Abstract
The aromatic nitrogen heterocyclic compound purine is the core structural framework of many important biomolecules, particularly nucleobases. Purine and purine derivatives have been observed in carbonaceous chondrites, and it has been hypothesized that the exogenous delivery of these compounds, along with many other biologically relevant compounds, may have played a role in the emergence of life. Numerous experiments in our laboratory have demonstrated that the nucleobases used by life to encode genetic material could have been produced abiotically under astrophysically relevant conditions. Specifically, the UV photoprocessing of pyrimidine and purine in simple ices of astrophysical interest has resulted in the production of all five biological nucleobases, namely, uracil (RNA), cytosine (RNA and DNA), thymine (DNA), adenine (RNA and DNA), and guanine (RNA and DNA). Additionally, follow-up work has examined the photochemistry of pyrimidine in more complex astrophysical ice mixtures to better understand the formation of these compounds under realistic conditions. In this work, we examine the photochemistry of purine in more complex ices of astrophysical interest and compare our results with those from simpler ice mixtures. We also examine the effects of competing parallel synthesis of organic compounds in the ices (unrelated to purine). Finally, we discuss the astrophysical and astrobiological implications of our findings.
Export citation and abstract BibTeX RIS
1. Introduction
Purine is a fused bicyclic aromatic nitrogen heterocyclic compound (N-heterocycle) of astrophysical interest. Aromatic compounds, including polycyclic aromatic hydrocarbons (PAHs), are believed to form in the outflows of late-type carbon (AGB) stars. In these environments, acetylene polymerization is thought to lead to the formation of PAHs (Frenklach & Feigelson 1989). It has been shown that if HCN is introduced during the acetylene polymerization process, then aromatic nitrogen heterocycles should also form (Ricca et al. 2001; Hamid et al. 2014). Additionally, although purine was not specifically identified, it was demonstrated that N-heterocycles can also form from the ultraviolet (UV) irradiation of small homocyclic aromatic hydrocarbons (e.g., benzene, naphthalene) in ices containing H2O and NH3 (Materese et al. 2015). To date, no small N-heterocycles have been firmly identified in the gas phase in space (Simon & Simon 1973; Kuan et al. 2004; Charnley et al. 2005; Brünken et al. 2006), but several of them have been detected in a number of carbonaceous chondrites including Murchison, Murray, Orgueil, Allan Hills 83100, Lewis Cliff 90500, and Lonewolf Nunataks 94102 (Folsome et al. 1971; Hayatsu et al. 1975; Stoks & Schwartz 1979; Callahan et al. 2011). Isotopic measurements taken for some of these compounds suggest an extraterrestrial origin rather than terrestrial contamination (Martins et al. 2008). Based on these arguments, it is hypothesized that pyrimidines, purines, and other small heterocycles, could be present in interstellar dense clouds, where they condense out in icy grain mantles.
Purine is an especially important molecule because of its relevance to astrobiology and potential role in the origins of life. Along with pyrimidine, purine serves as the core structural framework for the nucleobases that comprise the genetic material of all known life. The biological nucleobases based on pyrimidine include uracil, cytosine, and thymine; those based on purine include adenine and guanine. Understanding the abiotic origin of genetic material is a fundamental topic for astrobiology, and numerous hypotheses involving terrestrial and extraterrestrial pathways have been proposed. To our knowledge, no experimental conditions have been shown to produce the pyrimidine- and purine-based nucleobases together simultaneously in a terrestrial environment. We note, however, that additional pathways for the abiotic formation of nucleobases in an asteroidal or planetary parent body context have also been suggested (e.g., Matthews & Minard 2008; Ménor-Salván & Marín-Yaseli 2012; Pearce & Pudritz 2015; Rotelli et al. 2016; Saladino et al. 2016).
The Astrochemistry Laboratory at the NASA Ames Research Center has performed extensive research into the formation of nucleobases under astrophysically relevant conditions. Initial experiments studied the photochemistry of pyrimidine in H2O ice, and showed that the nucleobase uracil was readily formed, along with numerous other oxygen-bearing pyrimidine derivatives (Nuevo et al. 2009). When NH3 was added to the ice mixture, numerous aminopyrimidines and amino-hydroxy-pyrimidines, including the nucleobase cytosine were detected in addition to the oxygen-bearing pyrimidines previously observed from the earlier work (Nuevo et al. 2012). Further experiments involving mixtures of H2O, CH4, and pyrimidine also yielded methylpurines and trace amounts of the nucleobase thymine (Materese et al. 2013). Finally, experiments were performed using complex astrophysical ice mixtures containing H2O, CH3OH, NH3, and CH4 to test whether there were any significant changes from the results of the simpler ice mixtures (Nuevo et al. 2014). Notably, in these experiments cytosine production was greatly reduced and was only observed in experiments that received a larger UV dosage, while thymine was completely undetectable (Nuevo et al. 2014). More recently, we conducted a series of experiments where the photochemistry of purine in simple ices containing H2O and NH3 was studied (Materese et al. 2017). These experiments resulted in the formation of a wide range of aminopurines, hydroxypurines, and amino-hydroxypurines, including the nucleobases adenine and guanine.
The nature of the photochemistry of purine in simpler ices that are more relevant to astrophysical environments in which the ices may be layered can be found in Materese et al. (2017). In this work, we examine the photochemistry of purine intimately mixed in complex ice mixtures of astrophysical interest. The components of the ice mixtures selected for these experiments matched those used in our prior work involving pyrimidine (Nuevo et al. 2014), i.e., H2O:CH3OH:NH3:CH4:purine. The relative proportions of these compounds were generally chosen to be consistent with observations of ices in astrophysical environments (Dartois et al. 1999, 2002; Ehrenfreund & Charnley 2000; Gibb et al. 2004; Bottinelli et al. 2010; Whittet et al. 2011; Boogert et al. 2015), but the concentration of CH3OH was widely varied to evaluate the impact of parallel organic chemical pathways (i.e., synthesis of complex organic compounds unrelated to purine chemistry) on the abundances of the substituted purines formed. The results are compared with those obtained from our experiments involving simple ices (Materese et al. 2017).
2. Experimental Methods
Glass bulbs containing mixtures of H2O, CH3OH, NH3, and CH4 were prepared using established experimental procedures that have been previously used in the NASA Ames Astrochemistry Laboratory (e.g., Allamandola et al. 1988; Bernstein et al. 1995, 1999; Nuevo et al. 2009, 2012, 2014; Materese et al. 2013, 2015, 2017). Gas mixtures with pressures ranging from approximately 25 to 35 mbar were prepared using a vacuum manifold with an operating background pressure of approximately 5 × 10−6 mbar. The compositions of the experimental gas mixtures were determined by their partial pressure with an accuracy of 0.05 mbar. The following liquids and gases were used in these experiments: H2O (liquid; purified to 18.2 MΩ cm by a Millipore Direct-Q UV 3 device), CH3OH (liquid; Aldrich HPLC grade, 99.9% purity), NH3 (gas; Matheson, anhydrous, 99.99%), CH4 (gas, Matheson Tri-Gas, Research purity, 99.999%), H218O (liquid; Cambridge Isotope Laboratory, 97% 18O), and 15NH3 (gas; Cambridge Isotope Laboratory, 98% 15N). Purine (solid; Sigma-Aldrich, 98%) was prepared in a glass tube fitted with resistive heaters to allow for simultaneous deposition with the volatile gas mixture. All liquid reagents were freeze-pump-thawed three times and purine was pumped under vacuum prior to use to remove excess dissolved gases. Purine was sublimated at 70 °C, which yielded a deposition rate of 4.3 × 10−8 mol hr−1. The deposition rate of purine was previously estimated by independent experiments (Materese et al. 2017).
Volatile gases and purine were simultaneously deposited onto an aluminum foil substrate attached to a cryo-cooled cold finger mounted inside a vacuum chamber evacuated by a diffusion pump (Edwards BRV 25). The vacuum chamber operates with a background pressure of ∼8 × 10−8 mbar at room temperature, and a pressure of 2 × 10−8 at low temperature (20 K) because of the additional cryopumping. The residual pressure in the system is largely due to residual H2O vapor. The ultra-high vacuum grade aluminum foils used as substrates are pre-baked in air over night at a temperature of 500 °C to remove contaminant organics. Each foil was mounted on the sample head of a closed-cycle helium cryo-cooler (APD Cryogenics) that was typically cooled to ∼20 K.
Experimental ice mixtures were selected to be similar to the ices used in our previous experiments involving the photochemistry of pyrimidine in complex, astrophysically relevant ices (Nuevo et al. 2014) and varied over the following ranges H2O:CH3OH:NH3:CH4:purine (20:0–10:2:3:0.02). The concentrations of purine used are higher than would be expected in any astrophysical ice, but represent a compromise between having concentrations low enough to limit purine-purine interactions in the ices while generating enough products to allow for their detection in the GC-MS chromatograms. Additional experiments were run using H218O:CH318OH:NH3:CH4:purine, H2O:CH3OH:15NH3:CH4:purine, and H2O:13CH3OH:NH3:13CH4:purine ice mixtures in order to verify that the products identified in the resulting samples were not due to contamination. While being deposited, purine and ice mixtures were simultaneously irradiated with UV photons produced from a microwave-powered H2 UV lamp. This type of lamp was chosen to model the radiation field of stars and protostars, and primarily emits Lyα photons (121.6 nm) and a continuum centered around 160 nm with an estimated total flux of ∼1015 photons cm−2 s−1 (Warnek 1962; Chen et al. 2014). In a typical experiment, the simultaneous deposition/irradiation lasted between 44 and 48 hr. The total photon doses for typical experiments are equivalent to about ∼105 years in the diffuse interstellar medium (ISM) and more than 108 years in the dense ISM (Mathis et al. 1983; Prasad & Tarafdar 1983; Shen et al. 2004). However, it should be noted that ice-coated grains in protostellar disks may be exposed to much higher UV photon doses, with an estimated total fluence of 1.5 × 1020 photons cm−2 accumulated over a period of 106 years (Ciesla & Sandford 2012).
At the conclusion of each experiment, samples were warmed to ∼280 K over 3 hr, and then removed from the vacuum system for analysis. Refractory materials ("residues") were recovered from the foils with 25 μL of DMF (dimethylformamide) (Thermo, silylation grade) and 75 μL of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (Restek). The solutions containing the residues were then transferred to pre-baked (500 °C) vials and heated to 80 °C for 2 hr to convert functionalized hydrogen moieties such as OH, NH, and NH2 into their trimethylsilyl (TMS) derivatives.
Samples were analyzed using a Thermo Trace gas chromatograph (GC) equipped with an Agilent DB-17HT column (length: 30 m, inner diameter: 0.25 mm, film thickness: 0.15 μm), coupled to a DSQ II mass spectrometer (MS). Samples (1 μL) were injected in splitless injection mode and with an injector temperature of 250 °C into a helium (carrier gas; Madco, ultra-high purity research grade, 99.99%) flow of 1.3 mL minute−1. The column was heated using the following profile: the temperature was held constant at 100 °C for 2 minutes, and was then ramped up by 10 °C minute−1 to a final temperature of 300 °C, where it was held for 5 minutes. Chromatograms and mass spectral data were analyzed using XcaliburTM software (Thermo Finnigan). A series of standards were used to unambiguously identify a suite of purine derivatives and estimate their abundances in our samples. These standards included 2-aminopurine (nitrate salt; Sigma, 99% purity), adenine (Sigma, 99%), hypoxanthine (Aldrich, 98%), guanine (TCI, 98%), isoguanine (TCI, 95%), 2,6-diaminopurine (Aldrich, 98%), and xanthine (California Corporation for Biochemical Research, purity not reported). Additional tentative identifications were made using comparisons with the NIST mass spectral database and simple deduction from the GC-MS data for numerous compounds for which standards were not available.
3. Results
Our previous experiments involving the photoprocessing of purine in ices containing only H2O and NH3 resulted in the formation of a large suite of OH- and NH2-substituted purine derivatives (Materese et al. 2017). Every compound for which we had a standard was identified, namely hypoxanthine, adenine, 2-aminopurine, xanthine, isoguanine, guanine, and 2,6-diaminopurine. Additionally, based on measured masses, we tentatively identified all other possible singly substituted –OH and –NH2 isomers of purine for which the sidegroup is attached to a peripheral carbon, as well as some multiply substituted isomers. In those experiments, the relative abundances of singly substituted purine derivatives were orders of magnitude larger than doubly substituted purines. We found no evidence for sidegroup addition to any of the nitrogens in the purine but found evidence for sidegroup addition to all three of the unfused carbons in the molecule.
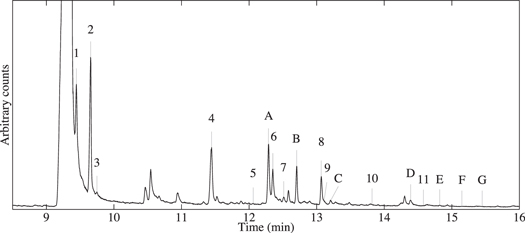
All of the purine derivatives identified in our earlier experiments with simple H2O:NH3:purine ices were also detected in some or all of the residues produced for the present study. These include hypoxanthine (A), adenine (B), 2-aminopurine (C), xanthine (D), isoguanine (E), guanine (F), and 2,6-diaminopurine (G) (Figure 1, Table 1). The identities of these compounds were confirmed by comparison of their retention times (Rt) and mass spectra with commercially available standards (Figure 2). Experiments involving isotopically labeled reactants were used to confirm that the compounds identified were not a result of contamination (see the bottom trace of Figure 2 for an example). Their abundances were determined by comparison of integrated single-ion chromatograms of sample peaks with those of corresponding standards of known concentrations. Notably, only hypoxanthine and adenine were detected in all residues (produced from all tested starting ice mixtures), and the abundances of all 7 purine derivatives formally identified in the produced residues decreased with increasing CH3OH abundance in the initial ice mixture (Table 1).
Figure 1. Total-ion chromatogram (TIC) of the residue produced from a UV-irradiated H2O:CH3OH:NH3:CH4:purine (20:0.2:2:3:0.02) ice mixture. Purine derivatives that have been identified by matching with commercially available standards are labeled A–G and reported in Table 1. Tentatively identified, unconfirmed purines are labeled 1–11 (see Table 2).
Download figure:
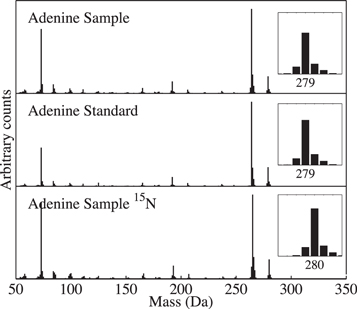
Standard image High-resolution imageFigure 2. Detection of adenine in a residue. This serves as an example of a purine derivative that was identified using a commercial standard. (Top trace) Mass spectrum of the peak identified as adenine in a residue produced from a UV-irradiated H2O:CH3OH:NH3:CH4:purine (20:0.2:2:3:0.02) ice. (Middle trace) Mass spectrum of the adenine standard. (Bottom trace) Mass spectrum of the peak identified as adenine in a residue produced from a UV-irradiated H2O:CH3OH:15NH3:CH4:purine (20:0.2:2:3:0.02) ice mixture. Peaks are shifted in mass as expected for a singly substituted aminopurine. Note that the masses include the mass of the TMS derivatizing sidegroups.
Download figure:
Standard image High-resolution imageTable 1. Purine Derivatives Identified by Matching with Commercially Available Standards
| Compound | Retention Time (Rt) | Intrinsic  Derivatized Mass Derivatized Mass |
H2O:CH3OH:NH3:CH4:Purine Ice Mixtures | |||
|---|---|---|---|---|---|---|
| (minutes) | (Da) | 20:0:2:3 | 20:0.2:2:3 | 20:1:2:3 | 20:10:2:3 | |
| (A) Hypoxanthine | 12.29 | 136  280 280 |
7 nmol | 6 nmol | 5 nmol | 1 nmol |
| (B) Adenine | 12.70 | 135 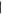 279 279 |
4 nmol | 3 nmol | 2 nmol | 0.2 nmol |
| (C) 2-Aminopurine | 13.32 | 135 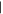 279 279 |
3 nmol | n.d. | n.d. | n.d. |
| (D) Xanthine | 14.39 | 152 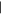 368 368 |
0.2 nmol | 0.1 nmol | 0.09 nmol | n.d. |
| (E) Isoguanine | 14.82 | 151  367 367 |
0.07 nmol | 0.06 nmol | 0.06 nmol | n.d. |
| (F) Guanine | 15.15 | 151 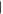 367 367 |
0.06 nmol | n.d. | n.d. | n.d. |
| (G) 2,6-Diaminopurine | 15.45 | 150 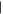 366 366 |
0.03 nmol | n.d. | n.d. | n.d. |
Note. Abundances were determined by comparison of the integrated peaks of the sample chromatogram with those of a chromatogram of a solution of standards of known concentration. The "n.d." notation indicates that the compound was not detected.
Download table as: ASCIITypeset image
Numerous other purine derivatives were tentatively identified in the produced residues based on their mass spectra alone. Since there were no standards available for these compounds, all identifications are partial (i.e., specific isomers cannot be determined) and should be considered to be tentative. Additionally, true abundances of these compounds cannot be determined because there are no reference standards of known concentrations for comparison. Once again, experiments involving isotopically labeled reactants were used to confirm that the compounds identified were not a result of contamination (see Figure 3 for an example). In lieu of providing actual abundances, we have reported the relative intensities of the peaks tentatively assigned to these compounds by scaling them against one the most abundant known standards, i.e., hypoxanthine (Table 2).
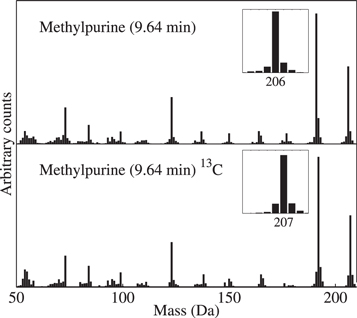
Figure 3. Example of purine derivative (methylpurine) that was tentatively identified in the absence of a commercial standard. (Top trace) Mass spectrum of the peak tentatively identified as a methylpurine isomer in a residue produced from a UV-irradiated H2O:CH3OH:NH3:CH4:purine (20:5:2:3:0.02) ice mixture. (Bottom trace) Mass spectrum of the same peak tentatively identified as a 13C-labeled methylpurine isomer in a residue produced from a UV-irradiated H2O:13CH3OH:NH3:13CH4:purine (20:5:2:3:0.02) ice mixture. Note that the masses include the mass of the TMS derivatizing sidegroup.
Download figure:
Standard image High-resolution imageTable 2. Purine Derivatives Partially Identified by Their Mass Spectra
| Compound | Retention Time (Rt) | Intrinsic  Derivatized Mass Derivatized Mass |
H2O:CH3OH:NH3:CH4:Purine Ice Mixtures | |||
|---|---|---|---|---|---|---|
| (minutes) | (Da) | 20:0:2:3 | 20:0.2:2:3 | 20:1:2:3 | 20:10:2:3 | |
| (1) Methylpurine | 9.43 | 134 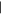 206 206 |
10 | 20 | 2 | 1 |
| (2) Methylpurine | 9.64 | 134 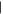 206 206 |
30 | 50 | 10 | 7 |
| (3) Dimethylpurine | 9.76 | 148 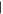 220 220 |
1 | 1 | 0.2 | n.d. |
| (4) Hydroxypurine | 11.46 | 136 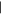 280 280 |
30 | 20 | 8 | 3 |
| (5) Hydroxy-methylpurine | 12.06 | 150  294 294 |
0.3 | 0.3 | n.d. | n.d. |
| (6) Hydroxypurine | 12.37 | 136 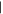 280 280 |
5 | 4 | 1 | n.d. |
| (7) Dihydroxypurine | 12.51 | 152 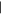 368 368 |
1 | 0.4 | 0.2 | n.d. |
| (8) Purinemethanol | 13.10 | 150 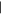 294 294 |
n.d. | 5 | 20 | 40 |
| (9) Amino-hydroxypurine | 13.14 | 151  367 367 |
0.4 | 0.1 | 0.04 | n.d. |
| (10) Dihydroxypurine | 13.82 | 152 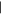 368 368 |
0.2 | 0.1 | 0.06 | n.d. |
| (11) Methoxypurine | 14.64 | 150 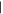 222 222 |
n.d. | n.d. | n.d. | 7 |
Note. Since no standards were commercially available for these compounds, integrated peaks were scaled as a percentage of a 50 nmol hypoxanthine standard. The "n.d." notation indicates that the compound was not detected.
Download table as: ASCIITypeset image
Two of the three possible singly substituted methylpurine derivatives (Figure 1, peaks 1 and 2) were tentatively identified in residues from all ice mixtures, and one of the possible dimethylpurine isomers (peak 3) was detected in residues from most ice mixtures (Figures 1 and 2, Table 2). In addition to hypoxanthine, the two remaining singly substituted hydroxypurine derivatives (peaks 4 and 6) and two dihydroxypurine derivatives (peaks 7 and 10) were detected in residues from nearly all ice mixtures. Two peaks (Rt = 12.06 minutes and Rt = 13.10 minutes) in the chromatogram had derivatized parent masses (294 Da) that are consistent with either a hydroxy-methylpurine, i.e., a purine with both CH3 and OH sidegroups, or a purinemethanol, i.e., a purine with a single CH2OH sidegroup. The peak at Rt = 12.06 minutes (5) most likely corresponds to a hydroxy-methylpurine because its abundance does not increase with increasing levels of CH3OH in the initial ice mixture. Additionally, the relatively low apparent abundance of this compound across all ice mixtures is consistent with a doubly substituted purine derivative rather than a singly substituted variant. In contrast, the peak at Rt = 13.10 minutes (8) is more consistent with a purinemethanol because its relative abundance in the final residue increases with increasing CH3OH concentration in the initial ice. Along with guanine and isoguanine, a peak consistent with an additional amino-hydroxypurine derivative was detected in the residues from most ice mixtures (9). Finally, a peak with a parent mass consistent with a methoxypurine was detected in ice mixtures with the highest concentration of CH3OH (11). Most of the partially identified purine derivatives detected in these experiments tended to decrease in abundance as the relative abundance of CH3OH was increased in the initial ice mixture. Purinemethanol and methoxypurine were both exceptions to this rule since their formation strongly depends on the presence of CH3OH in the ice.
4. Discussion
The photoirradiation of purine in complex ices leads to the formation of biological nucleobases and numerous other purine derivatives, including most of the species observed in our experiments on simpler ice mixtures (Materese et al. 2017). As was observed in our previous experiments with pyrimidine, the formation of nucleobases from the photoirradiation of purine in ices is a stochastic, kinetically driven process whose products are not strictly dictated by thermodynamic considerations (Sandford et al. 2015).
The addition of an NH2 group to purine appears to be slightly favored over the addition of an OH group, as suggested by comparing the abundances of hypoxanthine (6-hydroxypurine) and adenine (6-aminopurine) (Table 1). Indeed, in the present work, the adenine-to-hypoxanthine abundance ratio is greater than the NH3-to-H2O abundance ratio in all the starting ice mixtures. This was also demonstrated in our previous work when purine is mixed with simple H2O:NH3 ice mixtures (Materese et al. 2017). It is not currently possible to evaluate the relative preference of CH3, OCH2, and CH2OH substitutions relative to –OH and –NH2, since no relevant standards are available.
As a general rule, as the number of functional group additions increases, the relative abundance of purine derivatives decreases significantly. The compounds found in the residue produced in these experiments are the products of chains of stochastic additions and dissociations. These chains yield products whose relative abundances decrease in a nonlinear fashion for each required addition. For example, the residue from an H2O:CH3OH:NH3:CH4:purine (20:0.2:2:3:0.02) ice contained approximately 6 nmol of hypoxanthine (one OH group), but about 60 times less xanthine (two OH groups) (Table 1). This is primarily because products that require more additions require more photons to be produced, and their production pathway has more potential ways to be disrupted.
The absolute abundances of the amino- and hydroxypurines for which we have standards are all lower than the absolute abundances observed in our previous experiments using simpler H2O:NH3:purine ices (Materese et al. 2017). Additionally, we note that as the relative abundance of CH3OH in our initial ice mixtures increases, the abundance of most non-methanol related purine derivatives decreases. One possible common cause for these effects is that the inclusion of reactive and readily available carbon sources (e.g., CH3OH and CH4) in the ice leads to parallel production pathways for complex organic compounds that do not involve purine. The irradiation of CH3OH or CH4 in H2O- and NH3-containing ices is known to produce a vast suite of organic compounds (Bernstein et al. 1995; Dworkin et al. 2001, 2004; Muñoz Caro et al. 2002; Elsila et al. 2007; Nuevo et al. 2008, 2011, 2014) that consume reactants that would otherwise be available to react with purine. Complex organics formed in these experiments that are not related to purine may or may not appear in our chromatograms based on their size, volatility, stability, and whether or not they possess functional groups that can be derivatized. In contrast to ices containing additional carbon sources, the irradiation of simple H2O:purine and H2O:NH3:purine ice mixtures (Materese et al. 2017) does not result in a comparable level of parallel competing chemistry. The inclusion of CH3OH and CH4 in our ices also lowers the relative abundances of H2O and NH3 in the reactant ices, which will further decrease the abundances of photoproduced purine derivatives that require these constituents.
Finally, it is notable that the apparent absolute abundances of methyl- and dimethylpurines do not appear to be positively correlated with an increase in the abundance of methanol in the reactant ices. This result parallels our previous findings involving the photoprocessing of pyrimidine in ices containing methanol, which did not yield significant quantities of methylpyrimidines (Materese et al. 2013; Nuevo et al. 2014). Rather, CH4 seems to be the best source of CH3 groups that can be added to pyrimidine and purine, and its abundance does not vary in the ice mixtures studied in the present work.
5. Astrophysical Implications
The results of this work, taken in combination with our previous work simulating the chemistry of pyrimidine in ices, suggest that numerous biological and non-biological nucleobases form when complex mixed molecular ices of astrophysical interest are exposed to UV radiation. However, the absolute abundances of the nucleobases observed in these experiments decline as alternative carbon sources to purine become more abundant in the ices. If the ice mixtures studied in these experiments are reasonable analogs of the ices observed in the ISM or in protostellar disks, then it is reasonable to assume that the abundances of nucleobases produced in these ices in space would be lower than those predicted by our previous experiments involving simple ice mixtures (Materese et al. 2017). This decline in the production of nucleobases in realistic astrophysical ices may, however, be mitigated if different ices are condensed in layers, as hypothesized by some studies (Ehrenfreund & Charnley 2000; Boogert et al. 2015). In this case, experiments involving simpler ice analogs may provide a more accurate prediction of their formation efficiencies.
While the identifications of the photoproducts produced in these experiments are in qualitative agreement with the nucleobases observed in meteorites (Callahan et al. 2011), there are large differences in their relative abundances. There are several possible causes for these discrepancies: (1) There may be additional photochemical processing of complex organic residues that was not accounted for in these experiments; for example, Ciesla & Sandford (2012) demonstrated that organic-laden grains in the solar nebula may experience significant radiation processing with no surrounding ice. (2) The chemistry that occurs during aqueous alteration of the meteorite parent body or some other parent body process can degrade or alter any original organic materials made via ice photolysis. (3) There may be alternative formation pathways that are not captured in these experiments. (4) The process used to extract nucleobases from meteorites may alter their compositions or bias their relative detection efficiencies.
As we observed with our previous work involving purine in simple ices (Materese et al. 2017), the most abundant photoproducts identified in these experiments were adenine and hypoxanthine. The relatively high abundance of adenine is of astrobiological significance because it is one of the five main biological nucleobases. Additionally, the high abundance of hypoxanthine may also be of astrobiological interest, because it has been suggested as a base-pairing alternative for guanine in DNA and RNA during the emergence of life (Crick 1968; Nishikura 2010; Rios & Tor 2013; Cafferty & Hud 2015).
Overall, our experiments simulating the photochemistry of pyrimidine and purine in various ice analogs of astrophysical interest demonstrate that all the nucleobases required for DNA and RNA can be produced abiotically. The relative abundances of these nucleobases can vary widely depending on their identity and the composition of the ices in which they are produced. Although thymine was detected as a photoproduct in experiments involving the photoprocessing of pyrimidine in simple ices (Materese et al. 2013), it was notably the only nucleobase (among pyrimidines or purines) not observed in any of our experiments involving more complex ice mixtures (Nuevo et al. 2014). Based on this, our results are most consistent with the RNA world hypothesis, in which RNA was the first type of genetic material used by early life, with DNA coming along later as a biological innovation.
6. Conclusion
Laboratory experiments simulating the UV photoprocessing of purine in complex ices of astrophysical interest have led to the production of biologically relevant nucleobases and other functionalized purines. The relative abundances of these functionalized purines—methylpurines, methoxypurines, purinemethanols, hydroxypurines, aminopurines, and numerous di-substituted variants—strongly depend on the number of functional group additions to purine and the composition of the starting ice. In general, photoproducts that require the addition of one functional group to purine are far more abundant than products that require two or more. Additionally, as the relative abundances of reactive carbon sources in the form of methanol and methane increase in the ice, the abundance of functionalized purines decreases. This is likely caused by a growing parallel competing chemistry that uses the reactants to make other products that might have otherwise reacted with purine. These results suggest that if true astrophysical ices are complex and contain numerous carbon sources that are intimately mixed, then the absolute abundances of nucleobases formed via photochemistry may be attenuated relative to those observed in simpler ice mixtures. Alternatively, if ices are layered, then the results obtained for simpler ice mixtures may provide a better prediction of the distribution of products formed under these conditions.
Taken with our previous results involving the photochemistry of pyrimidine in complex ices, these results have interesting astrobiological implications. If nucleobases produced in space by the processes described in this work played a role in the emergence of life, then our work is consistent with the RNA world hypothesis, which suggests that RNA was the earliest form of genetic material and that DNA was not developed until life was further evolved. Additionally, because of its large relative abundance, our work is also consistent with the notion that hypoxanthine, a base-pairing substitute for guanine, may have been used in primitive forms of RNA and thus played a role in the early development of life.
This material is based upon work supported by the National Aeronautics and Space Administration through the NASA Astrobiology Institute under Cooperative Agreement Notice NNH13ZDA017C issued through the Science Mission Directorate. The authors are grateful for the helpful suggestions provided by an anonymous reviewer.