Abstract
Rhythm perception can be distorted following prolonged exposure to an isochronous rhythm. It has been suggested that this might arise from the neural adaptation of temporal interval selective neurons. However, the rhythm in our daily life is not always isochronous, and the mechanism that rules the anisochronous rhythm is unclear. Here, we used a sensory adaptation paradigm to investigate whether rhythm perception can be affected by adaptation to the anisochronous rhythm. In Experiments 1 and 2, the direction of tempo change (accelerating vs. decelerating) judgment task and the rhythmic isochronism (isochronous vs. anisochronous) judgment task were used to evaluate participants’ perception of rhythms, respectively. We found that after adaptation to a decelerating rhythm, participants tended to perceive the subsequent isochronous rhythm as accelerating. In Experiment 3, visual test rhythms followed the auditory adapting rhythm. In this situation, we did not find any adaptation effects on subsequent rhythm perception. Our results suggest that adaptation to the decelerating rhythm can induce a modality-specific rhythm aftereffect, which is consistent with the temporal order contingent duration aftereffect. It implies a unified timing mechanism for duration and rhythm perception.
Similar content being viewed by others
Introduction
A fundamental function of the brain is to realize temporal properties of events in our daily life. To ensure our survival, it is necessary for the brain to be sensitive to a variety of temporal properties, such as the duration and rhythm information. Compared with the perception of the single duration, the rhythm perception refers to the perception of complex temporal information, requiring the processing of structured information over time. The rhythm perception is essential for various kinds of human activity, such as the perception of speech and music and the execution of skilled movements (Flaugnacco et al., 2014; Kraus & Chandrasekaran, 2010; Thaut, McIntosh, McIntosh, & Hoemberg, 2001). Gaining knowledge about how the brain processes the rhythm information will be of interest to the fields of language and music perception, action control, etc.
Although the ecological importance of accurate timing is obvious (e.g., for speaking, driving a car, and playing the piano), the brain is prone to distorting the perceived time (Eagleman, 2008). Among those misperceptions, a notable example influencing time perception is perceptual adaptation. For example, after adaptation to a relatively fast auditory rhythm, a subsequent moderately fast auditory rhythm is perceived as being slower, while after adaptation to a relatively slow auditory rhythm, the same moderate auditory rhythm appears faster (Becker & Rasmussen, 2007; Levitan, Ban, Stiles, & Shimojo, 2015; Motala, Heron, McGraw, Roach, & Whitaker, 2018). This negative rhythm aftereffect is similar to the duration aftereffect, which shows that adaptation to a relatively short or long sensory event would lengthen or shorten the perceived duration of a subsequent event (Heron et al., 2012; Li, Yuan, & Huang, 2015; Walker, Irion, & Gordon, 1981). Studies have found that both the rhythm and the duration aftereffects occur in vision, audition, and touch, but are sensory-specific, and tuned around the adapting rhythm and duration (Becker & Rasmussen, 2007; Heron et al., 2012; Li et al., 2015; Motala et al., 2018). These studies suggest that they may originate from a unified adaptation mechanism. This idea has been further supported by a recent study that found that adaptation to a given rate of the rhythm strongly influenced the perceived duration of a single empty or filled interval (Motala, Heron, McGraw, Roach, & Whitaker, 2020). However, a growing body of evidence suggests that there are separate mechanisms for duration and rhythm perception (Johnston, Arnold, & Nishida, 2006; Pariyadath & Eagleman, 2007). For example, Pariyadath and Eagleman (2007) have found that when streams of stimuli were presented, the duration of an oddball stimulus was perceived to last longer, while the perceived rate of an auditory beep or visual flicker was unchanged by the oddball status. Moreover, fMRI studies have found distinct neural substrates of duration-based and beat-based timing (Grube, Cooper, Chinnery, & Griffiths, 2010; Teki, Grube, Kumar, & Griffiths, 2011). How the brain processes the rhythm information is, however, still debated.
Unlike the single duration, a rhythm is usually constructed from sets of several intervals. We not only perceive the rate of the rhythm, but also the tempo changes of the rhythm, which commonly take place in our environment, and play a critical role in interpretation of the rhythm structure, as well as the apparent duration of a sensory event (Binetti, Lecce, & Doricchi, 2012; Horr & Di Luca, 2015; Matthews, 2011, 2013). However, although previous studies have found the rhythm aftereffect in perception of the rate of isochronous rhythm, we know little about whether the perceived tempo changes of the anisochronous rhythm can also be adapted. It has been suggested that the duration aftereffect is contingent on the temporal order (Walker & Irion, 1979; Walker et al., 1981). For example, after adaptation to a pair of tones of the same pitch, the first shorter and the second longer, a pair of test tones of equal and intermediate duration was regarded as the first tone sounding longer than the second. In structure, the anisochronous rhythm is composed of successive rhythmic events that specify successive duration intervals. Thus, if the rhythm perception is simply the perceptual aggregate of its component duration intervals, we expect that adaptation to an anisochronous rhythm would induce a similar aftereffect. That is, after adaptation to a decelerating rhythm, the subsequently isochronous rhythm will be regarded as accelerating, and vice versa.
To test this hypothesis, the present study used the sensory adaptation paradigm to test whether adaptation to an accelerating or decelerating rhythm modulates subsequent processing of the rhythm. Here, the accelerating and decelerating rhythms were constructed from sets of two consecutive intervals and the tempo changes were defined by their durational relationship. If the first interval is longer than the second one, the rhythm is speeding up; vice versa, it’s slowing down. Specifically, participants were asked to complete a perceptual timing task of whether the test rhythm was accelerating or decelerating (Experiment 1) or of whether the test rhythm was isochronous or anisochronous (Experiment 2), after repetitive exposure to an accelerating or decelerating rhythm. Moreover, to examine the modality specificity of the adaptation effect, in Experiment 3 we implemented the visual test rhythms after the auditory adapting rhythm.
Experiment 1
Method
Participants
The participants in Experiment 1 were 14 volunteers (nine female; mean age: 21.8 ± 1.7 years), all of whom were naïve to the purpose of the experiment. They were all right-handed and reported normal or corrected-to-normal vision and normal hearing. All of them provided informed consent before the experiment and were paid for their time. This sample size is comparable to the size of groups used in the previous study (Walker et al., 1981). The study was carried out in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the Shaanxi Normal University of China.
Apparatus and stimuli
Participants sat in a quiet room and stared at a central fixation cross (0.4° × 0.4°), which was presented on the screen with a gray background. The viewing distance was set to approximately 57 cm. The rhythm was composed of a three-beat auditory sequence. The beat was a 10-ms presentation of a 2,000-Hz tone with a 2-ms fade-in and fade-out. The rhythms were presented through headphones (~55 dB SPL). There were two adapting rhythms and seven test rhythms. They differed in terms of the amount and direction of tempo change, and were constructed from sets of two intervals. The first interval was defined as the stimulus-onset asynchrony (SOA) between the first and second beats, while the second interval was the SOA between the second and third beats (Fig. 1A). In the accelerating rhythms, the first interval was longer than the second interval, and this pattern was reversed in the decelerating rhythms. Stimuli presentation and data collection were implemented with computer programs designed with Matlab (The MathWorks, Natick, MA, USA) and Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997).
(A) Schematic diagram of the auditory rhythm, which was composed of a three-beat auditory sequence. (B) Schematic illustration of the two adaptation blocks (AA: adapt to accelerating rhythm; AD: adapt to decelerating rhythm), each of which began with the initial adaptation phase followed by top-up/test trials
Procedure
There were four adaptation blocks in Experiment 1. Each adaptation block began with an initial adaptation phase followed by top-up/test trials. During the initial adaptation phase, a clearly accelerating (first/second interval: 710/310 ms) or decelerating (first/second interval: 310/710 ms) adapting rhythm was repeated 80 times with an inter-rhythm interval (IRI) of 1,500–2,000 ms (Fig. 1B). Thus, the mean adapting frequency was about 1.08 Hz. In each top-up/test trial, one of seven test rhythms (first/second interval: 420/600, 450/570, 480/540, 510/510, 540/480, 570/450, and 600/420 ms) was presented randomly following a top-up adaptation period, where four top-up rhythms that were the same as the adapting rhythms in the initial adaptation phase were presented. After the test rhythm disappeared, participants were asked to indicate whether the test rhythm was accelerating or decelerating by pressing one of two labeled keys on a QWERTY keyboard (the “F” and “J” keys were used). The response mapping was counterbalanced across participants. During the entire block, participants were asked to stare at the fixation on the screen. The color of the fixation was always blue except during the IRI between the last top-up rhythm and the test rhythm. The color pattern was blue (500–750 ms) – red (500 ms) – blue (500–750 ms) during this period. This was to remind participants that the response period would begin shortly. There were two adaptation conditions: “adapt to accelerating rhythm” (AA) and “adapt to decelerating rhythm” (AD). Thus, for each adaptation condition, participants completed two blocks of 35 test trials with five trials for each of the test rhythms. Both the order of trials in each block and the order of blocks were selected randomly. After each block, participants took a break of at least 3 min to wash out any potential carry-over effect between blocks. Moreover, a baseline (BA) performance was collected before the adaptation blocks. That is, participants completed a pretest block of 70 trials, which were similar to these in the adaptation blocks except that there was no adaptation phase and no top-up adaptation period. The total experiment lasted approximately 80 min.
Measurements
In Experiment 1, for each participant, the proportion of “accelerating” responses to the test rhythms for each condition was plotted as a function of the difference between first and second intervals (FSD: 0, ± 60, ± 120, ± 180) and fitted with a logistic function (Fig. 2A): \(y=\frac{1}{1+{e}^{-\frac{\left(x-x0\right)}{b}}}\), where x0 is the FSD corresponding to the point of subjective isochronism (PSI; 50% response level on the psychometric function) and b provides an estimate of the rhythm discrimination threshold (DT; approximately half the offset between the 27% and 73% response levels). The PSI refers to the relative point in the FSD where participants were equally likely to classify the test rhythm as “accelerating” or “decelerating.” The DT was taken as a measure of participants’ sensitivity at judging the direction of tempo change. The PSI and DT values were obtained for all observers in all of the conditions, and were analyzed with the repeated-measures analysis of variance (ANOVA), respectively. Bonferroni post hoc tests were used if the ANOVA was significant. Moreover, to examine the robustness of statistical comparisons, we computed the 95% confidence interval (CI) for each comparison using a bootstrapping procedure based on 1,000 iterations. All tests were two-tailed.
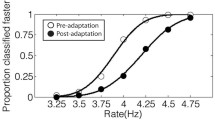
Results of Experiment 1. (A) Psychometric functions (averaged across 14 participants) showing the proportion of “accelerating” responses to the test rhythms, which was fitted with a logistic function of the difference between the first and second intervals (FSD) in each condition (BA: baseline without adaptation; AA: adapt to accelerating rhythm; AD: adapt to decelerating rhythm). (B) Points of subjective isochronism (PSIs) in the three conditions. Error bars represent standard errors in each condition. * p < 0.05, *** p < 0.001
Results and discussion
A repeated-measures ANOVA on the PSI showed that the main effect of adaptation was significant (F(2, 26) = 10.591, p < 0.001, ηp2 = 0.449; Figs. 2B and S2A). Bonferroni post hoc tests demonstrated that the PSI in the AD condition was significantly smaller than the PSIs in AA (p < 0.001, Cohen’s d = -1.409; 95% CI [−85.31, −42.40], p = 0.001) and BA (p = 0.017, Cohen’s d = -0.881; 95% CI [-90.55, −26.83], p = 0.021) conditions. However, there was no significant difference on the PSIs between AA and BA conditions (p = 1.000, Cohen’s d = 0.091; 95% CI [-24.42, 31.92], p = 0.755). Moreover, the repeated-measures ANOVA on the DT showed that the main effect of adaptation was not significant (F(2, 26) = 1.835, p = 0.180, ηp2 = 0.124), which suggests participants’ rhythm discrimination sensitivity was not affected by the rhythm adaptation. In sum, Experiment 1 found that only adaptation to the decelerating rhythm modulated subsequent rhythm perception. That is, after adaptation to a decelerating rhythm, participants tended to judge the subsequent isochronous rhythm as accelerating.
Experiment 2
The tempo change discrimination task used in Experiment 1 was based on the comparative judgment and could be confounded by the decision bias (Schneider & Komlos, 2008). It is unclear whether the adaptation effect observed in Experiment 1 originated at a perceptual level of information processing or at a decisional level. Therefore, in Experiment 2, we attempted to replicate the aftereffect from Experiment 1 using an equality judgment task – the rhythmic isochronism judgment task, which is less vulnerable to decision bias (Schneider & Komlos, 2008). In this task, participants were asked to judge whether the test rhythm was “isochronous” or “anisochronous.” If the aftereffect observed in Experiment 1 emerged from nondecisional processes, it should also be observed in the rhythmic isochronism judgment task.
Method
Participants
Sixteen volunteers (ten female; mean age: 18.4 ± 0.7 years) participated in Experiment 2. They were naïve to the purpose of the experiment. All but one of the participants were right-handed and they had normal or corrected-to-normal vision and normal hearing. All of them provided informed consent before the experiment and were paid for their time.
Apparatus, stimuli, and procedure
The apparatus, stimuli, and procedure were identical to those in Experiment 1 except that participants had to judge whether the test rhythm was“isochronous” or “anisochronous” once the test rhythm was disappeared.
Measurements
The proportion of “isochronous” responses to the test rhythms for each condition for each participant was plotted as a function of the FSD and fitted with a Gaussian function (Fig. 3A): \(y=a{e}^{-\frac{{\left(x-u\right)}^2}{2{\sigma}^2}}\), where u is the FSD corresponding to the point of subjective isochronism (PSI; the mean of the Gaussian distribution) and σ provides an estimate of the rhythm discrimination threshold (DT; the standard deviation of the Gaussian function). Here, the PSI refers to the relative point in the FSD where the test rhythm was most likely to be perceived as isochronous, and the DT was taken as a measure of participants’ sensitivity at judging isochronism. In this way, the PSI and DT values were obtained for all observers in all of the conditions.
Results of Experiment 2. (A) Psychometric functions (averaged across 16 participants) showing the proportion of “isochronous” responses to the test rhythms, which was fitted with a Gaussian function of the difference between the first and second intervals (FSD) in each condition (BA: baseline without adaptation; AA: adapt to accelerating rhythm; AD: adapt to decelerating rhythm). (B) Points of subjective isochronism (PSIs) in the three conditions. Error bars represent standard errors in each condition. * p < 0.05, ** p < 0.01
Results and discussion
As in Experiment 1, a repeated-measures ANOVA was performed on the PSI (Figs. 3B and S2B). The main effect of adaptation was significant (F(2, 30) = 8.063, p = 0.002, ηp2 = 0.350). Specifically, the PSI in the AD condition was significantly larger than the PSIs in AA (p = 0.031, Cohen’s d = 0.732; 95% CI [7.27, 35.14], p = 0.018) and BA (p = 0.004, Cohen’s d = 0.980; 95% CI [12.66, 34.57], p = 0.003) conditions. However, there was no significant difference on the PSIs between the AA and BA conditions (p = 1.000, Cohen’s d = 0.074; 95% CI [-9.78, 12.99], p = 0.771). We also found that the main effect of adaptation on the DT was significant (F(2, 30) = 8.356, p = 0.001, ηp2 = 0.358). That is, the DT in the AA condition was significantly larger than that in the BA condition (p = 0.004, Cohen’s d = 0.985; 95% CI [10.27, 31.67], p = 0.002). However, there were no significant differences on the DTs between the AA and AD conditions (p = 0.130, Cohen’s d = 0.552; 95% CI [1.55, 20.63], p = 0.059), and between the AD and BA conditions (p = 0.195, Cohen’s d = 0.498; 95% CI [0.36, 23.37], p = 0.100). These results provide further evidence of the aftereffect resulting from adaptation to the decelerating rhythm, suggesting it emerges on a perception level rather than on a decisional level.
Experiment 3
Previous studies have suggested that both the duration aftereffect and the rhythm aftereffect are sensory-specific, indicating there are independent timing mechanisms for different modalities (Becker & Rasmussen, 2007; Heron et al., 2012). Thus, it is possible that the aftereffect observed in Experiments 1 and 2 also involves the sensory-specific timing mechanism. To test this possibility, in Experiment 3 we presented auditory adapting rhythms and determined whether they would influence the perception of rhythm from another sense – visual rhythm.
Method
Participants
Participants were 16 new volunteers (ten female; mean age: 19.1 ± 0.9 years) who were naïve to the experimental conditions. All participants were right-handed and had normal or corrected-to-normal vision and normal hearing. All of them provided informed consent before the experiment and were paid for their time.
Apparatus, stimuli, and procedure
The apparatus, stimuli, and procedure of Experiment 3 were identical to those in Experiment 1, with the following exceptions. The test rhythms were presented visually. Specifically, the test rhythms were composed of a single white disc (0.8° × 0.8°, 20 ms) that blinked on and off three times and was located at 0.8° of the upper of the central fixation cross. Since studies have suggested the inferior visual (vs. auditory) performance in temporal processing (Goldstone & Lhamon, 1972, 1974; Grondin, Meilleur-Wells, Ouellette, & Macar, 1998; Ulrich, Nitschke, & Rammsayer, 2006), to match the difficulty of the visual rhythm perception to the auditory rhythm perception, we used seven visual test rhythms (first/second intervals: 400/640, 440/600, 480/560, 520/520, 560/480, 600/440, and 640/400 ms) with larger FSDs (0, ± 80, ± 160, ± 240) than those in Experiments 1 and 2. All visual rhythms were present on the CRT monitor (85-Hz refresh rate, 1,600 × 1,200 pixels) with a gray background.
Measurements
As in Experiment 1, for each participant, the proportion of “accelerating” responses to the test rhythms for each condition was plotted as a function of the FSD (0, ± 80, ± 160, ± 240) and fitted with the logistic function (Fig. 4A). The data from two participants in Experiment 3 were excluded from further analyses due to their improper performance according to the fitting coefficient (R2 < 0.6). The PSI and DT values were calculated for the remaining 14 participants for each condition.
Results of Experiment 3. (A) Psychometric functions (averaged across 14 participants) showing the proportion of “accelerating” responses to the visual test rhythms, which was fitted with the logistic function of the difference between the first and second intervals (FSD) in each condition (BA: baseline without adaptation; AA: adapt to accelerating rhythm; AD: adapt to decelerating rhythm). (B) Points of subjective isochronism (PSIs) in the three conditions. Error bars represent standard errors in each condition
Results and discussion
The results of the repeated-measures ANOVA showed that there were no significant main effects of adaptation on the PSI (F(2, 26) = 0.304, p = 0.740, ηp2 = 0.023; Figs. 4B and S2C) and DT (F(2, 26) = 0.991, p = 0.385, ηp2 = 0.071). To test whether the lack of the adaptation effect was due to the visual rhythm perception itself, we investigated the rhythm aftereffect within the visual modality in a control experiment (see the Online Supplementary Material (OSM) for detail). The results showed that adaptation to the visual rhythm modulated subsequent visual rhythm perception (Fig. S1, OSM). Moreover, a comparison across experiments showed that the rhythm aftereffect within the visual modality (control experiment) was comparable to that within the auditory modality (Experiment 1). This rules out the possibility that the lack of the rhythm aftereffect in Experiment 3 was due to the visual rhythm perception itself. These results demonstrated that the adaptation effect on subsequent rhythm perception could not transfer to an unadapted modality, suggesting the modality specificity of the rhythm aftereffect.
General discussion
In the present study, we investigated the effect of adaptation to the accelerating or decelerating rhythm on subsequent rhythm perception. We found a rhythm aftereffect specific to the decelerating rhythm adaptation. That is, after adaptation to the decelerating rhythm, participants tended to perceive subsequent isochronous rhythm as accelerating.
We observed the rhythm aftereffect not only in the direction of the tempo change judgment task, but also in the rhythmic isochronism judgment task. Previous research has suggested that comparative judgments tend to be influenced by decision biases, whereas equality judgments should not (Birngruber, Schröter, & Ulrich, 2014; Schneider & Komlos, 2008; Valsecchi, Vescovi, & Turatto, 2010). Accordingly, our results suggest the rhythm aftereffect is of perceptual origin. Moreover, the participants’ rhythm discrimination threshold (i.e., DT) was not significantly different between the decelerating rhythm adaptation and no-adaptation conditions. It further suggests that the rhythm aftereffect cannot result from the change in the task difficulty after adaptation.
Previous studies have investigated the isochronous rhythm adaptation and found a bi-directional negative rhythm aftereffect (Becker & Rasmussen, 2007; Levitan et al., 2015; Motala et al., 2018). It has been suggested that this rhythm aftereffect arises from the adaptation of the duration-tuned neurons: prolonged exposure to a fast (slow) train of beats (shorter (longer) interval between beats) diminished the response of neurons tuned to the fast (slow) beats, so that the overall output from the bank of duration-tuned neurons would be shifted towards the slow (fast) end of the spectrum, given the suppressed firing rate of the adapted neurons responsible for coding fast (slow) temporal duration (Becker & Rasmussen, 2007). This idea is identical to the duration channel-based model (Heron et al., 2012), and consistent with the neurophysiological findings, which show the sub-second duration-tuned neurons in the nervous systems of animals (Casseday et al., 1994; Duysens et al., 1996; Faure et al., 2003) and human beings (Harvey et al., 2020; Hayashi et al., 2015; Hayashi and Ivry, 2020; Protopapa et al., 2019). From this perspective, the perception of the rhythm is based on the perception of the interval between its component signals. Within this framework, the rhythm aftereffect observed here may result from the simultaneous adaptations of the intervals between beats of the anisochronous rhythm. This is conceivable given that previous studies have found that the duration aftereffect is contingent on the temporal order (Walker & Irion, 1979; Walker et al., 1981). In particular, our results demonstrated the rhythm aftereffect was only observed after adaptation to the decelerating rhythm, which is in line with previous studies that found the temporal order contingent duration aftereffect was only observed after the short-long (first short second long) inspections (Walker & Irion, 1979; Walker et al., 1981). This explanation is also consistent with a recent study suggesting temporal rate and duration are not a distinct perceptual metric (Motala et al., 2020). Our results offer further evidence that the duration and rhythm perceptions involve a common timing mechanism.
The present rhythm aftereffect is similar to the frequency-change aftereffect produced by adaptation to the unidirectional frequency change (Takuro, 2001). In the frequency-change aftereffect, adaptation to an upward or downward frequency change results in the subsequent constant frequency being perceived as if its frequency was changing in the opposite direction to the adapting sweeps. This aftereffect is assumed to be mediated by neural mechanisms sensitive to the frequency changes. Indeed, neurons responding selectively to a particular direction of frequency change have been identified in the auditory pathway of various animals (Britt & Starr, 1976; Tian & Rauschecker, 1994). According to these studies, it seems to be possible that the rhythm aftereffect observed in the present study could result from the adaptation of the neurons that are sensitive to frequency changes. However, we noted that the frequency-change aftereffect is bi-directional (i.e., both upward and downward adaptations induce the aftereffect), which is different from the present rhythm aftereffect (i.e., only adaptation to the decelerating rhythm induces the aftereffect). This implies different adaptation mechanisms are involved in these two aftereffects.
In the present study, we observed that participants tended to perceive the isochronous rhythm as the accelerating rhythm even in the no-adaptation condition when they were asked to judge the direction of tempo change of the test rhythm. This means that participants tended to perceive the second interval to be shorter than the first interval even when they were actually the same in duration. This suggests that the temporal order modulates the temporal perception. This is consistent with a general phenomenon known as time-order error (TOE) for duration, in which the duration seems shorter for the second stimulus than for the first one when they are short in duration (Allan, 1977; Hellström & Rammsayer, 2015). To some extent, the TOE could also explain why we did not observe the rhythm aftereffect after adaptation to the accelerating rhythm. That is, the TOE effect is contrary to the effect of the accelerating rhythm adaptation, and might have weakened it. Moreover, the temporal prediction may also play a critical role in rhythm perception (Morillon, Schroeder, Wyart, & Arnal, 2016). Within the Bayesian approach, the temporal performance is described with prior knowledge (Sadakata, Desain, & Honing, 2006; Shi, Church, & Meck, 2013). The rhythmic pattern extracted from corpora of musical scores has suggested that the frequency of occurrence of the accelerating rhythmic pattern is greater than that of the decelerating rhythmic pattern (Sadakata, Desain, & Honing, 2006). Utilizing this empirical prior, participants could be prone to perceiving the isochronous rhythm as accelerating rhythm. It is possible that when the adapting rhythm was consistent with the prior (accelerating rhythm), the temporal prediction effect from the prior on subsequent rhythm perception would be enhanced. And this effect might weaken the accelerating rhythm adaptation effect.
A fundamental question on temporal perception is whether the brain uses modality-specific timing mechanisms or a unified timing mechanism. Using temporal aftereffects, previous studies have widely explored this question. However, the debates remained. Although most studies on duration and rhythm aftereffects have suggested modality-specific timing mechanisms (Becker & Rasmussen, 2007; Heron et al., 2012; Li et al., 2015; Motala et al., 2018), there were studies indicating a unified timing mechanism (Levitan et al., 2015; Zhang, Chen, & Zhou, 2012). Here, we found that the auditory rhythm aftereffect induced by the decelerating rhythm adaptation did not generalize to a visual modality. This result is consistent with a previous study on temporal order contingent duration aftereffect, which is modality-specific(Walker et al., 1981). It supports the modality-specific timing mechanisms, instead of the amodal timing mechanism or the idea that the perception of visual rhythms is recoded into an auditory signal (Barakat, Seitz, & Shams, 2015; Guttman, Gilroy, & Blake, 2005).
It has been suggested that the interval between two taps in the spontaneous tempo, measured by the natural speed of tapping, varies from 380 to 880 ms, and the interval in the preferred tempo (i.e., a regular succession judged as being neither too slow nor too fast) is close to 500 ms (Fraisse, 1982). Accordingly, in the present study, we picked tempos whose intervals are close to the intervals in the spontaneous tempo and preferred tempo. Although tempos used here are representative, it’s worth thinking about whether the rhythm aftereffect could be observed with faster or slower tempos. Previous studies have found that duration adaptation occurred not only for sub-second durations, but also for supra-second durations and across sub- and supra-second durations (Li, Xiao, Yin, Liu, & Huang, 2017; Shima, Murai, Hashimoto, & Yotsumoto, 2016). Given that the present study implies a unified timing mechanism for duration and rhythm perception, it is possible that similar rhythm aftereffects will be found with faster or slower tempos. This hypothesis should be addressed in the future.
In sum, we have demonstrated that adaptation to a decelerating rhythm induced a rhythm aftereffect, which is modality-specific. This aftereffect is consistent with the temporal order contingent duration aftereffect. The present results offer further evidence that rhythm perception is the perceptual aggregate of its component duration intervals, and contribute to our understanding of rhythm perception.
References
Allan, L. G. (1977). The time-order error in judgments of duration. Canadian Journal of Psychology, 31(1), 24-31.
Barakat, B., Seitz, A. R., & Shams, L. (2015). Visual rhythm perception improves through auditory but not visual training. Current Biology, 25(2), R60-R61.
Becker, M. W., & Rasmussen, I. P. (2007). The rhythm aftereffect: support for time sensitive neurons with broad overlapping tuning curves. Brain and Cognition, 64(3), 274-281.
Binetti, N., Lecce, F., & Doricchi, F. (2012). Time-dilation and time-contraction in an anisochronous and anisometric visual scenery. Journal of Vision, 12(7), 8, 1-19.
Birngruber, T., Schröter, H., & Ulrich, R. (2014). Duration perception of visual and auditory oddball stimuli: Does judgment task modulate the temporal oddball effect? Attention, Perception, & Psychophysics, 76, 814-828.
Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10(4), 433-436.
Britt, R., & Starr, A. (1976). Synaptic events and discharge patterns of cochlear nucleus cells. II. Frequency-modulated tones. Journal of Neurophysiology, 39(1), 179-194.
Casseday, J., Ehrlich, D., & Covey, E. (1994). Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science, 264(5160), 847-850.
Duysens, J., Schaafsma, S., & Orban, G. (1996). Cortical off response tuning for stimulus duration. Vision Research, 36(20), 3243-3251.
Eagleman, D. M. (2008). Human time perception and its illusions. Current Opinion in Neurobiology, 18(2), 131-136.
Faure, P. A., Fremouw, T., Casseday, J. H., & Covey, E. (2003). Temporal masking reveals properties of sound-evoked inhibition in duration-tuned neurons of the inferior colliculus. Journal of Neuroscience, 23(7), 3052-3065.
Flaugnacco, E., Lopez, L., Terribili, C., Zoia, S., Buda, S., Tilli, S., . . . Schön, D. (2014). Rhythm perception and production predict reading abilities in developmental dyslexia. Frontiers in Human Neuroscience, 8, 392.
Fraisse, P. (1982). Rhythm and tempo. In D. Deutsch (Ed.), The Psychology of Music (pp. 149–180). New York, NY: Academic Press.
Goldstone, S., & Lhamon, W. T. (1972). Auditory-visual differences in human temporal judgment. Perceptual and Motor Skills, 34(2), 623-633.
Goldstone, S., & Lhamon, W. T. (1974). Studies of auditory-visual differences in human time judgment: 1. Sounds are judged longer than lights. Perceptual and Motor Skills, 39(1), 63-82.
Grondin, S., Meilleur-Wells, G., Ouellette, C., & Macar, F. (1998). Sensory effects on judgments of short time-intervals. Psychological Research, 61(4), 261-268.
Grube, M., Cooper, F. E., Chinnery, P. F., & Griffiths, T. D. (2010). Dissociation of duration-based and beat-based auditory timing in cerebellar degeneration. Proceedings of the National Academy of Sciences of the United States of America, 107(25), 11597-11601.
Guttman, S. E., Gilroy, L. A., & Blake, R. (2005). Hearing what the eyes see: auditory encoding of visual temporal sequences. Psychological Science, 16(3), 228-235.
Hayashi, M. J., Ditye, T., Harada, T., Hashiguchi, M., Sadato, N., Carlson, S., . . . Kanai, R. (2015). Time adaptation shows duration selectivity in the human parietal cortex. PLoS Biology, 13(9), e1002262.
Hayashi, M. J., & Ivry, R. B. (2020). Duration selectivity in right parietal cortex reflects the subjective experience of time. Journal of Neuroscience, 40(40), 7749-7758.
Harvey, B. M., Dumoulin, S. O., Fracasso, A., & Paul, J. M. (2020). A network of topographic maps in human association cortex hierarchically transforms visual timing-selective responses. Current Biology, 30(8), 1424-1434.
Hellström, Å., & Rammsayer, T. H. (2015). Time-order errors and standard-position effects in duration discrimination: an experimental study and an analysis by the sensation-weighting model. Attention, Perception, & Psychophysics, 77(7), 2409-2423.
Heron, J., Aaen-Stockdale, C., Hotchkiss, J., Roach, N. W., McGraw, P. V., & Whitaker, D. (2012). Duration channels mediate human time perception. Proceedings of the Royal Society B: Biological Sciences, 279(1729), 690-698.
Horr, N. K., & Di Luca, M. (2015). Filling the blanks in temporal intervals: the type of filling influences perceived duration and discrimination performance. Frontiers in Psychology, 6, 114.
Johnston, A., Arnold, D. H., & Nishida, S. (2006). Spatially localized distortions of event time. Current Biology, 16(5), 472-479.
Kraus, N., & Chandrasekaran, B. (2010). Music training for the development of auditory skills. Nature Reviews Neuroscience, 11(8), 599-605.
Levitan, C. A., Ban, Y. H., Stiles, N. R., & Shimojo, S. (2015). Rate perception adapts across the senses: evidence for a unified timing mechanism. Scientific Reports, 5, 8857.
Li, B., Xiao, L., Yin, H., Liu, P., & Huang, X. (2017) Duration aftereffect depends on the duration of adaptation. Frontiers in Psychology, 8, 491.
Li, B., Yuan, X., & Huang, X. (2015). The aftereffect of perceived duration is contingent on auditory frequency but not visual orientation. Scientific Reports, 5, 10124.
Matthews, W. J. (2011). How do changes in speed affect the perception of duration? Journal of Experimental Psychology: Human Perception and Performance, 37(5), 1617-1627.
Matthews, W. J. (2013). How does sequence structure affect the judgment of time? Exploring a weighted sum of segments model. Cognitive Psychology, 66, 259-283.
Morillon, B., Schroeder, C. E., Wyart, V., & Arnal, L. H. (2016). Temporal prediction in lieu of periodic stimulation. Journal of Neuroscience, 36(8), 2342-2347.
Motala, A., Heron, J., McGraw, P. V., Roach, N. W., & Whitaker, D. (2018). Rate after-effects fail to transfer cross-modally: evidence for distributed sensory timing mechanisms. Scientific Reports, 8, 924.
Motala, A., Heron, J., McGraw, P. V., Roach, N. W., & Whitaker, D. (2020). Temporal rate is not a distinct perceptual metric. Scientific Reports, 10, 8654.
Pariyadath, V., & Eagleman, D. (2007). The effect of predictability on subjective duration. Plos One, 11, e1264.
Pelli, D. G. (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision, 10(4), 437-442.
Protopapa, F., Hayashi, M. J., Kulashekhar, S., van der Zwaag, W., Battistella, G., Murray, M. M., . . . Bueti, D. (2019). Chronotopic maps in human supplementary motor area. PLoS Biology, 17(3), e3000026.
Sadakata, M., Desain, P., & Honing, H. (2006). The Bayesian way to relate rhythm perception and production. Music Perception, 23(3), 269-288.
Schneider, K. A., & Komlos, M. (2008). Attention biases decisions but does not alter appearance. Journal of Vision, 8(15), 3, 1-10.
Shi, Z., Church, R. M., & Meck, W. H. (2013). Bayesian optimization of time perception. Trends in Cognitive Sciences, 17(11), 556-564.
Shima, S., Murai, Y., Hashimoto, Y., & Yotsumoto, Y. (2016). Duration adaptation occurs across the sub- and supra-second systems. Frontiers in Psychology, 7, 114.
Takuro, K. (2001). Aftereffect of adaptation to uni-directional frequency change: evidence for selective processing mechanism. Acoustical science technology, 22(1), 49-51.
Teki, S., Grube, M., Kumar, S., & Griffiths, T. D. (2011). Distinct neural substrates of duration-based and beat-based auditory timing. Journal of Neuroscience, 31(10), 3805-3812.
Thaut, M. H., McIntosh, K. W., McIntosh, G. C., & Hoemberg, V. (2001). Auditory rhythmicity enhances movement and speech motor control in patients with Parkinson's disease. Functional Neurology, 16(2), 163-172.
Tian, B., & Rauschecker, J. P. (1994). Processing of frequency-modulated sounds in the cat's anterior auditory field. Journal of Neurophysiology, 71(5), 1959-1975.
Ulrich, R., Nitschke, J., & Rammsayer, T. (2006). Crossmodal temporal discrimination: assessing the predictions of a general pacemaker-counter model. Perception & Psychophysics, 68(7), 1140-1152.
Valsecchi, M., Vescovi, M., & Turatto, M. (2010). Are the effects of attention on speed judgments genuinely perceptual? Attention, Perception, & Psychophysics, 72(3), 637-650.
Walker, J. T., & Irion, A. L. (1979). Two new contingent aftereffects: perceived auditory duration contingent on pitch and on temporal order. Perception & Psychophysics, 26(3), 241-244.
Walker, J. T., Irion, A. L., & Gordon, D. G. (1981). Simple and contingent aftereffects of perceived duration in vision and audition. Perception & Psychophysics, 29(5), 475-486.
Zhang, H., Chen, L., & Zhou, X. (2012). Adaptation to visual or auditory time intervals modulates the perception of visual apparent motion. Frontiers in Integrative Neuroscience, 6, 100.
Funding
This work was supported by the National Natural Science Foundation of China (Grant numbers 32000744, 31671125) and Fundamental Research Funds for the Central Universities (Grant number GK202003095).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethics approval and informed consent
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the local ethics committee of the Shaanxi Normal University of China. Informed consent was obtained from all individual participants included in the study.
Additional information
Open practices statement
None of the experiments was preregistered, but all the data and materials are available from the corresponding author on reasonable request.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 288 kb)
Rights and permissions
About this article
Cite this article
Li, B., Wang, K. & Chen, L. The rhythm aftereffect induced by adaptation to the decelerating rhythm. Psychon Bull Rev 29, 467–475 (2022). https://doi.org/10.3758/s13423-021-02014-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-021-02014-8