 | 1.10/0.7 | 1.02/0.8 | 0.86/0.54 | 0.41/0.3 | 0.10/0.2 |
| K | 0.22/0.9 | 1.17/0.8 | 0.91/ 0.8 | 0.86/0.55 | 0.60/0.4 | 0.16/0.2 |
| Ca | 0.26/1.3 | 1.71/0.8 | 1.54/0.7 | 1.41/0.70 | 0.98/0.3 | 0.20/0.2 |
Note. From left to right: I for the insertion into the bulk, S1 and S2 for the substitution in place of one or four molecules of H2O in the bulk, A(O) and A(H) for the adsorption on the surface, over an H or an O, respectively. Only significant examples are presented in Figures 1–3. In the end, all three metals, Na, K and Ca, are quasi neutral and ready to be released with the surrounding water molecules by any kind of surface abrasion.
Download table as: ASCIITypeset image
4. Concluding Remarks
Before drawing any conclusions, it must be noted that this work is not a study of neutral atoms interacting with ice starting with adsorption from the gas phase followed by possible migration in the solid. This might be the case of neutral alkali metals deposition from the exosphere onto the surface of Europa. In this spirit, Vondrak et al. (2006) have shown that, indeed, alkali atoms can be self-ionized due to the interaction with water ice surface, without inducing OH− production by electron attachment in the case that the ice temperature is below 100 K and the coverage is very weak. Previous calculations by Mundy et al. (2000) have also shown that the production of OH− due to the alkali solvation and ionization probably requires the presence of neutral alkali dimers (Ferro & Allouche 2003), which is very unlikely.
In contrast, here the starting species are not the neutral atoms but the ionized ones. The aim of this Letter has been to follow how sodium, potassium, and calcium, originally dispersed as positive ions in the ocean water (Zhang 2001), may travel through a crust of ice. The different steps of this journey have been characterized, providing some insight in a key mechanism that precedes the ejection of these elements in the exosphere of Europa.
Our calculations show that the straightforward insertion of the three metals in an already-formed ice is not favored, whereas embedding, a process that can be seen as a formal replacement of H2O molecules by metals in the ice lattice, is more favorable when it takes place simultaneously with the formation of an organized solid. Thus, sodium and potassium behave very similarly, with a small energetic advantage for Na in small cavities and for K in larger cavities, which might be simply linked to their different ionic radius (larger for K). Calcium is also found to be very stable in the cavities within the ice lattice. Even when reaching the topmost ice layer, Na, K, and Ca remain firmly attached to the ice. The consequence is that the three metals remain stable in their respective environments during the internal convection, pushing them to the surface.
We have also found a systematic and progressive charge decrease of the metallic ions in the electron–rich environment of ice. Na and K lose 20% of their charge as soon as they are encapsulated in the cavities, and bear only a little more than half of their original positive charges when reaching the topmost layer. For Ca, a doubly charged ion at the origin, there is an immediate gain of one electron when incorporated in the ice cavities and then it behaves similarly to the other two singly charged alkali ions. Once transported toward the surface, all three metals are weakly adsorbed on the ice, with very close adsorption energies (0.1–0.2 eV), and is almost neutral with similar positive charges (+0.2). It suggests that the journey through the ice has no decisive impact on the relative proportions of the atoms reaching the surface. Whether or not little fractionation occurs should be linked to the composition of the underlying ocean materials. Such a fractionation could be eventually estimated via the subsurface stabilization of Ca compared with Na and K, but it should be marginal because all metals are pushed up on top of the ice where they are in situations favorable for ejection in the gas phase.
Indeed, Na and K present rather different initial relative abundances in the ocean water. If this latter factor is the effective discriminating parameter, it implies an abundance of Na exposed to sputtering greater than that of K by more than an order of magnitude. It must be stressed that the ratio of the molalities of the two metals is found to be ∼30, and corresponds to an abundance ratio close to the one reported in the observations in the exosphere. With an initial relative abundance differing from Na by less than one order of magnitude, Ca should be observed being ejected together with Na, though in smaller abundance (five times less, which amounts to a ratio Ca/K ∼ 6). If the convection is not vigorous enough to push all elements up to the surface, Ca would be differentiated due to the much more substantial stabilization in the subsurface substitution sites. This implies that Ca would be much less abundant than Na or K in Europa's exosphere.
The prospective conclusion of this Letter is that the next robotic missions, such as the NASA Europa Clipper mission (Pappalardo et al. 2017) and the ESA JUICE mission (Grasset et al. 2013), both dedicated to the exploration of the Jovian system in the late 2020s, should be able to measure the composition of Europa's exosphere more precisely via mass spectrometry, and thereby provide an answer to the origin of the observed alkalis that would clarify the composition of the subsurface ocean and the nature of the chemical exchanges with the surface.
O.M. acknowledges support from CNES. O.M. also acknowledges support from the A*MIDEX project (no ANR-11-IDEX-0001-02) funded by the "Investissements d'Avenir" French Government program, managed by the French National Research Agency (ANR).






