ABSTRACT
In an effort to further our interest in understanding the basic chemistry of interstellar molecules, here we carry out an extensive investigation of the stabilities of interstellar carbon chains; Cn, H2Cn, HCnN and CnX (X = N, O, Si, S, H, P, H−, N−). These sets of molecules account for about 20% of all the known interstellar and circumstellar molecules. Their high abundances, therefore, demand serious attention. High-level ab initio quantum chemical calculations are employed to accurately estimate the enthalpy of formation, chemical reactivity indices, global hardness and softness, and other chemical parameters of these molecules. Chemical modeling of the abundances of these molecular species has also been performed. Of the 89 molecules considered from these groups, 47 have been astronomically observed, and these observed molecules are found to be more stable with respect to other members of the group. Of the 47 observed molecules, 60% are odd-numbered carbon chains. Interstellar chemistry is not actually driven by thermodynamics, but it is primarily dependent on various kinetic parameters. However, we found that the detectability of the odd-numbered carbon chains could be correlated due to the fact that they are more stable than the corresponding even-numbered carbon chains. Based on this aspect, the next possible carbon chain molecule for astronomical observation in each group is proposed. The effect of kinetics in the formation of some of these carbon chain molecules is also discussed.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
As more and more interstellar and circumstellar molecules are observed, our understanding of the chemistry of these molecules is either strengthened or challenged depending on whether the new molecules fall within or outside the boundaries of their expected basic chemistry. These observations also put a constraint on the different chemical models. The fields of astronomy, astrophysics, astrochemistry and astrobiology have witnessed dramatic changes in the last few decades as a result of the advances in science and technology. In addressing the chemical origin of life and for the proper understanding of the solar system, there has been a constant and consistent searching for molecules in the interstellar and circumstellar media, resulting in the unique observation of about 200 different molecular species to date. The year 2014 has seen unparalleled observations of nine new interstellar and circumstellar molecules, including the first branched chain molecule in space, namely, isopropyl cyanide, an important biological molecule; urea, the sulfur analog (ethyl mercaptan) of the well known interstellar alcohol; ethanol, among others and the confirmation of many others (Agúndez et al. 2014; Anderson & Ziurys 2014; Belloche et al. 2014; Cernicharo et al. 2014; Kolesniková et al. 2014; Remijan et al. 2014).
Almost on a regular basis, carbon chain molecules such as Cn, H2Cn, HCnN, and CnX (X = N, O, Si, S, H, P, H−, N−) are being astronomically observed. To date, the largest claimed straight-chain interstellar molecule is the cyanopolyne, HC11N, a linear carbon chain molecule. Among the carbon chains, there seems to be no easily understood trend or pattern within the observed molecules. In the CnN and CnN− chains, with the exception of C2N, only the odd-numbered carbon chains are observed from n = 1 to n = 6 (Jefferts et al. 1970; Friberg et al. 1980; Guélin et al. 1998; Cernicharo et al. 2008; Thaddeus et al. 2008; Agúndez et al. 2010; Anderson & Ziurys 2014). Whereas in the CnH chains, both odd- and even-numbered chains are observed between 1 and 8, but in the CnH−, only even-numbered chains are observed (Swings & Rosenfeld 1937; Tucker et al. 1974; Guélin et al. 1978; Thaddeus et al. 1985a; Cernicharo et al. 1986, 2007; Suzuki et al. 1986; Cernicharo & Guélin 1996; Guélin et al. 1997; McCarthy et al. 2006; Brünken et al. 2007a). Among the cyanopolyne chains, between 6 and 12 carbon atoms, only odd-numbered chains are observed (Snyder & Buhl 1971; Turner 1971; Avery et al. 1976; Broten et al. 1978; Kroto et al. 1978; Guélin & Cernicharo 1991; Bell et al. 1997; Cernicharo et al. 2004), while in the CnSi group, all the molecules between carbon 1 and 4 have been observed (Thaddeus et al. 1984; Cernicharo et al. 1989; Ohishi et al. 1989; Apponi et al. 1999). This type of confusing trend is observed among the different groups of linear interstellar carbon chains.
As part of our interest in understanding the basic chemistry of interstellar and circumstellar molecules, the present work focuses on the interstellar and circumstellar carbon chains; Cn, H2Cn, HCnN and CnX (X = N, O, Si, S, H, P, H−, N−). High-level quantum chemical calculations are applied to accurately determine the enthalpies of formation, chemical reactivity indices, global hardness and softness, and other chemical parameters of these molecules. Chemical modeling of the abundances of these molecular species has also been performed, and the results are used in accounting for the general observation of more odd carbon chains than even carbon chains, and the dominance of the even carbon chains in a few cases. The possible candidates for astronomical observation among the carbon chain molecules are also considered.
2. COMPUTATIONAL DETAILS
The results of the quantum chemical calculations reported in this work were obtained using the Gaussian 09 suite of programs (Frisch et al. 2009). In order to estimate accurate enthalpies of formation, which are in good agreement with the experimental values (where available) for the different carbon chains considered in this work, the G4 composite method was employed. The choice of this method is borne out of experience, as it has been shown to compute enthalpies of formation to chemical accuracy (Curtiss et al. 1998, 2007a, 2007b). The geometries of these molecules were optimized at the G4 level, and harmonic vibrational frequency calculations were used to characterize the stationary nature of all the structures with equilibrium species possessing only real frequencies. The reported zero-point corrected standard enthalpies of formation of the carbon chains were calculated from the optimized geometries. The enthalpies of formation were calculated from atomization energies. With the G4 computational method and accurate experimental values of the standard enthalpy of formation of the constituent elements involved, very high accurate enthalpies of formation can be estimated for different sets of molecular systems. Atomization energies (sometimes referred to as the total dissociation energies) were evaluated using the calculated values of the energies (sum of electronic and zero-point energy corrections). In calculating the enthalpy of formation (ΔfH) at 0 K for all the molecules reported in this study, the experimental values of the standard enthalpy of formation of elements C, H, O, N, P, Si and S reported in the literature were used (Ochterski 2000). Chemical reactivity indices such as global hardness (η) and softness (S) have been calculated for different carbon chains, which were examined in this study. For the computation of S and η, we use the DFT method with the B3LYP/6-311g++(d, p) basis set of the Gaussian 09 program. First, we calculate the ionization potential (I) of the desired species by computing the energy (ground-state optimized energy) difference between the cation and neutral and electron affinity (A) by computing the energy difference between the neutral and anion. Chemical hardness is calculated by η = (I-A) (Parr & Pearson 1983) and softness is calculated by 1/η. The chemical modeling of the abundances of these carbon chain species is described, together with the obtained results, in the discussion section.
3. RESULTS AND DISCUSSION
The results of the quantum chemical calculations at the G4 level of theory, which were obtained for all the carbon chain molecules, are presented and discussed in this section (3.1–3.11). In all the cases, the reported zero-point corrected standard enthalpies of formation (ΔfH°) are in kcal/mol. Section 3.12 examines the effect of kinetics on the formation processes of these carbon chains. In Section 3.13, chemical reactivity indices and chemical modeling supporting the observed results in the previous sections are presented and discussed before the final conclusion.
3.1. Cn Chains
Apart from their importance in astrophysics, astrochemistry, and astrobiology, pure carbon chain molecules such as C2, C3, and C5 are of great interest to the combustion chemistry, particularly for application in flames and propellants. Table 1 shows the enthalpy of formation and the current astronomical status of the carbon chain (Cn) molecules considered in this study. Rotational spectroscopy has been the dominant tool in the chemical examination of interstellar and circumstellar media with about 90% of all known interstellar and circumstellar molecules observed via their rotational transitions. However, the application of rotational spectroscopy in this field is hampered by the fact that it is limited to molecules with a permanent dipole moment (Etim & Arunan 2015). The Cn chain molecules have no permanent dipole moment; hence, their pure rotational transitions cannot be measured. All the known interstellar Cn chain molecules have been observed in the infrared region through their vibrational transitions.
Table 1. ΔfH° for Cn Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status |
|---|---|---|
| 2 | 194.432 | aObserved |
| 3 | 244.785 | bObserved |
| 4 | 261.328 | Not observed |
| 5 | 305.822 | cObserved |
| 6 | 310.379 | Not observed |
| 7 | 356.501 | Not observed |
| 8 | 363.736 | Not observed |
Notes.
aSouza & Lutz (1977). bHinkle et al. (1988), Cernicharo et al. (2000). cBernath et al. (1989).Download table as: ASCIITypeset image
As can be seen from Table 1, there is a steady increase in the enthalpy of formation of the Cn chains as the chain length increases. C4 is the exception, as it is yet to be observed. All the lower members of the chains with lower enthalpies of formation have been detected (Souza & Lutz 1977; Hinkle et al. 1988; Bernath et al. 1989; Cernicharo et al. 2000). With respect to the effect of thermodynamics, the lower members of the chains with lower enthalpies of formation are more stable than the higher members of the chains with higher enthalpies of formation. Figure 1 shows the plot of the enthalpy of formation of the Cn chain molecules estimated at the G4 level. The lower members of the chains remain the most likely candidates for astronomical observation compared to the higher members of the chains. As C4 is yet to be observed and C5 has already been observed, it is likely that C7, which has a larger enthalpy of formation, may be observed before C6, which has a lower enthalpy of formation.
Figure 1. Plot showing the ΔfH° for Cn chain molecules.
Download figure:
Standard image High-resolution image3.2. CnO Chains
The CnO group presents an interesting set of carbon chain molecules containing two (carbon and oxygen) of the four most important biological elements. Unlike the Cn chains, which lack a permanent dipole moment, the CnO chains all possess a permanent dipole moment. Hence, all the known interstellar/circumstellar CnO molecules have been observed via their rotational transition spectra. The enthalpy of formation and current astronomical status for all the CnO molecules considered in this study are presented in Table 2, while Figure 2 depicts the plot of the enthalpy of formation for these molecules. Of the six molecules considered in the CnO group, the first three (n = 1–3) have been astronomically observed (Smith & Stecher 1971; Matthews et al. 1984; Ohishi et al. 1991). The trend of the enthalpy of formation is the same as in the case of the Cn chain molecules. The odd-numbered carbon chain molecules are found to be more stable (lower enthalpy of formation value) than their corresponding even-numbered ones. The interstellar chemistry of oxygen and sulfur is well coupled, with over 80% of all the known S-containing interstellar molecules having their corresponding O-containing molecules as known interstellar molecules. Also, in most of the cases, the abundance of the S-compound relative to its O-analog is approximately equal to the cosmic S/O ratio (Frerking et al. 1979; Linke et al. 1979). The high probability of C5O as an interstellar molecule stems from two important facts; C5O has lower enthalpy of formation compared with C4O and C6O, and consequently, it is more stable in interstellar medium (ISM), and can thus possibly be observed. Second, C5S, the sulfur analog of C5O, has recently been observed (Agúndez et al. 2014) and the O-containing molecules are known to be more abundant in ISM than in their corresponding S-analog. Hence, astronomical observation of C5S is a clear indication of the presence of C5O in detectable abundance in ISM.
Figure 2. Plot showing the ΔfH° for CnO chain molecules.
Download figure:
Standard image High-resolution imageTable 2. ΔfH° for CnO Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status |
|---|---|---|
| 1 | −23.127 | aObserved |
| 2 | 91.369 | bObserved |
| 3 | 75.328 | cObserved |
| 4 | 148.640 | Not observed |
| 5 | 133.782 | Not observed |
| 6 | 204.526 | Not observed |
Notes.
aSmith & Stecher (1971). bOhishi et al. (1991). cMatthews et al. (1984).Download table as: ASCIITypeset image
3.3. CnS Chains
IRC+10216, the carbon-star envelope, has emerged as one of the richest sources of molecules, with over eighty different molecular species so far observed in it. Alongside other molecular clouds, all the known CnS molecules were observed in IRC+10216. As discussed in the previous section, the interstellar chemistry of oxygen and sulfur is very similar in many ways. Of the four (CS, C2S, C3S, C5S) observed molecules in the CnS group (Penzias et al. 1971; Cernicharo et al. 1987; Saito et al. 1987; Yamamoto et al. 1987; Agúndez et al. 2014), 3 (CS, C2S, C3S), three have the corresponding O-analogs known as interstellar molecules. However, C5S has been astronomically observed, but not C4S and C6S. This could be correlated with its enthalpy of formation value (Table 3) compared to those of C4S and C6S. As shown in Table 3 and Figure 3, all the odd-numbered CnS chains have lower enthalpy of formation than their corresponding even-numbered CnS chains. Hence they are more stable and likely more abundant, and can thus be more easily observed than their even-numbered counterparts.
Figure 3. Plot showing the ΔfH° for CnS chain molecules.
Download figure:
Standard image High-resolution imageTable 3. ΔfH° for CnS Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status |
|---|---|---|
| 1 | 72.404 | aObserved |
| 2 | 141.307 | bObserved |
| 3 | 136.292 | cObserved |
| 4 | 196.958 | Not observed |
| 5 | 190.684 | dObserved |
| 6 | 254.207 | Not observed |
| 7 | 243.688 | Not observed |
| 8 | 313.195 | Not observed |
Notes.
aPenzias et al. (1971). bSaito et al. (1987). cYamamoto et al. (1987). dCernicharo et al. (1987). eAgúndez et al. (2014).Download table as: ASCIITypeset image
3.4. CnN Chains
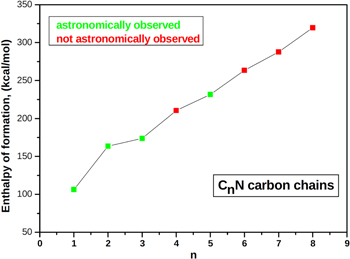
The long carbon chain radicals like CnN have long been thought to be present in the interstellar and circumstellar media in detectable abundance and supposed to play an important role in astrophysics. A number of fundamental interstellar molecules were first detected in space before being observed in the gas phase in the terrestrial laboratory. These include HCO+, C2H, HNC, and  (Guélin & Thaddeus 1977) among others, and thus most of them were termed "non-terrestrial." The cyanoethynyl radical, C3N, is one such molecule whose identification was guided by the electron spin resonance spectrum of a related species, C4H (butadinyl radical). The astronomical observations of C2N and C5N were hindered by the lack of accurate spectroscopic data. Their astronomical observations became possible immediately after their laboratory microwave spectra were measured (Jefferts et al. 1970; Friberg et al. 1980; Guélin et al. 1998; Anderson & Ziurys 2014). Table 4 and Figure 4 indicate a constant gradual increase in the enthalpy of formation of the CnN chain molecules. A good correlation between the observations of the lower chains (except for C4N) with the enthalpy of formation can be seen.
(Guélin & Thaddeus 1977) among others, and thus most of them were termed "non-terrestrial." The cyanoethynyl radical, C3N, is one such molecule whose identification was guided by the electron spin resonance spectrum of a related species, C4H (butadinyl radical). The astronomical observations of C2N and C5N were hindered by the lack of accurate spectroscopic data. Their astronomical observations became possible immediately after their laboratory microwave spectra were measured (Jefferts et al. 1970; Friberg et al. 1980; Guélin et al. 1998; Anderson & Ziurys 2014). Table 4 and Figure 4 indicate a constant gradual increase in the enthalpy of formation of the CnN chain molecules. A good correlation between the observations of the lower chains (except for C4N) with the enthalpy of formation can be seen.
Figure 4. Plot showing the ΔfH° for CnN chain molecules.
Download figure:
Standard image High-resolution imageTable 4. ΔfH° for CnN Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status |
|---|---|---|
| 1 | 106.413 | aObserved |
| 2 | 163.544 | bObserved |
| 3 | 173.701 | cObserved |
| 4 | 210.486 | Not observed |
| 5 | 231.723 | dObserved |
| 6 | 263.488 | Not observed |
| 7 | 287.663 | Not observed |
| 8 | 319.404 | Not observed |
Notes.
aJefferts et al. (1970). bAnderson & Ziurys (2014). cFriberg et al. (1980). dGuélin et al. (1998).Download table as: ASCIITypeset image
3.5. CnSi Chains
Every unique astronomical observation of molecule(s) in the interstellar or circumstellar medium has always been a concerted effort between the laboratory spectroscopists and the astrophysicists. The successful detection (after a number of failed attempts) of SiC in IRC+10216 (Cernicharo et al. 1989), came immediately after the successful measurements of its laboratory spectrum. Whether cyclic molecules are chemically less abundant than their related linear species in the interstellar or circumstellar medium has been the question for decades, due to the fact that of about 200 known interstellar and circumstellar molecules, only about ten are cyclic. However, of the four known CnSi molecules, two are cyclic. The ground-state geometry of SiC2 is shown to have a compact symmetric (C2v) ring, in contrast to the earlier belief that SiC2 is linear. The proper elucidation of its ground-state geometry became instrumental for its astronomical observation (Thaddeus et al. 1984). Figure 5 shows the most stable geometry for the SiC3 molecule, which is the rhomboidal isomer. The experimentally determined bond lengths of c-SiC3 through isotopic substitution measurement (Apponi et al. 1999) and the theoretically calculated bond lengths at the G4 level are in good agreement, as shown in Figures 5(A) and (B), respectively. Table 5 and Figure 6 show the enthalpies of formation for all the CnSi molecules considered in this study. There is a steady increase in the enthalpy of formation value as the carbon chain increases, except for c-C2Si. The observed molecules are also the most stable molecules (Thaddeus et al. 1984; Cernicharo et al. 1989; Ohishi et al. 1989; Apponi et al. 1999). The enthalpy of formation for the linear C3Si molecule, 221.687 kcal/mol, is about 10 kcal/mol higher than the most stable isomer, which is cyclic in structure. The detection of more silicon–carbon molecules in IRC+10216 in particular (where all CnSi chains have been observed) and other astronomical sources await laboratory spectra of such molecules.
Figure 5. Experimental (A) and theoretical geometry (B) of the c-SiC3 molecule.
Download figure:
Standard image High-resolution imageFigure 6. Plot showing the ΔfH° for CnSi chain molecules.
Download figure:
Standard image High-resolution imageTable 5. ΔfH° for CnSi Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status |
|---|---|---|
| 1 | 177.113 | Observed (IRC+10216) |
| c-C2Si | 151.989 | Observed (IRC+10216) |
| c-C3Si | 228.773 | Observe (IRC+10216) |
| 4 | 216.930 | Observed (IRC+10216) |
| 5 | 269.727 | Not observed |
| 6 | 277.600 | Not observed |
Notes.
aCernicharo et al. (1989). bThaddeus et al. (1984). cApponi et al. (1999). dOhishi et al. (1989).Download table as: ASCIITypeset image
3.6. HCnN Chains
The largest claimed interstellar linear molecule, the cyanopolyne HC11N, belongs to the HCnN chains. The enthalpies of formation of these molecules follow a unique trend, with the odd-numbered carbon chains being more stable than their preceding and the next even-numbered carbon chains, as shown in Table 6. For instance, HC3N is more stable than both HC2N and HC4N; HC5N is more stable than both HC4N and HC6N, etc. Figure 7 depicts the zigzag pattern of the enthalpies of formation of the HCnN chains. Of the eight molecules belonging to the HCnN carbon chain, which have been detected in interstellar and circumstellar media (Snyder & Buhl 1971; Turner 1971; Avery et al. 1976; Broten et al. 1978; Kroto et al. 1978; Guélin & Cernicharo 1991; Bell et al. 1997; Cernicharo et al. 2004), only two (HC2N and HC4N) are even-numbered carbon chains, while the remaining six are odd-numbered (HCN, HC3N, HC5N, HC7N, HC9N, and HC11N). A very good correlation between the observations of more odd-numbered HCnN chains and their enthalpy of formation values, in comparison to the even-numbered HCnN chains, can be seen. The odd-numbered HCnN chains are found to be more stable, and thus may be more easily observed compared to the even-numbered HCnN chains. From this analogy, HC13N is a more probable candidate for astronomical observation than both HC12N and HC14N. Accurate laboratory rest frequencies of HC13N will be crucial for its astronomical searches and possible detection.
Figure 7. Plot showing the ΔfH° for HCnN chain molecules.
Download figure:
Standard image High-resolution imageTable 6. ΔfH° for HCnN Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status |
|---|---|---|
| 1 | 32.192 | aObserved |
| 2 | 116.586 | bObserved |
| 3 | 88.738 | cObserved |
| 4 | 161.602 | dObserved |
| 5 | 140.566 | eObserved |
| 6 | 211.115 | Not observed |
| 7 | 191.824 | fObserved |
| 8 | 263.903 | Not oberved |
| 9 | 242.928 | gObserved |
| 10 | 317.217 | Not observed |
| 11 | 292.191 | hObserved |
| 12 | 372.551 | Not observed |
| 13 | 342.655 | Not observed |
| 14 | 428.028 | Not observed |
Notes.
aSnyder & Buhl (1971). bGuélin & Cernicharo (1991). cTurner (1971). dCernicharo et al. (2004). eAvery et al. (1976). fKroto et al. (1978). gBroten et al. (1978). hBell et al. (1997).Download table as: ASCIITypeset image
3.7. CnH Chains
Discoveries of cyanopolynes set the pace for the recognition of long chain molecules in space, such as CnH, CnH−, etc. The acetylenic chain (CnH) radicals from n = 1 to n = 8 have all been observed in space, (Swings & Rosenfeld 1937; Tucker et al. 1974; Guélin et al. 1978; Thaddeus et al. 1985a; Cernicharo et al. 1986; Suzuki et al. 1986; Cernicharo & Guélin 1996; Guélin et al. 1997). The steady increase in the enthalpy of formation of these chains, as the carbon chain increases, is shown in Table 7 and Figure 8. Most of the CnH radicals could be termed "non-terrestrial", as they were first observed in space using the results from quantum chemical calculations prior to being studied in the laboratory. They are believed to grow steadily in the recombination phase of the interstellar medium (Suzuki 1983). In the carbon-rich astronomical sources, such as TMC and IRC+10216, where all the known CnH chain radicals have been detected, successive members of the CnH chains (n > 8) are more likely to exist.
Figure 8. Plot showing the ΔfH° for CnH chain molecules.
Download figure:
Standard image High-resolution imageTable 7. ΔfH° for CnH Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status | |
|---|---|---|---|
| 1 | 142.025 | aObserved | 142.025 |
| 2 | 148.023 | bObserved | 134.951 |
| 3 | 173.615 | cObserved | 173.615 |
| 4 | 194.790 | dObserved | 194.790 |
| 5 | 224.678 | eObserved | 224.678 |
| 6 | 247.560 | fObserved | 247.560 |
| 7 | 279.702 | gObserved | 279.702 |
| 8 | 304.480 | hObserved | 304.481 |
Notes.
aSwings & Rosenfeld (1937). bTucker et al. (1974). cThaddeus et al. (1985a). dGuélin et al. (1978). eCernicharo et al. (1986). fSuzuki et al. (1986). gGuélin et al. (1997). hCernicharo & Guélin (1996).Download table as: ASCIITypeset image
3.8. CnP Chains
Just like sulfur and oxygen, phosphorus (the 18th most cosmically abundant element) and nitrogen are in the same group in the periodic table. Whereas the similarity between the interstellar molecules of sulfur and oxygen is well established with almost all S-containing interstellar molecules having the O-analog as a known interstellar molecule, this is not the case in terms of the interstellar chemistry of N-containing molecules and the P-analog, with only very few known P-containing molecules compared with the large number of N-containing interstellar/circumstellar molecules. This is due to the cosmic ratio of phosphorus and nitrogen compared with the cosmic ratio of sulfur and oxygen. Table 8 and Figure 9 display the enthalpy of formation for all the CnP chains considered in this study. The only two astronomically observed molecules in this group, CP and C2P (Guélin et al. 1990; Halfen et al. 2008), are also the most stable, and thus they may be detectable. As with the CnH chains, there is a corresponding increase in the enthalpy of formation value for every successive increase in the carbon chain.
Figure 9. Plot showing the ΔfH° for CnP chain molecules.
Download figure:
Standard image High-resolution imageTable 8. ΔfH° for CnP Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status |
|---|---|---|
| 1 | 125.335 | aObserved |
| 2 | 153.136 | bObserved |
| 3 | 174.674 | Not observed |
| 4 | 199.132 | Not observed |
| 5 | 221.889 | Not observed |
| 6 | 247.577 | Not observed |
Notes.
aGuélin et al. (1990). bHalfen et al. (2008).Download table as: ASCIITypeset image
3.9. H2Cn Chains
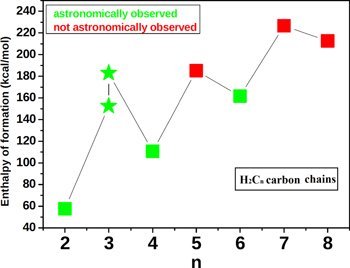
Interstellar acetylene is a well known starting material in the formation processes of many interstellar and circumstellar species (Ridgway et al. 1976; Lacy et al. 1989). For the molecules with the empirical formula, H2C3, both the linear and the cyclic isomers have been observed in the Taurus molecular cloud, i.e., TMC-1 (Thaddeus et al. 1985b; Cernicharo et al. 1991). The cyclic isomer is now recognized as a common feature of many molecular clouds/astronomical sources. The polyacetylenic chains, C4H2 and C6H2, have been observed in the proto-planetary nebula CRL 618 (Cernicharo et al. 2001) via their infrared vibrational spectra. The first three carbon chains in the H2Cn group have been astronomically observed, as shown in Table 9 and Figure 10. The next most likely observable member of this group is neither H2C5 nor H2C7, but rather H2C6.The even-numbered carbon chains in H2Cn are more stable than their corresponding odd-numbered carbon counterparts, thus probably accounting for the astronomical observation of more even-numbered than odd-numbered carbon chains in this group.
Figure 10. Plot showing the ΔfH° for H2Cn chain molecules.
Download figure:
Standard image High-resolution imageTable 9. ΔfH° for H2Cn Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status |
|---|---|---|
| 2 | 57.612 | aObserved |
| 3 (linear) | 152.590 | bObserved |
| 3 (cyclic) | 183.036 | cObserved |
| 4 | 110.685 | dObserved |
| 5 | 185.283 | Not observed |
| 6 | 161.678 | dObserved |
| 7 | 226.691 | Not observed |
| 8 | 212.684 | Not observed |
Notes.
aRidgway et al. (1976), Lacy et al. (1989). bCernicharo et al. (1991). cThaddeus et al. (1985b). dCernicharo et al. (2001).Download table as: ASCIITypeset image
3.10. CnN− Chains
Among the ion molecules in interstellar and circumstellar media, the positive ions have long been known and observed, while the negative ions have only been known for less than a decade. Of the eight CnN− chains considered here, only three have been astronomically observed; CN−, C3N−, and C5N− (Cernicharo et al. 2008; Thaddeus et al. 2008; Agúndez et al. 2010). The observed molecules (odd-numbered carbon chains) are found to be more stable than the others. CN− is more stable than C2N−; C3N− is more stable than both C2N− and C4N−; and C5N− is more stable than both C4N− and C6N− (Table 10). Figure 11 depicts the plot of the enthalpy of formation of these molecules, which shows a zigzag pattern as noted in the case of the HCnN chains. Following the order of their astronomical observations, C7N can be considered as the next probable candidate as it is more stable than both C6N− and C8N−.
Figure 11. Plot showing the ΔfH° for CnN− chain molecules.
Download figure:
Standard image High-resolution imageTable 10. ΔfH° for CnN− Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status |
|---|---|---|
| 1 | 15.459 | aObserved |
| 2 | 99.183 | Not observed |
| 3 | 72.910 | bObserved |
| 4 | 137.056 | Not observed |
| 5 | 124.358 | cObserved |
| 6 | 182.629 | Not observed |
| 7 | 176.752 | Not observed |
| 8 | 232.983 | Not observed |
Notes.
aAgúndez et al. (2010). bThaddeus et al. (2008). cCernicharo et al. (2008).Download table as: ASCIITypeset image
3.11. CnH− Chains
Molecular anions have long been predicted to be detectable in astronomical sources, and are currently estimated by chemical formation models to be in higher abundance than their neutral counterparts. The first known interstellar molecular anion, C6H−, was shown to be the carrier of the unidentified series of lines of IRC+10216 via laboratory measurements of its rotational spectrum. The astronomical observations of C4H− and C8H− came immediately after the successful measurements of their rotational spectra (McCarthy et al. 2006; Brünken et al. 2007a; Cernicharo et al. 2007). Theoretically estimated enthalpy of formation for the CnH− (n = 1–10) chains using the G4 composite method is presented in Table 11. The same is also displayed in Figure 12. The even-numbered carbons chains are more stable than the preceding and the next odd-numbered carbon chains; C2H− is more stable than CH− and C3H, C4H− is more stable than C3H− and C5H−, and so on. The rotational spectrum of C2H− is known (Brünken et al. 2007b), and its astronomical observation is a function of time. The three astronomically detected CnH− carbon chains are all even-numbered. These are the most stable, which might account for their observation, rather than the preceding and the next odd-numbered chain in each case, which have not been observed.
Figure 12. Plot showing the ΔfH° for CnH− chain molecules.
Download figure:
Standard image High-resolution imageTable 11. ΔfH° for CnH− Chains and Current Astronomical Status
| n | ΔfH° (kcal/mol) | Astronomical Status |
|---|---|---|
| 1 | 135.131 | Not observed |
| 2 | 92.858 | aTentatively observed |
| 3 | 196.282 | Not observed |
| 4 | 188.822 | bObserved |
| 5 | 268.466 | Not observed |
| 6 | 227.126 | cObserved |
| 7 | 270.745 | Not observed |
| 8 | 267.406 | dObserved |
| 9 | 279.286 | not observed |
| 10 | 256.98 | not observed |
Notes.
aAgúndez et al. (2010) and references therein. bCernicharo et al. (2007). cMcCarthy et al. (2006). dBrünken et al. (2007a).Download table as: ASCIITypeset image
Table 12 gives the summary of all the different carbon chains, which have been considered in this study. It is obvious from the table, that in each group the number of odd- and even-numbered carbon chains observed depends on the trend of the enthalpy of formation of the molecules in that group. The odd-numbered carbon chains are found to be more stable than their corresponding even-numbered carbon chains in most of the group (Figure 13). This might have resulted in the observation of more interstellar odd-numbered (60%) than the even-numbered carbon chains.
Figure 13. Percentage of detected odd- and even-numbered carbon chains.
Download figure:
Standard image High-resolution imageTable 12. Carbon Chains which have been Considered in this Study
| Chain | Total Number Considered | Odd Observed | Even Observed |
|---|---|---|---|
| Cn | 7 | 2 | 1 |
| CnO | 6 | 2 | 1 |
| CnS | 8 | 3 | 1 |
| CnN | 8 | 3 | 1 |
| CnSi | 6 | 2 | 2 |
| HCnN | 14 | 6 | 2 |
| CnH | 8 | 4 | 4 |
| CnP | 6 | 1 | 1 |
| CnN− | 8 | 3 | 0 |
| CnH− | 10 | 0 | 3 |
| H2CnN | 8 | 2 | 3 |
| Total | 89 | 28 | 19 |
Download table as: ASCIITypeset image
3.12. Effect of Kinetics on the Formation of Carbon Chains
The effect of kinetics on the formation of carbon chain species both in the terrestrial laboratory and in the interstellar medium cannot be overemphasized. Although there is hardly consensus as to how any molecule (especially the complex ones with six atoms and above) is formed in the interstellar medium, C2 addition is the widely acclaimed route for the formation of the linear carbon chains. In the terrestrial laboratory, unsaturated molecules like acetylene, diacetylene, etc., are used as the source of the C2 in producing the carbon chains, while in the interstellar medium, acetylene, which produces the C2 is generally accepted as the starting material for the formation of these carbon chains (Cherchneff et al. 1993; Cherchneff & Glassgold 1993; McCarthy et al. 1997; Thaddeus et al. 1998). With respect to the carbon chains discussed here, a good correlation between the enthalpy of formation and observed abundances has been seen. For instance, in the CnO chains, where the odd-numbered carbon chains are more stable than their progressive even-numbered chains, C2 addition to the lower chains to form the higher chains, clearly favors the odd-numbered chains compared to their corresponding even-numbered chains. A C2 addition to CO to form C3O is likely to have a lower barrier compared to C2 addition to C2O to form C4O, which could account for the delayed observation of C4O. This is also applicable to the CnS chains, where a C2 addition to C3S to form C5S (astronomically observed) is more favorable than a C2 addition to C2S to form C4S (yet to be observed). And this continues to the higher members of the chains. Also, in the HCnN and CnN− chains, C2 addition to form higher members of the chains is likely to favor the odd-numbered carbon chains compared to the even-numbered ones, which explains the trends in the astronomical observation of these species. In the case of CnSi, H2Cn, and CnH− chains, where the even-numbered are more stable than their corresponding odd-numbered chains, it is crystal clear that C2 addition to the lower members of the chains is more likely to favor the even-numbered carbon chains compared to their odd-numbered counterparts. For instance, a C2 addition to HC5N to form HC7N is could be more favorable than C2 addition to HC4N to form HC6N, which has not been detected. Correlation between the effects of kinetics on the formation of these species can be seen even for the chains with a linear increase in the enthalpy of formation such as in the CnH, CnP, CnN and Cn chains.
3.13. Other Studies Supporting the Above Results
The correlation between the thermodynamics and observation of the linear interstellar carbon chains has been discussed in the previous sections. Here, it is also correlated with the chemical reactivity indices such as global hardness (η) and softness (S). Soft species are more reactive and unstable in comparison to the hard species. Larger values of S indicate that the species are more unstable, and larger values of η indicate that the species are more stable. All the parameters are pointed out in Table 13. For the sake of simplicity, in Figure 14, we have shown the softness values for the carbon chain species.
Figure 14. Variation of global softness with the number of carbon atoms present in carbon chains.
Download figure:
Standard image High-resolution imageTable 13. Global Reactivity Indices for the Carbon Chain Molecules
| Carbon Chains | Electron Affinity (ev) | Ionization Potential (ev) | Global Hardness (η) | Global Softness (S) | Electrophilicity Index (ω) |
|---|---|---|---|---|---|
| C2 | 4.315589604 | 12.38118018 | 8.06559057 | 0.12398348 | 4.320547246 |
| C3 | 4.299943058 | 10.03321861 | 5.73327555 | 0.17442036 | 4.479104517 |
| C4 | 3.223841626 | 10.48334934 | 7.25950771 | 0.13775039 | 3.23518983 |
| C5 | 5.061235976 | 8.983784335 | 3.92254836 | 0.25493631 | 6.286174748 |
| C6 | 4.573961705 | 9.574978885 | 5.00101718 | 0.19995932 | 5.003795043 |
| C7 | 5.054541975 | 8.581572853 | 3.52703088 | 0.28352459 | 6.589948956 |
| C8 | 4.638425476 | 8.973879391 | 4.33545392 | 0.23065636 | 5.342429173 |
| CO | −1.15085110 | 14.19743128 | 15.3482824 | 0.06515387 | 1.386256536 |
| C2O | 2.34194785 | 11.3391474 | 8.99719955 | 0.1111457 | 2.600425363 |
| C3O | 1.07569326 | 11.91692674 | 10.8412335 | 0.09224043 | 1.946367251 |
| C4O | 2.9618232 | 10.00853788 | 7.04671468 | 0.1419101 | 2.984196789 |
| C5O | 2.124637729 | 10.36919758 | 8.24455985 | 0.1212921 | 2.366650306 |
| C6O | 3.324768653 | 9.175651811 | 5.85088316 | 0.17091437 | 3.338395837 |
| CH | 0.120138265 | 11.00240651 | 10.8822682 | 0.09189261 | 1.421015815 |
| C2H | 3.111703509 | 11.56853937 | 8.45683586 | 0.11824754 | 3.185433869 |
| C3H | 1.926647692 | 9.361641627 | 7.43499393 | 0.1344991 | 2.142326491 |
| C4H | 3.47244484 | 10.36144233 | 6.88899749 | 0.145159 | 3.472501523 |
| C5H | 2.593898064 | 8.655995991 | 6.06209793 | 0.16495939 | 2.609660009 |
| C6H | 3.647169143 | 9.20098561 | 5.55381647 | 0.18005636 | 3.715352351 |
| C7H | 2.975782641 | 7.758237977 | 4.78245534 | 0.20909761 | 3.01150744 |
| C8H | 3.823907089 | 8.65754704 | 4.83363995 | 0.20688343 | 4.028710732 |
| CN | 4.962404226 | 15.61593357 | 10.6535293 | 0.09386561 | 4.968634956 |
| C2N | 2.940870434 | 11.09087072 | 8.15000029 | 0.12269938 | 3.019781486 |
| C3N | 4.493470428 | 12.46142655 | 7.96795612 | 0.1255027 | 4.509759578 |
| C4N | 3.312577953 | 9.828969953 | 6.516392 | 0.15345915 | 3.312804873 |
| C5N | 4.888198779 | 10.5489832 | 5.66078442 | 0.17665396 | 5.262225376 |
| C6N | 3.605127553 | 9.117174544 | 5.51204699 | 0.1814208 | 3.67052773 |
| C7N | 4.971356771 | 9.555305054 | 4.58394828 | 0.21815255 | 5.7544253 |
| C8N | 3.803988355 | 8.626553273 | 4.82256492 | 0.20735854 | 4.005087743 |
| CP | 3.624420425 | 10.72087752 | 7.09645709 | 0.14091539 | 3.624829446 |
| C2P | 2.731533249 | 9.229122182 | 6.49758893 | 0.15390324 | 2.752122361 |
| C3P | 3.658761193 | 9.590434952 | 5.93167376 | 0.16858648 | 3.699234095 |
| C4P | 3.154316538 | 8.617627938 | 5.4633114 | 0.18303917 | 3.170665797 |
| C5P | 3.821539698 | 8.940409387 | 5.11886969 | 0.19535563 | 3.977131533 |
| C6P | 3.431410071 | 8.207797269 | 4.7763872 | 0.20936326 | 3.545335153 |
| HCN | −0.70335987 | 13.50925535 | 14.2126152 | 0.07036003 | 1.442301049 |
| HC2N | 1.977478559 | 10.69984312 | 8.72236456 | 0.11464781 | 2.303195479 |
| HC3N | −0.05679016 | 11.341542 | 11.3983322 | 0.08773213 | 1.396537913 |
| HC4N | 2.023765125 | 9.568992381 | 7.54522726 | 0.13253411 | 2.226441001 |
| HC5N | 0.9548475 | 11.55373638 | 10.5988889 | 0.09434951 | 1.845295677 |
| HC6N | 2.439038072 | 8.919565466 | 6.48052739 | 0.15430843 | 2.488568184 |
| HC7N | 1.592246982 | 9.470487167 | 7.87824019 | 0.1269319 | 1.941805593 |
| HC8N | 2.732295168 | 8.471257899 | 5.73896273 | 0.17424752 | 2.733934842 |
| HC9N | 2.030105378 | 8.964491463 | 6.93438609 | 0.14420887 | 2.179016971 |
| HC10N | 2.90070643 | 8.139006888 | 5.23830046 | 0.19090161 | 2.908273187 |
| HC11N | 2.433160413 | 8.604403205 | 6.17124279 | 0.16204191 | 2.467651466 |
| H2C2 | 0.677808389 | 11.27155432 | 10.5937459 | 0.09439532 | 1.684806183 |
| l-H2C3 | 1.945042588 | 10.29523704 | 8.35019445 | 0.11975769 | 2.242828688 |
| c-H2C3 | −1.08125684 | 9.15234512 | 10.2336010 | 0.0977517 | 0.79575879 |
| H2C4 | 1.859653262 | 10.34897952 | 8.48932626 | 0.11779498 | 2.194678204 |
| H2C5 | 2.618905327 | 9.231217459 | 6.61231213 | 0.15123303 | 2.654620033 |
| H2C6 | 2.548155726 | 10.02097349 | 7.47281776 | 0.13381833 | 2.642627825 |
| H2C7 | 3.056083438 | 6.991176246 | 3.93509281 | 0.25412361 | 3.206640608 |
| H2C8 | 2.988626415 | 8.396290533 | 5.40766412 | 0.18492273 | 2.996125755 |
| CSi | 2.293974178 | 10.54732331 | 8.25334913 | 0.12116293 | 2.497454647 |
| C2Si | 1.678207747 | 9.765921174 | 8.08771343 | 0.12364434 | 2.024182854 |
| C3Si | 2.66475651 | 9.066534157 | 6.40177765 | 0.15620661 | 2.687206357 |
| C4Si | 2.343199574 | 9.39489394 | 7.05169437 | 0.14180989 | 2.442371155 |
| C5Si | 3.048681941 | 8.506959236 | 5.45827729 | 0.18320799 | 3.058035434 |
| C6Si | 2.784622661 | 8.774501573 | 5.98987891 | 0.16694828 | 2.788314986 |
| CS | 0.299352446 | 11.50772193 | 11.2083695 | 0.08921904 | 1.554719952 |
| C2S | 2.689736562 | 10.40672208 | 7.71698552 | 0.12958428 | 2.778242044 |
| C3S | 1.702779628 | 10.75483733 | 9.0520577 | 0.11047212 | 2.143051687 |
| C4S | 3.159758815 | 9.441370985 | 6.28161217 | 0.1591948 | 3.159787407 |
| C5S | 2.481569466 | 9.63361942 | 7.15204995 | 0.13982005 | 2.565309993 |
| C6S | 3.452934277 | 8.804488519 | 5.35155424 | 0.1868616 | 3.509363981 |
| C7S | 2.959483021 | 9.001607792 | 6.04212477 | 0.16550469 | 2.95979682 |
| C8S | 3.693210806 | 8.368290018 | 4.67507921 | 0.21390012 | 3.889768375 |
| CN− | −6.72932108 | 4.962404226 | 11.6917253 | 0.08553058 | 0.033378256 |
| C2N− | −4.94071675 | 2.940870434 | 7.88158719 | 0.126878 | 0.06342925 |
| C3N− | −5.60834808 | 4.493470428 | 10.1018185 | 0.09899208 | 0.015380302 |
| C4N− | −3.36525919 | 3.312577953 | 6.67783715 | 0.14974908 | 5.19501E-05 |
| C5N− | −3.91196313 | 4.888198779 | 8.80016191 | 0.11363427 | 0.013537195 |
| C6N− | −2.21244886 | 3.605127553 | 5.81757642 | 0.17189289 | 0.04167444 |
| C7N− | −2.62399385 | 4.971356771 | 7.59535063 | 0.13165949 | 0.090682329 |
| C8N− | −1.34579346 | 3.803988355 | 5.14978182 | 0.19418298 | 0.146674226 |
| CH− | −3.69620405 | 0.120138265 | 3.81634232 | 0.262031 | 0.418864631 |
| C2H− | −4.15879760 | 3.111703509 | 7.27050111 | 0.1375421 | 0.018850249 |
| C3H− | −5.00950713 | 1.926647692 | 6.93615483 | 0.1441721 | 0.171276856 |
| C4H− | −5.56902763 | 3.47244484 | 9.04147247 | 0.11060145 | 0.06077079 |
| C5H− | −3.65484275 | 2.593898064 | 6.24874082 | 0.16003224 | 0.022516609 |
| C6H− | −4.06075498 | 3.647169143 | 7.70792413 | 0.12973662 | 0.002773984 |
| C7H− | −2.53308061 | 2.975782641 | 5.50886326 | 0.18152565 | 0.00444704 |
| C8H− | −2.99548368 | 3.823907089 | 6.81939077 | 0.14664067 | 0.012579667 |
3.13.1. Chemical Modeling
The need for chemical modeling is ever increasing, because of the recent development of various sensitive ground-based and space-borne facilities as well as the vast data produced by the low-temperature laboratory experiments. Chemical modeling plays a very important role in the understanding of the empirical outcome of the observational results. We use our large gas-grain chemical network (Das et al. 2013, 2015a, 2015b; Majumdar et al. 2014a, 2014b) for the purpose of chemical modeling. We assume that gas and grains are coupled through accretion and thermal/non-thermal/cosmic ray evaporation processes. Details of these processes and chemical network were already presented in Das et al. (2015a, 2015b). The gas-phase chemical network is principally adopted from the UMIST 2006 (Woodall et al. 2007) database. Since the UMIST 2006 database does not consider some of the carbon chain molecules, we include their reactions from the UMIST 2012 network (McElroy et al. 2013). These databases contain different assorted reaction pathways and rate coefficients from the literature and, therefore, our calculated thermodynamical parameters do not seem to play any role in this modeling.
3.13.2. Physical Aspects
Chemical enrichment of a molecular cloud is interrelated with the surrounding physical conditions and age of the cloud. Depending on the densities (nH), temperatures (T) and visual extinction (AV) parameters, Snow & McCall (2006) classified various types of clouds. Das et al. (2016) also assumed these classifications in their model. For the diffuse cloud region, they considered that nH may vary between 100–500 cm−3, T may vary between 30–100 K and AV = 0.2, for the translucent cloud region, we used nH = 501–10,000 cm−3, T = 15–30 K, AV = 1–2, and for the dense cloud region, we used nH = 10,001–1,000,000 cm−3, T = 10 K, and AV = 5–10. In order to consider a more realistic condition, they considered different temperatures and extinction parameters for various regions of the clouds. They used constant slopes for T and AV in respective number density windows. In diffuse and translucent cloud regions, gas and grains are not well coupled. So, temperature between these two phases might vary. However, in dense cloud regions, gas and grains are strongly coupled and the temperature would be more or less the same for both phases. They assumed that the grain temperature is always fixed at 10 K for all the clouds and for the gas temperature (Tgas), we assume Tgas = T. Here, we present the results for the dense and translucent cloud regions. Following Das et al. (2015a), we use nH = 2 × 104 cm−3, Tgas = 10 K, Tgr = 10 K, AV = 5.05 for dense cloud and nH = 5 × 103 cm−3, Tgas = 22.89 K, Tgr = 10 K, and AV = 1.47 for translucent cloud.
We assume that initially most of the hydrogen was in the form of molecular hydrogen. Our adopted initial condition is shown in Table 14. The choice of our initial condition was based on the study of Leung et al. (1984). These initial low-metalicity elemental abundances are often used to calculate molecular abundances in cold, dense clouds. Gas-phase species are allowed to accrete on the grains with a sticking coefficient of 0.3. Surface species are allowed to migrate through the grain surface, depending upon their binding energies. Binding energies of the surface species are taken from some past studies (Allen & Robinson 1977; Tielens & Allamandola 1987; Hasegawa & Herbst 1993; Hasegawa et al. 1992).
Table 14. Initial Abundances
| Species | Abundance w.r.t. total H |
|---|---|
| H2 | 5.00 × 10−01 |
| He | 1.00 × 10−01 |
| N | 2.14 × 10−05 |
| O | 1.76 × 10−04 |
| H3+ | 1.00 × 10−11 |
| C+ | 7.30 × 10−05 |
| S+ | 8.00 × 10−08 |
| Si+ | 8.00 × 10−09 |
| Fe+ | 3.00 × 10−09 |
| Na+ | 2.00 × 10−09 |
| Mg+ | 7.00 × 10−09 |
| P+ | 3.00 × 10−09 |
| Cl+ | 4.00 × 10−09 |
| e- | 7.31 × 10−05 |
| HD | 1.60 × 10−05 |
Download table as: ASCIITypeset image
Chemical evolution of the carbon chain molecules is shown in Figure 15 (for the dense cloud region) and Figure 16 (for the translucent cloud region). For the dense cloud region, gas-phase abundances of the carbon chain molecules are decreased at the latter stages (∼106 years). This happened due to the heavy depletion of the gas-phase species. Since the depletion timescale is inversely proportional to the number density of a cloud, for the translucent cloud region no such depletion is prominent around the latter stages. The abundance of interstellar species is not driven by the thermodynamic stability of that species. It primarily depends on some other factors like: formation and destruction rate of the species, relative concentration of the interacting species, size of the species (here it depends on the number of carbons in it), etc. For example, for the formation of CnX, it would require some extra reaction steps in comparison to the formation of Cn-1X. So, while comparing the abundances of any species with the effect of thermodynamics, it should be with the appropriate weighted average. The peak and final abundances of these species after 106 years are shown in Table 15. Keeping in mind all these aspects, the abundances might be correlated with the energy and stability values of several carbon chain species. Few prominent examples of these correlations could be seen from Figures 15 and 16 and Table 15. Some examples are:
- (a)
- (b)
Figure 15. Chemical evolution of carbon chains for dense cloud.
Download figure:
Standard image High-resolution imageFigure 16. Chemical evolution of carbon chains for translucent cloud.
Download figure:
Standard image High-resolution imageTable 15. Abundances of Carbon Chain Molecules
| Carbon Chains | Peak Abundance (Final Abundance) with Respect to Total H in all Forms | |
|---|---|---|
| Translucent Cloud | Dense Cloud | |
| C2 | 5.32E-08(4.53E-08) | 4.62E-08 (4.94E-12) |
| C3 | 1.58E-13 (1.17E-13) | 3.97E-10 (3.44E-16) |
| C4 | 1.51E-11 (2.03E-12) | 1.32E-10 (2.31E-14) |
| C5 | 1.69E-11 (1.17E-11) | 8.62E-11 (1.23E-13) |
| C6 | 1.23E-12 (7.90E-13) | 3.69E-12 (4.40E-14) |
| C7 | 7.68E-15 (6.90E-15) | 5.37E-13 (3.66E-18) |
| C8 | 2.40E-20 (7.91E-21) | 4.86E-15 (3.29E-25) |
| CO | 6.26E-06 (1.08E-07) | 2.93E-05 (1.09E-07) |
| C2O | 2.46E-11 (1.23E-13) | 1.67E-10 (7.85E-16) |
| C3O | 5.07E-16 (5.35E-18) | 1.43E-13 (5.85E-18) |
| CH | 2.22E-07 (5.68E-08) | 3.72E-07 (5.43E-10) |
| C2H | 1.19E-09 (2.98E-10) | 2.89E-09 (1.13E-13) |
| C3H | 2.88E-11 (1.12E-11) | 2.97E-10 (2.49E-14) |
| C4H | 2.97E-11 (5.16E-12) | 3.51E-10 (1.86E-13) |
| C5H | 4.86E-12 (1.45E-12) | 2.79E-11 (9.76E-14) |
| C6H | 3.49E-11 (3.40E-11) | 1.51E-11 (1.96E-13) |
| C7H | 9.51E-15 (5.67E-15) | 1.23E-12 (2.73E-17) |
| C8H | 2.08E-17 (6.61E-18) | 1.04E-12 (2.76E-20) |
| CN | 3.60E-07 (1.58E-09) | 4.25E-06 (4.08E-10) |
| C2N | 1.29E-11 (4.14E-14) | 8.32E-10 (3.19E-14) |
| C3N | 5.88E-13 (7.75E-15) | 3.45E-11 (5.93E-13) |
| C4N | 2.68E-13 (3.21E-16) | 5.90E-11 (4.11E-15) |
| C5N | 1.53E-14 (7.44E-16) | 4.14E-13 (2.63E-16) |
| C6N | ||
| C7N | 4.16E-16 (1.62E-17) | 1.61E-12 (4.86E-13) |
| C9N | 1.19E-21(2.54E-23) | 4.64E-14(7.61E-17) |
| CP | 2.53E-12 (2.53E-12) | 7.83E-12 (1.63E-14) |
| C2P | 9.57E-16 (3.55E-16) | 1.79E-13 (1.21E-17) |
| C3P | 1.82E-20 (9.47E-21) | 1.31E-17 (6.57E-22) |
| C4P | 4.83E-20 (3.27E-20) | 4.45E-17 (9.13E-23) |
| C5P | ||
| C6P | ||
| HCN | 1.06E-10 (2.06E-12) | 1.33E-07 (1.27E-11) |
| HC2N | 3.06E-14 (4.14E-17) | 1.21E-12 (1.23E-17) |
| HC3N | 1.13E-14 (5.67E-17) | 2.46E-09 (3.28E-14) |
| HC5N | 1.66E-14 (2.67E-16) | 8.37E-13 (1.10E-14) |
| HC7N | 2.37E-15 (1.06E-16) | 4.86E-12 (1.35E-15) |
| HC9N | 1.17E-20 (2.36E-22) | 1.53E-14 (1.03E-19) |
| H2C2 | 1.76E-11 (4.77E-12) | 2.75E-10 (8.38E-13) |
| H2C3 | 2.33E-11(7.51E-11) | 4.48E-10 (4.95E-14) |
| H2C4 | 1.21E-14 (2.87E-15) | 1.69E-12 (3.58E-17) |
| H2C5 | 8.96E-12 (9.26E-13) | 5.64E-11 (1.05E-12) |
| H2C6 | 1.90E-11 (7.85E-12) | 2.11E-11 (2.05E-12) |
| H2C7 | 3.18E-14 (5.86E-15) | 5.77E-12 (1.06E-15) |
| H2C8 | 8.27E-17 (1.71E-17) | 1.93E-12 (1.22E-18) |
| H2C9 | 4.73E-18 (1.21E-18) | 1.42E-12(5.32E-21) |
| CSi | 1.47E-12 (1.47E-12) | 1.51E-12 (1.97E-14) |
| C2Si | 1.04E-14 (1.04E-14) | 2.14E-12 (1.64E-19) |
| C3Si | 1.48E-14 (1.07E-14) | 3.57E-16 (5.17E-18) |
| C4Si | 4.04E-19 (1.73E-19) | 3.57E-16 (1.32E-22) |
| CS | 1.79E-10 (9.97E-11) | 1.58E-08 (7.11E-12) |
| C2S | 2.32E-15 (2.32E-15) | 1.93E-12 (2.46E-16) |
| C3S | 2.24E-16 (2.19E-16) | 3.53E-13 (3.98E-17) |
| C4S | 1.07E-20 (1.04E-20) | 1.27E-17 (5.04E-21) |
| CN− | 4.71E-16 (1.82E-18) | 1.85E-14 (2.52E-22) |
| C3N− | 2.03E-15 (6.99E-18) | 1.07E-13 (5.09E-16) |
| C5N− | 2.12E-14 (2.01E-16) | 3.46E-13 (1.04E-16) |
| CH− | 6.46E-19 (8.64E-23) | 1.30E-16 (1.80E-28) |
| C2H− | 1.65E-16 (1.34E-18) | 1.07E-14 (7.42E-22) |
| C3H− | 2.24E-14 (1.49E-17) | 6.53E-13 (4.20E-21) |
| C4H− | 3.32E-12 (1.29E-13) | 4.46E-11 (6.74E-15) |
| C5H− | 1.58E-12 (1.35E-13) | 1.31E-11 (1.32E-14) |
| C6H− | 1.00E-11 (4.81E-12) | 1.02E-11 (4.01E-14) |
| C7H− | 1.11E-14 (2.44E-15) | 2.81E-12 (1.71E-17) |
| C8H− | 7.99E-18 (8.47E-19) | 7.78E-13 (5.64E-21) |
| C9H− | 1.01E-18 (5.61E-23) | 3.05E-13 (2.93E-19) |
4. CONCLUSION
A comprehensive investigation of different interstellar carbon chain molecules; Cn, H2Cn, HCnN and CnX (X = N, O, Si, S, H, P, H−, N−) with a total of 89 species has been carried out using high-level quantum chemical simulation to determine their accurate enthalpies of formation, chemical reactivity indices, global hardness and softness, and other chemical parameters of these molecules. Chemical modeling of the abundances of these molecular species has also been performed. From the results, in all the groups considered, the most stable molecules have been astronomically observed. The next possible candidate for astronomical observations in each of the carbon chain groups considered is proposed. The effect of kinetics on the formation of some of these molecular species is shown to be correlated with our observations.
EEE acknowledges a research fellowship from the Indian Institute of Science, Bangalore. A.D., P.G., and S.K.C. are grateful to DST (Grant No. SB/S2/HEP-021/2013) for the partial financial support.