Abstract
Retroactive cue interference refers to situations in which a target cue X is paired with an outcome in phase 1 and a nontarget cue Z is paired with the same outcome in phase 2, with less subsequent responding to X being seen as a result of the phase 2 training. Two conditioned suppression experiments with rats were conducted to determine whether retroactive cue interference is similarly modulated by a manipulation that influences retroactive outcome interference (e.g., extinction). Both experiments used an ABC renewal-like design in which phase 1 training, phase 2 training, and testing each occurred in different contexts. Experiment 1 found that training the target association in multiple contexts without altering the number of training trials during phase 1 decreased retroactive cue interference (i.e., increased responding consistent with the target association). Experiment 2 found that training the interfering association in multiple contexts without altering the number of interference trials during phase 2 increased retroactive cue interference (i.e., decreased responding consistent with the target association). The possibility of similar mechanisms underlying cue interference and outcome interference is discussed.
Similar content being viewed by others
Throughout their lives, organisms learn many relationships between events. In some cases, an organism may learn that a particular event X is associated with event P in one situation and event Q in a second situation. Within the associative literature, numerous reports have shown that such circumstances lead to what is commonly called associative interference. Associative interference refers to reduced control of behavior by a given cue when (1) two different outcomes have followed that cue at different times, which is known as associative outcome interference (e.g., latent inhibition [Lubow & Moore, 1959], extinction, and counterconditioning [Pavlov, 1927]), or (2) when a given target cue has been followed by an outcome that, at another time, has been preceded by a nontarget cue, which is known as associative cue interference (e.g., Matute & Pineño, 1998).
Associative interference results from situations in which two competing associations have a common element—specifically, a common subsequent event (i.e., the outcome) in the case of cue interference (i.e., X–O and Y–O, where X and Y are cues and O is an outcome) or a common antecedent event (i.e., the cue) in the case of outcome interference (i.e., X–O1 and X–O2, where X is a cue and O1 and O2 are two different outcomes)—and it is most commonly seen when the two types of training trials are phasic (i.e., blocked) rather than interspersed. Outcome interference, usually in the form of acquisition followed by extinction, has been given far more attention in the learning literature than has cue interference. Both types of interference can be further characterized as proactive or retroactive according to which association is targeted for testing. For example, in cue interference, if the pairings of the interfering cue with the common outcome occur before the target pairings, the interference is defined as proactive. If the pairings of the interfering cue with the common outcome come after the target pairings, the interference is defined as retroactive. Our interest in conducting the present experiments was cue interference, and the present experiments were cast in a retroactive interference design.
A small number of models have been developed in a Pavlovian framework to account for the reduction of acquired behavior observed when interacting associations are trained apart (i.e., associative interference), in contrast to interacting associations that are trained together, which ordinarily result in what is commonly called cue or outcome competition. One such model is Bouton’s (1993) model of associative interference, which was developed to account for interference between outcomes trained in successive phases. Bouton posited that both associations (e.g., X–O1 followed by X–O2) are learned and that each potentially interferes with retrieval of the other, thereby producing the observed behavioral deficit. Moreover, when the two associations are acquired in different contexts, the association acquired in the context most similar to the test context is the association most apt to be retrieved.
Recently, Miller and Laborda (2011; Laborda & Miller, 2012) provided an account of whether the target or interfering association will be expressed depending not only on the relative efficacies of their respective retrieval cues (modulated by test context similarity), but also on the relative strengths of the two associations. While considerable attention has been paid to interference between outcomes, much less attention has been paid to developing accounts of interference between cues. Bouton’s (1993) account of outcome interference does not lend itself to explaining cue interference without some revision. Miller and Escobar (2002) proposed a modification of Bouton’s model that anticipates parallel effects between outcome interference and cue interference (for precursors of this account, see Escobar, Arcediano, & Miller, 2001a; Escobar, Matute, & Miller, 2001b; Pineño, Ortega, & Matute, 2000). In short, the model encompasses two independent mechanisms, both of which are always in force; but specific procedures can favor the expression of one to a greater degree than the other. One mechanism is that of the comparator hypothesis (Miller & Matzel, 1989; Stout & Miller, 2007), which relies on a form of associative competition that modulates the expression of the target-cue-outcome association as a result of the associative status of other cues that are associated with the target cue. Hence, this mechanism is activated only when the competing cues are linked by a within-compound association (i.e., are trained in compound, as in overshadowing and blocking). The second mechanism is based on differential facilitation by the test context of retrieval of two different cue–outcome associations with one element in common. To maximize such differential facilitation, the two interacting associations need to be trained apart from one another (e.g., in different contexts and/or at different times). Clearly, it is the latter mechanism that should prevail in the present experiments, because X and Y were trained independently and had the same outcome. Important for the present research, the Miller and Escobar model accounts for associative interference in terms of the test context not only facilitating the retrieval of associations acquired in that context, but also down-modulating (i.e., inhibiting) the retrieval of all other associations with one, but not both, elements in common with the positively primed associations (a process similar to that applied to the retrieval-induced forgetting phenomenon of Anderson, Bjork, & Bjork [1994], in which retrieval of the subsequent event is impaired, and Ortega-Castro & Vadillo [2013], in which retrieval of the antecedent event is impaired).
Regardless of the specific details and mechanisms of each account of interference, each model attempts to explain the recovery from interference that is observed as a result of select manipulations. Recovery from retroactive outcome interference is exemplified by spontaneous recovery from extinction (i.e., the recovery of responding to an extinguished cue after a long retention interval; e.g., Brooks & Bouton, 1993; Pavlov, 1927) and renewal (i.e., the recovery of responding to an extinguished cue when it is tested outside of the extinction context; e.g., Bouton & Bolles, 1979). Renewal after retroactive outcome interference is typically reported when the first outcome involves the formation of an excitatory association (e.g., Bouton & Bolles, 1979; Bouton & King, 1983; Bouton & Ricker, 1994). However, Nelson (2002) reported renewal-like recovery from retroactive outcome interference when phase 1 target training consisted of inhibitory training of the target cue and the phase 2 training consisted of excitatory training of the target cue.
Similar to renewal following outcome interference, renewal-like context specificity of retroactive cue interference has also been reported in human causal learning (Matute & Pineño, 1998, Experiment 3). Moreover, Miguez, Cham, and Miller (2012) provided evidence of renewal-like context specificity of (and spontaneous recovery from) retroactive cue interference in a fear-conditioning paradigm with rats as subjects, which parallels the observations of these types of recovery in the associative outcome interference literature. Specifically, they observed spontaneous recovery from retroactive cue interference after a 21-day retention interval, relative to the low responding to the target cue observed when testing occurred after a 1-day retention interval (see also Escobar et al., 2001b; Luque, Morís, & Cobos, 2010; Pineño et al., 2000).
More central to the focus of the present research, Miguez et al. (2012) also reported renewal-like context specificity of retroactive cue interference in the form of greater responding to the target cue when it was tested in an irrelevant context (which did not prime retrieval of either the target association or the interfering association), relative to testing the target cue in the context where the interfering association was trained (i.e., a form of ABC renewal, where the first letter denotes the context in which the target association was trained during phase 1, the second letter denotes the context in which the interfering association was trained during phase 2, and the third letter denotes the context in which testing occurred; see also Escobar et al., 2001b). These prior findings indicate the generality of recovery effects following both retroactive outcome interference and retroactive cue interference. Moreover, they suggest that the processes responsible for associative cue interference may be the same as those responsible for associative outcome interference. Further evidence bearing on this tentative conclusion would come from examination of the consequences of other manipulations on the two types of interference. Similar consequences would lend support, whereas divergent consequences would argue against similar underlying processes.
One manipulation that has been examined in detail in associative outcome interference is training in multiple contexts. Specifically, training the interfering association in multiple contexts has been found to prevent recovery (i.e., renewal) from associative outcome interference (Chelonis, Calton, Hart, & Schachtman, 1999; Glautier & Elgueta, 2009; Glautier, Elgueta, & Nelson, 2013; Gunther, Denniston, & Miller, 1998; Laborda & Miller, 2013; Neumann, 2006; Pineño & Miller, 2004; Vansteenwegen et al., 2007; but for negative results that suggest that the effect, like many behavioral effects, is parameter dependent, see Betancourt et al., 2008; Bouton, García-Gutiérrez, Zilski, & Moody, 2006; Neumann, Lipp, & Cory, 2007). Moreover, training the target association in multiple contexts has been found to enhance recovery (i.e., renewal) from retroactive outcome interference (e.g., Gunther et al., 1998). These observations come from a variety of preparations and species. For example, enhancement of retroactive outcome interference has been reported as a result of training the interfering association in multiple contexts in conditioned taste aversion in rats (Chelonis et al., 1999), fear conditioning in rats (Gunther et al., 1998; Laborda & Miller, 2013), fear conditioning in humans (Neumann, 2006; Vansteenwegen et al., 2007), and contingency tasks in humans (Glautier & Elgueta, 2009; Glautier et al., 2013; Pineño & Miller, 2004). However, to date, there has been no assessment of the effect of training in multiple contexts on associative cue interference.
On the basis of the observations concerning the effect of training in multiple contexts on outcome interference, if renewal of retroactive (or proactive) cue interference arises from processes similar to those that produce renewal following retroactive (or proactive) outcome interference (e.g., extinction), then two predictions follow: (1) Training the target association in multiple contexts should reduce interference when the target cue is tested in a neutral context, and (2) training the interfering association in multiple contexts should enhance interference when the target cue is tested in a neutral context. Testing these predictions is not meant to differentiate among accounts of cue interference, because all contemporary models of associative interference assume that the attenuation in behavioral control produced by interference between associations trained in different phases is due to differential priming (for retrieval) of the target and interfering associations at the moment of testing (e.g., Miller & Escobar, 2002). Rather, our question is empirical: Is cue interference influenced by training in multiple contexts in the same way as is outcome interference?
Using a lick suppression procedure with rats, the present series of experiments was designed to determine whether training the target association in multiple contexts would increase responding consistent with the target association following retroactive cue interference treatment (Experiment 1) and whether training the interfering association in multiple contexts would decrease responding consistent with the target association following retroactive cue interference treatment (Experiment 2). Renewal following cue interference has already been reported (e.g., Miguez et al., 2012), so our goal was not to demonstrate renewal in cue interference per se, and, in fact, we did not include a control to assess renewal. Rather, we used a renewal-like procedure (without a renewal control condition) to assess whether training in multiple contexts modulated cue interference in a manner similar to that seen in outcome interference. In both experiments, we used a sensory preconditioning procedure, because retroactive cue interference is severely attenuated if the target cue acquires high biological significance in phase 1 (i.e., prior to the interference treatment; Escobar et al., 2001b), which appears to be an instance of the general difficulty of attenuating through indirect means the response to a cue that has already acquired the potential to elicit a vigorous response (e.g., Denniston, Miller, & Matute, 1996).
Experiment 1: training the target association in multiple contexts
Experiment 1 was designed to determine whether training a target association in multiple contexts (relative to target training entirely in one context, with the total amount of target training being held constant) would decrease retroactive cue interference, as evidenced by stronger responding based on the target association following target and subsequent interference treatment. On the basis of previously described reports of reduced outcome interference as a result of target training occurring in multiple contexts and the seeming similarity of cue interference to outcome interference, training the target association in multiple contexts was expected to facilitate retrieval of that association at test (i.e., less interference was expected).
Experiment 1 (see Table 1) used a 2 (phase 1: ABC vs. [ADE]BC) × 2 (phase 2 training: pair vs. unpair) between-subjects factorial design. Subjects in Condition ABC received three pairings of the target cue (X) with the outcome (O) in context A during phase 1. Subjects in Condition [ADE]BC received one X–O pairing in each of contexts A, D, and E during phase 1. Orthogonal to phase 1 treatment, in phase 2, subjects in Condition Pair received four pairings of the interfering conditioned stimulus (CS) (Z) with the outcome (i.e., Z–O) in context B, whereas subjects in Condition Unpair received four unpaired presentations of Z and O in context B. During phase 3, all subjects received four O–unconditioned-stimulus (US) pairings in context C in order to provide a motivational basis for responding based on the X–O association. At test, all subjects were tested on X and on O in context C.
We expected to see relatively high responding to X in both groups within Condition Unpair, because these subjects had no training with a potentially interfering association (Z–O). Additionally, we expected lower responding in Group ABC–Pair relative to Group ABC–Unpair, because the former group had been trained on an interfering association, whereas the latter group had not. Critically, if cue interference is reduced by training the target association in multiple contexts, we would expect more behavioral control (e.g., greater suppression) in Group [ADE]BC–Pair than in Group ABC–Pair. The last anticipated difference, assuming that it is observed, could potentially be explained in terms of associative competition between X and its training context. That is, lower conditioned suppression in Group ABC–Pair could result from greater Context A–O associative strength in Group ABC–Pair (as compared with Group [ADE]BC–Pair) overshadowing the X–O association during phase 1, relative to each phase 1 training context overshadowing the X–O association in Group [ADE]BC–Pair. However, this potential difference in overshadowing would also be expected to result in greater conditioned suppression by Group [ADE]BC–Unpair relative to Group ABC–Unpair. That is, this mechanism anticipates a main effect of number of phase 1 training contexts rather than an interaction. A second mechanism potentially able to account for the expected difference between Groups ABC–Pair and [ADE]BC–Pair is based on the facilitation of priming of the target association (X–O) at test that could result in Group [ADE]BC–Pair from greater generalization of the context(s) of the target training phase to the test context. That is, the target X–O association might be more readily retrieved in the test context by Group [ADE]BC–Pair due to more total generalization of facilitated retrieval in the test context as a consequence of target training in multiple contexts. Thus, the priming mechanism account would be supported by an interaction between phase 1 (ABC vs. [ADE]BC) and phase 2 (pair vs. unpair) training.
Method
Subjects
The subjects were 36 male and 36 female, experimentally naive, Sprague Dawley descended rats obtained from our own breeding colony. Body weight ranges were 174–347 g for females and 191–333 g for males. Subjects were randomly assigned to one of four groups (ABC–Pair, [ADE]BC–Pair, ABC–Unpair, and [ADE]BC–Unpair), counterbalanced within groups for sex. A larger group size was used for Condition Pair (each n = 24) than for Condition Unpair (each n = 12), because Condition Unpair was a control condition in which, on the basis of pilot data, relatively strong conditioned suppression was expected in all subjects. The animals were individually housed in standard hanging stainless steel wire mesh cages in a vivarium maintained on a 16:8-h light:dark cycle. Experimental manipulations occurred near the middle portion of the light phase. The animals received free access to Purina Lab Chow, whereas water availability was limited to 20 min per day following a progressive deprivation schedule initiated 1 week prior to the start of the experiment. From the time of weaning until the start of the study, all animals were handled for 30 s, three times per week.
Apparatus
Six identical copies of Chamber V, 6 of Chamber R, 12 of Chamber Modified R, 12 of Chamber O, and 12 of Chamber Modified O were used. Chamber V was a 27-cm-long box in a truncated-V shape (29.5-cm height, 21.5 cm wide at top, and 5.5 cm wide at bottom). The floor was made up of two 27-cm-long, 2-cm-wide stainless steel plates, with a 1.5-cm gap between the two plates. The ceiling was clear Plexiglas, the front and back walls were black Plexiglas, and the sidewalls were stainless steel. Each copy of Chamber V was housed in a separate sound- and light-attenuating environmental isolation chest. Each chamber was illuminated by a 7-W (nominal at 120 VAC but driven at 50 VAC) light bulb, which was mounted on the inside wall of the environmental enclosure approximately 30 cm from the center of the experimental chamber. The light entered the chamber primarily by reflection from the ceiling of the environmental chest.
Chamber R was rectangular, measuring 24.0 × 9.0 × 12.5 cm (l × w × h). The walls and ceiling of Chamber R were clear Plexiglas, and the floor was made up of stainless steel rods measuring 0.5 cm in diameter, spaced 1.3 cm apart (center to center). Each copy of Chamber R was housed in a separate light- and sound-attenuating environmental isolation chamber. Each chamber was dimly illuminated by a 2-W (nominal at 120 VAC but driven at 50 VAC) incandescent house light mounted on an inside wall of the environmental chest located approximately 30 cm from the animal enclosure. Chamber Modified R was identical to Chamber R, except for four modifications. The four modifications were the following: (1) A different instance of Chamber R was used, (2) a clear Plexiglas floor was placed over the grid floor, (3) the house light was off, and (4) a daily drop of 98 % methyl salicylate was placed onto a small block of wood located inside the isolation chest.
Chamber O was a rectangular enclosure measuring 30.5 × 27.5 × 27.3 cm (l × w × h). The side walls of each chamber were made of stainless steel, and the front and back walls and ceiling of each chamber were made of clear Plexiglas. Each instance of Chamber O was housed in a separate environmental chest and was dimly illuminated by a house light (1.12-W, #1820 incandescent bulb) mounted on a wall of the experimental chamber. The floor was constructed of 0.3-cm diameter stainless steel rods, spaced 1.7 cm center-to-center and connected by NE-2 neon bulbs that allowed a 0.5-s, 0.6-mA constant-current footshock to be delivered by means of a high voltage AC circuit in series with a 1.0-MΩ resistor. Chamber O could be equipped with a water-filled lick tube that extended 1 cm into a cylindrical niche, which was 4.5 cm in diameter and was left–right centered, with its bottom 1.75 cm above the floor of the apparatus and 5.0 cm deep. There was a photobeam detector 1 cm in front of the lick tube that was broken whenever the subject licked the tube. Chamber Modified O was Chamber O with five modifications: (1) A different instance of Chamber O was used, (2) a clear Plexiglas floor was placed over the grid floor, (3) the house light was off, (4) a daily drop of lemon extract was placed onto a small block of wood located inside the isolation chest, and (5) no lick tube was available.
Three 45-Ω speakers on the inside walls of each isolation chest could deliver a click train (6 Hz) 6 dB above background (all auditory cues were measured on the C-scale), a complex tone (500 Hz + 520 Hz) 6 dB above background, and a white noise 6 dB above background. Ventilation fans in each enclosure provided a constant 76-dB background noise. The light intensities inside the two illuminated chambers were approximately equal, due to the difference in opaqueness of the walls of Chambers V and R.
CS X was the click train. CS Z was the complex tone. The outcome (O) was the white noise. Each of these cues was 5 s in duration, except during testing. The footshock served as the US. Contexts A, D, and E were Chamber R, Chamber V, and Chamber Modified R, counterbalanced within groups; context B was Chamber Modified O; context C was Chamber O.
Procedure
Acclimation
On day 1, all subjects were acclimated to contexts A, B, C, D, and E for 30 min each, with free access to the water-filled lick tubes only in context C. There were no presentations of X, Z, O, or US during this phase. At the end of acclimation, the water tubes were removed from context C until reacclimation.
Phase 1
On days 2–4, Condition ABC (i.e., Groups ABC–Pair and ABC–Unpair) received one daily X–O (i.e., click–white noise) pairing in context A, at 30 min into a 60-min session. On each of these trials, the onset of the outcome immediately followed the termination of X. Additionally, these two groups received 60 min of exposure daily to context D and context E. Condition [ADE]BC (i.e., Groups [ADE]BC–Pair and [ADE]BC–Unpair) received one X–O pairing in context A on day 4, one X–O pairing in context D on day 5, and one X–O pairing in context E on day 6, at 30 min into a daily 60-min session. On each of these 3 days, these two groups were also placed in the other two contexts for 60 min each. Hence, all groups received identical exposure and distribution of exposure to contexts A, D, and E. The daily order of training and context exposure sessions was equated across all groups.
Phase 2
On day 5, Condition Pair was presented with four Z–O (i.e., tone–white noise) pairings in context B, starting at 12, 24, 36, and 48 min into a 60-min session. On each of these trials, the onset of the outcome immediately followed the termination of Z. Condition Unpair received equivalent unpaired presentations of Z and O. Presentations of Z occurred at 12, 24, 36, and 48 min, whereas presentations of O occurred at 6, 18, 42, and 55 min into the 60-min session.
Phase 3
On day 6, all subjects received four O–US (i.e., white noise–footshock) pairings in context C, starting at 12, 24, 36, and 48 min into a 60-min session. On each of these trials, O and the US coterminated.
Reacclimation
On days 7 and 8, all subjects were reacclimated to context C in one daily 90-min session. Subjects had free access to the water-filled lick tubes, and no nominal stimuli were programmed to occur. These sessions were intended to restore baseline drinking that might have been disrupted by the footshock in phase 3.
Test
On day 9, all subjects were tested for conditioned lick suppression to X in context C. Upon placement of each subject in its test chamber, time spent drinking was recorded. Immediately after completion of an initial 5 cumulative seconds of licking in the absence of any nominal stimulus, subjects were presented with CS X. Thus, all subjects were drinking at the time of CS onset. Time to complete an additional 5 cumulative seconds of licking in the presence of CS X was recorded. The times recorded during the presentation of X were interpreted as reflecting subjects’ expectancy of the US following onset of the CS. A ceiling score of 9 min was imposed on the time to complete 5 cumulative seconds of drinking in the presence of X. Session duration was 10 min. Following the convention of our laboratory, all animals that took more than 60 s to complete their first 5 cumulative seconds of licking (i.e., prior to CS onset) during the test session were scheduled to be eliminated from the study, because such long latencies were considered indicative of an unusually great fear of the test context. In actuality, no rat from Experiment 1 or Experiment 2 met this elimination criterion. On day 10, testing on O occurred for all groups in context C in the same manner in which X had been tested on day 9.
Results and discussion
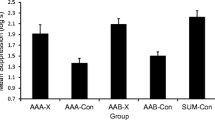
The results of testing on CS X in Experiment 1 are depicted in Fig. 1, and they support the hypothesis that training the target association in multiple contexts reduces retroactive cue interference. The robust test trial responding based on the target association (X–O) observed in Group [ADE]BC–Pair following retroactive cue interference treatment parallels the reduced retroactive outcome interference that is seen when the target association is trained in multiple contexts (e.g., Gunther et al., 1998, Experiment 2).
Results of Experiment 1. Mean times to complete 5 cumulative seconds of drinking in the presence of the target CS X. Therefore, 0.7 log s is the lowest possible score. Higher scores represent stronger responding to the target CS. Error bars represent standard errors of the means. See the text and Table 1 for details
All latencies were log (base 10) transformed to better normalize the distribution of the scores within groups. A 2 (ABC vs. [ADE]BC) × 2 (pair vs. unpair) analysis of variance (ANOVA) was conducted on the day 9 pre-CS scores (i.e., latencies to complete 5 cumulative seconds of drinking prior to CS onset). This analysis revealed no differences among groups, all ps > .32, which indicates similar baseline drinking across groups (ABC–Pair, M = 1.18; ABC–Unpair, M = 1.22; [ADE]BC–Pair, M = 1.17; [ADE]BC–Unpair, M = 1.22 log s). An analogous ANOVA performed on the day 9 suppression scores collected during presentation of CS X (i.e., latencies to complete 5 cumulative seconds of drinking in the presence of the CS) revealed an interaction, F(1, 68) = 4.54, p = .03, Cohen’s f = .25 (95 % CI = 0.00; 0.49), MSE = .102. A similar 2 × 2 ANOVA on the latencies to complete 5 cumulative seconds of drinking in the presence of O on day 10 found no significant main effects or interactions in either pre-CS scores (ABC–Pair, M = 1.32; ABC–Unpair, M = 1.32; [ADE]BC–Pair, M = 1.29; [ADE]BC–Unpair, M = 1.32 log s) or CS scores (ABC–Pair, M = 1.65; ABC–Unpair, M = 1.63; [ADE]BC–Pair, M = 1.59; [ADE]BC–Unpair, M = 1.65 log s), all ps > .60. The lack of a significant difference in the CS scores suggests that O was similarly excitatory for all four groups.
In order to test specific hypotheses and to identify the source of the day 9 interaction, planned comparisons were conducted using the error term from the ANOVA on suppression to CS X. Group ABC–Pair exhibited less suppression than did Group ABC–Unpair, F(1, 68) = 16.86, p < .01, Cohen’s f = .51 (95 % CI = 0.26; 0.76), evidencing the presence of retroactive cue interference. Group [ADE]BC–Pair took longer to resume drinking than did Group ABC-Pair, F(1, 68) = 18.55, p < .01, Cohen’s f = .51 (95 % CI = 0.26; 0.77), which is indicative of less retroactive cue interference in the group that received target acquisition in multiple contexts. Group [ADE]BC–Unpair and Group ABC–Unpair did not differ statistically, p = .66, which suggests that administering target training in multiple contexts specifically reduced cue interference, rather than facilitated acquisition per se. Notably, the mean lick suppression to CS X in these two groups was far below the maximum possible suppression of 2.7 log s, which indicates that this lack of a significant difference was not the result of a ceiling effect. This absence of a difference allows rejection of the view that target training in multiple contexts reduces cue interference due to a stronger X–O association arising from the training context(s) being less effective in competing with CS X for associative strength in Group [ADE]BC–Pair than in Group ABC–Pair. In contrast, the data are congruent with the view that target training in multiple contexts allows more generalization from the training contexts to the test context, thereby resulting in stronger contextual priming of retrieval of the target association at test (e.g., Miller & Escobar, 2002).
Experiment 2: training the interfering association in multiple contexts
Experiment 2 (Table 2) was designed to determine whether training the interfering association in multiple contexts would increase retroactive cue interference, analogous to how training the target association in multiple contexts was seen to decrease interference in Experiment 1. Like Experiment 1, Experiment 2 was embedded within a sensory preconditioning preparation in order to increase the resultant amount of cue interference. Experiment 2 also used a renewal-like procedure, this time to circumvent the issue of which interference training context to test in when multiple contexts are used for interference training.
According to associative models that account for interference effects, training the interfering association in multiple contexts should facilitate the retrieval of the interfering association at test in an associatively neutral test context more than when interference training occurs entirely in one context. This should result in increased interference, manifested as a reduction in conditioned suppression to the target CS. Experiment 2 used a 2 (phase 2 context: ABC vs. A[BDE]C) × 2 (phase 2 training: pair vs. unpair) factorial design. During phase 1, all subjects received three X–O associations in context A. During phase 2, subjects in Condition ABC received interference treatment exclusively in context B, while subjects in Condition A[BDE]C received a comparable amount of interference training distributed equally across contexts B, D, and E. Orthogonal to the phase 2 context treatment variable (ABC vs. A[BDE]C), subjects in Condition Pair received three Z–O pairings during phase 2, while subjects in Condition Unpair received three unpaired presentations each of Z and O. Training during phase 2 equated across groups the total number of Z and O presentations and exposure to contexts B, D, and E. During phase 3, all subjects received four O–US pairings in context C. At test, all subjects were tested on X and then O in context C. We expected to see relatively high conditioned suppression to X in both Condition Unpair groups, because no interfering association was trained in this condition. Additionally, we expected to observe low suppression in Group ABC–Pair relative to Group ABC–Unpair, because the latter group did not receive interference training. Critically, if training the interfering association in multiple contexts enhances interference, we would expect to observe less suppression to CS X in Group A[BDE]C–Pair than in Group ABC–Pair.
Method
Subjects and apparatus
Subjects were 24 male and 24 female, experimentally naive, Sprague Dawley descended rats obtained from our own breeding colony. Body weight ranges were 169–251 g for females and 212–333 g for males. Subjects were randomly assigned to one of four groups (ABC–Pair, A[BDE]C–Pair, ABC–Unpair, A[BDE]C–Unpair; ns = 12), counterbalanced within groups for sex. The reduction of Condition Pair group sizes in Experiment 2, relative to Experiment 1, was based on the large effect sizes obtained in Experiment 1. The animals were maintained and housed as in Experiment 1. Apparatuses were the same as in Experiment 1. CS X was the click train. CS Z was the complex tone. The outcome (O) was the white noise. X, Z, and O were 5 s in duration, except during testing. The footshock served as the US. Context A was Chamber Modified O; contexts B, D, and E were Chamber R, Chamber V, and Chamber Modified R, counterbalanced; context C was Chamber O.
Procedure
Acclimation
On day 1, all subjects were acclimated to each context as in Experiment 1, with the lick tube available only in context C.
Phase 1
On day 2, all subjects were presented with three X–O (i.e., click–white noise) pairings in context A, starting at 18, 30, and 48 min into a 60-min session. On each of these trials, the onset of the outcome immediately followed the termination of X.
Phase 2
On days 3–5, subjects in Condition ABC received one daily Z–O (i.e., tone–white noise) pairing in context B, beginning 30 min into a daily 60-min session. On each of these trials, the onset of the outcome immediately followed the termination of Z. Additionally, these groups received 60 min of exposure daily to contexts D and E. Subjects in Condition A[BDE]C received one Z–O pairing in context B on day 3, one Z–O pairing in context D on day 4, and one Z–O pairing in context E on day 5, each beginning 30 min into the session. Subjects in Condition A[BDE]C were also exposed to each of the other two contexts for 60 min on each of these 3 days.
Phase 3, reacclimation, and test
On day 6, all subjects received four O–US pairings in context C. On days 7 and 8, all subjects were reacclimated to context C in one daily 90-min session. On day 9, testing on CS X occurred in context C for all groups. On day 10, testing on O in context C occurred for all groups. All of these procedures were just as in Experiment 1.
Results and discussion
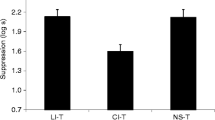
Group mean suppression to CS X in Experiment 2 is depicted in Fig. 2. The results support the hypothesis that training the interfering association in multiple contexts increases retroactive cue interference as assessed by reduced responding based on the target association. The following analyses support this conclusion.
Results of Experiment 2. Mean times to complete 5 cumulative seconds of drinking in the presence of the target CS X. Therefore, 0.7 log s is the lowest possible score. Higher scores represent stronger responding to the target CS. Error bars represent standard errors of the means. See the text and Table 2 for details
A 2 (pair vs. unpair) x 2 (ABC vs. A[BDE]C) ANOVA performed on latencies to complete 5 cumulative seconds of licking prior to the onset of the test stimulus on day 9 (i.e., baseline drinking) revealed no significant main effect or interaction, all ps > .08 (ABC–Pair, M = 1.17; ABC–Unpair, M = 1.24; [ADE]BC–Pair, M = 1.19; [ADE]BC–Unpair, M = 1.31 log s). An analogous ANOVA performed on suppression during CS X revealed an interaction, F(1, 44) = 8.79, p < .01, Cohen’s f = .43 (95 % CI = 0.13; 0.72), MSE = 0.11. The main effect of pair versus unpair was also statistically significant, F(1, 44) = 40.41, p < .001, Cohen’s f = .91 (95 % CI = 0.57; 1.26), MSE = 0.11. A 2 × 2 ANOVA on the data of day 10 (i.e., CS O test) found no main effects or interaction during either the pre-CS (ABC–Pair, M = 1.25; ABC–Unpair, M = 1.22; [ADE]BC–Pair, M = 1.34; [ADE]BC–Unpair, M = 1.35 log s) or the presentation of O, all ps >.16, the latter of which suggests that O was similarly excitatory across groups (ABC–Pair, M = 1.61; ABC–Unpair, M = 1.59; [ADE]BC–Pair, M = 1.71; [ADE]BC–Unpair, M = 1.57 log s).
To test specific hypotheses, planned comparisons were conducted using the error term from the ANOVA that had been performed on day 9 suppression to CS X. Group ABC–Pair exhibited less suppression to CS X than did Group ABC–Unpair, F(1, 44) = 5.76, p = .02, Cohen’s f = .34 (95 % CI = 0.04; 0.63), evidencing the presence of retroactive cue interference. Group A[BDE]C–Pair had shorter latencies to resume drinking after onset of CS X than did Group ABC–Pair, F(1, 44) = 7.91, p < .01, Cohen’s f = .40 (95 % CI = 0.11; 0.70), which is indicative of more retroactive cue interference in the group that received interference training in multiple contexts. Group A[BDE]C–Unpair and Group ABC–Unpair did not differ statistically, p = .17, which suggests that administering interference training in multiple contexts (as opposed to a single context) increased interference only when Z and O were paired. The present data mirror the results of Experiment 1 in finding that, in an associative cue interference situation, administering interference training in multiple contexts (without altering the total number of training trials) results in that phase of training having a greater effect on behavior at test in an associatively neutral context. Moreover, they suggest that training the interfering association in multiple contexts reduces the context specificity of associative interference, as proposed by Miller and Escobar (2002), although the present study did not include a control condition to directly assess the context specificity of retroactive cue interference. That is, there was no ABB control condition, because we were focally interested in the effects on cue interference of training in multiple contexts (and additionally, prior research with similar procedures [Miguez et al., 2012] had already demonstrated ABC renewal, relative to an ABB control condition, following retroactive cue interference).
General discussion
The results of Experiments 1 and 2 provide a further demonstration that reduction in acquired behavior to a target cue results when two associations share a common outcome (i.e., cue interference occurs). Within a Pavlovian preparation, less conditioned lick suppression was observed to the target CS (X) when an interfering association was trained (Z–O), relative to when a control treatment exposed the subjects to the same number of unpaired presentations of the interfering cue and the outcome. These observations are concordant with numerous other reports concerning associative cue interference (e.g., Cobos, López, & Luque, 2007; Escobar et al., 2001b; Luque et al., 2010; Luque, Morís, Cobos, & López, 2009; Luque, Cobos, & López, 2008; Matute & Pineño, 1998; Miguez et al., 2012; Vadillo, Orgaz, & Matute, 2008; Pineño et al., 2000).
What is novel in the present findings is that the results of Experiment 1 indicate that training the target association in multiple contexts reduces retroactive cue interference. Subjects that received target training (X–O pairings) during phase 1 in multiple contexts (A, D, and E) followed by interference training (Z–O pairings) during phase 2 (in context B) displayed less interference when tested on CS X in context C than did otherwise equivalent subjects that received target training in a single context. Without Z–O interference training, target training in multiple contexts had little effect, suggesting that the benefit of target training in multiple contexts was not due to better acquisition of the X–O association. Thus, the benefit of target training in multiple contexts observed here seems to be specific to associative interference situations, as opposed to facilitated learning in general or to unambiguous learning (i.e., without interference training) transferring better to a neutral context. Notably, training the target association in multiple contexts prevented any observable interference in Group [ADE]BC–Pair relative to Group [ADE]BC–Unpair. This result is analogous to observations in the outcome interference literature. For example, Gunther et al. (1998, Experiment 2) conducted acquisition training of a CS-US either in a single or in multiple contexts, with the total number of parings equivalent in the two conditions. Testing in a neutral context after extinction yielded lower responding (indicative of more effective extinction—that is, relatively strong outcome interference) in the group that received acquisition training (i.e., CS-US pairings) in a single context, as compared with subjects that received acquisition training in multiple contexts.
The observations of Experiment 2 supported our second hypothesis. That is, training the interfering association in multiple contexts increased the decrement in behavioral control by the target association (i.e., increased retroactive cue interference). When all subjects received target association training in a single context (context A) during phase 1 and were tested in a neutral context at test (context C), those that received phase 2 interference training in multiple contexts (contexts B, D, and E) exhibited a greater reduction in suppression to CS X than did those subjects that received interference training in a single context (context B). The observations of Experiment 2 are analogous to several other reports in the outcome interference literature in which the interfering association generalizes to a neutral context better at test when it has been trained in diverse contexts (e.g., Chelonis et al., 1999; Gunther et al., 1998, Experiment 1; Glautier & Elgueta, 2009; Glautier et al., 2013; Laborda & Miller, 2013; Neumann, 2006; Pineño & Miller, 2004; Vansteenwegen et al., 2007).
Only a few models have been proposed to account for interference effects within a Pavlovian framework. Of these, most of them (e.g., Bouton, 1993; Miller & Escobar, 2002; Miller & Laborda, 2011; Rosas, Aguilera, Álvarez, & Abad, 2006) can account for the consequences of training either the interfering association or the target association in multiple contexts, within an outcome interference design. However, only Miller and Escobar’s model was developed to account for cue interference, as well as outcome interference. Moreover, this account anticipates the data from both Experiments 1 and 2. According to this model, the reduction in interference observed in Group [ADE]BC–Pair relative to Group ABC–Pair of Experiment 1 could have been due to less competition between CS X and the target acquisition contexts during phase 1 or to more facilitation of retrieval of the X–O association at test in Group [ADE]BC–Pair. First, competition with X by the training context(s) may have been weaker in Group [ADE]BC–Pair because X had higher relative cue validity than the three target training contexts, whereas in Group ABC–Pair, the context A–O association was likely stronger due to three, rather than one, presentations of O in context A and, hence, better able to compete with X. Second, facilitated retrieval at test in context C of the X–O association in Group [ADE]BC–Pair, relative to Group ABC–Pair, might be expected, because in the former group, contexts A, D, and E could each partially generalize to context C, whereas in the latter group, only context A could partially generalize to context C. That is, as was noted by Gunther et al. (1998), context C should have had more features in common with any one of the three acquisition contexts (A, D, and E) than with a single context (A). Therefore, for Group [ADE]BC–Pair, context C should have better primed activation of the target association, while dampening retrieval of other associations (i.e., Z–O) that share one of the elements (e.g., O). Our use of a factorial design allowed us to differentiate the contribution of each of these two potential mechanisms to the benefit of training in multiple contexts. The observation that training the target association in multiple contexts produced differential conditioned suppression, as compared with training in a single context in Condition Pair but not in Condition Unpair, suggests that the effect is due not to differential acquisition of the target association, but to a facilitation of priming of the target association by the test context.
The Miller and Escobar (2002) model also anticipates the results of Experiment 2. The interaction between type of phase 2 treatment (i.e., pair vs. unpair) and number of contexts in which phase 2 treatment occurred supports the view that the greater interference observed in Group A[BDE]C–Pair, as compared with Group ABC–Pair, was due to facilitated retrieval of the interfering association in Group A[BDE]C–Pair and consequent inhibition of retrieval of the target association by the facilitated activation of the interfering association.
Although we have spoken here of target training in multiple contexts reducing cue interference, this conclusion has to be qualified, because the present experimental designs prevented us from differentiating between (1) target training in multiple contexts reducing cue interference (and interference training in multiple contexts increasing cue interference) and (2) target training in multiple contexts increasing the context specificity of subsequent cue interference (and interference training in multiple contexts decreasing the context specificity of subsequent cue interference). This is a question that needs to be addressed in future research. A likely way of answering it would be by comparing an ABB–Pair group with an [ADE]BB–Pair group—that is, using an experimental design that does not include a renewal manipulation. The present experiments used a renewal-like procedure because we feared that testing in context B, the context of interference training, would result in sufficiently strong interference that a floor effect for behavioral control by CS X might mask any effect of training in multiple contexts. Additionally, we repeat that these experiments were not intended to document renewal in cue interference; that has been done previously (e.g., Miguez et al., 2012). Rather, we simply used a renewal-like procedure to increase sensitivity to the effect of training in multiple contexts. That is why there was no control for renewal (i.e., an [ADE]BB group) in Experiment 1.
The preceding discussion assumes that the Z–O association impacted expression of the X–O association. However, one may question whether the Z–O association also affected the O–US association, which is presumably activated after activation of either the X–O link or the Z–O link at test. Critically, the absence of a significant difference between the pair and unpair conditions in responding to O speaks against this possibility. But this argument must be qualified, because the prior test on X may have reduced sensitivity to differences in suppression to O. However, in three prior retroactive interference experiments, Escobar and Miller (2003) independently manipulated (1) the times between onsets of X and O and between onsets of Z and O and (2) the durations of X and of Z and the durations of the outcomes paired with X and with Z. They observed far greater interference when the X–O and Z–O associations shared similar temporal features. In the present experiments, the X–O and Z–O associations shared similar temporal features, whereas the O–US association did not. Thus, it is more likely that in the immediate studies, the Z–O association interfered more with the X–O association than with the O–US association.
Taking a broader perspective, the results of these experiments indicate that analogous treatments regarding training in multiple contexts in outcome interference and cue interference have similar effects on behavior (Chelonis et al., 1999; Glautier & Elgueta, 2009; Glautier et al., 2013; Gunther et al., 1998; Laborda & Miller, 2013; Neumann, 2006; Pineño & Miller, 2004; Vansteenwegen et al., 2007). In both outcome interference and cue interference, training the target association in multiple contexts increases renewal-like context specificity of retroactive interference, and training the interfering association in multiple contexts decreases renewal-like context specificity of retroactive interference in both cues and outcomes. Previous research has looked at other treatments that have equivalent effects in cue and outcome interference. For example, Miguez et al. (2012; see also Escobar et al., 2001a; Luque et al., 2010) reported that a retention interval between retroactive interference treatment and testing in cue interference facilitates responding to the target cue (i.e., spontaneous recovery), as has been observed in retroactive outcome interference (e.g., Bouton & Peck, 1992; Pavlov, 1927). Other reports indicate that the similarity of the test context to the training context, relative to the similarity of the test context to the interference context, is a determinant of interference between both interfering cues (e.g., Amundson, Escobar, & Miller, 2003; Escobar et al., 2001a, 2001b; Miguez et al., 2012) and interfering outcomes (e.g., Bouton & Bolles, 1979; González-Martín, Cobos, Morís, & López, 2012; Rosas, García-Gutiérrez, & Callejas-Aguilera, 2006; Rosas, Vila, Lugo, & López, 2001). To date, the present article is the only report that has assessed the effects of training in multiple contexts in cue interference. Together with the growing literature reporting analogous phenomena across cue interference and outcome interference, these data seem to suggest that common mechanisms underlie the two types of interference. This poses a challenge for accounts of interference that cannot explain cue interference (e.g., Bouton, 1993).
References
Amundson, J. C., Escobar, M., & Miller, R. R. (2003). Proactive interference between cues trained with a common outcome in first-order Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes, 29, 311–322. doi:10.1037/0097-7403.29.4.311
Anderson, M. C., Bjork, R. A., & Bjork, E. L. (1994). Remembering can cause forgetting: Retrieval dynamics in long-term memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 20, 1063–1087. doi:10.1037/0278-7393.20.5.1063
Betancourt, R., Corada, L., Dominichetti, J. E., Laborda, M. A., Martínez, G., & Miguez, G. (2008). Efecto de la extinción en múltiples contextos sobre la renovación de la tolerancia asociativa al etanol. Psicothema, 20, 285–289.
Bouton, M. E. (1993). Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin, 114, 80–99. doi:10.1037/0033-2909.114.1.80
Bouton, M. E., & Bolles, R. C. (1979). Contextual control of the extinction of conditioned fear. Learning and Motivation, 10, 445–466. doi:10.1016/0023-9690(79)90057-2
Bouton, M. E., García-Gutiérrez, A., Zilski, J., & Moody, E. W. (2006). Extinction in multiple contexts does not necessarily make extinction less vulnerable to relapse. Behaviour Research and Therapy, 44, 983–994.
Bouton, M. E., & King, D. A. (1983). Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes, 9, 248–265. doi:10.1037/0097-7403.9.3.248
Bouton, M. E., & Peck, C. A. (1992). Spontaneous recovery in cross-motivational transfer (counterconditioning). Animal Learning & Behavior, 20, 313–321. doi:10.3758/BF03197954
Bouton, M. E., & Ricker, S. T. (1994). Renewal of extinguished responding in a second context. Animal Learning & Behavior, 22, 317–324.
Brooks, D. C., & Bouton, M. E. (1993). A retrieval cue for extinction attenuates spontaneous recovery. Journal of Experimental Psychology: Animal Behavior Processes, 19, 77–89. doi:10.1037/0097-7403.19.1.77
Chelonis, J. J., Calton, J. L., Hart, J. A., & Schachtman, T. R. (1999). Attenuation of the renewal effect by extinction in multiple contexts. Learning and Motivation, 30, 1–14. doi:10.1006/lmot.1998.1022
Cobos, P. L., López, F. J., & Luque, D. (2007). Interference between cues of the same outcome depends on the causal interpretation of the events. Quarterly Journal of Experimental Psychology, 60, 369–386. doi:10.1080/17470210601000961
Denniston, J. C., Miller, R. R., & Matute, H. (1996). Biological significance as determinant of cue competition. Psychological Science, 7, 325–331. doi:10.1111/j.1467-9280.1996.tb00383.x
Escobar, M., Arcediano, F., & Miller, R. R. (2001a). Conditions favoring retroactive interference between antecedent events (cue competition) and between subsequent events (outcome competition). Psychonomic Bulletin & Review, 8, 691–697. doi:10.3758/BF03196205
Escobar, M., Matute, H., & Miller, R. R. (2001b). Cues trained apart compete for behavioral control in rats: Convergence with the associative interference literature. Journal of Experimental Psychology: General, 130, 97–115. doi:10.1037/0096-3445.130.1.97
Escobar, M., & Miller, R. R. (2003). Timing in retroactive interference. Learning & Behavior, 31, 257–272. doi:10.3758/BF03195987
Glautier, S., & Elgueta, T. (2009). Multiple cue extinction effects on recovery of responding in causal judgments. International Journal of Comparative Psychology, 22, 254–270.
Glautier, S., Elgueta, T., & Nelson, J. B. (2013). Extinction produces context inhibition and multiple-context extinction reduces recovery in human predictive learning. Learning & Behavior, 41, 341–352. doi:10.3758/s13420-013-0109-7
González-Martín, E., Cobos, P. L., Morís, J., & López, F. J. (2012). Interference between outcomes, spontaneous recovery, and context effects as measured by a cued response reaction time task: Evidence for associative retrieval models. Journal of Experimental Psychology: Animal Behavior Processes, 38, 419–432. doi:10.1037/a0029517
Gunther, L. M., Denniston, J. C., & Miller, R. R. (1998). Conducting exposure treatment in multiple contexts can prevent relapse. Behaviour Research and Therapy, 36, 75–91. doi:10.1016/S0005-7967(97)10019-5
Laborda, M. A., & Miller, R. R. (2012). Reactivated memories compete for expression after Pavlovian extinction. Behavioural Processes, 90, 20–27. doi:10.1016/j.beproc.2012.01.012
Laborda, M. A., & Miller, R. R. (2013). Preventing return of fear in an animal model of anxiety: Additive effects of massive extinction and extinction in multiple contexts. Behavior Therapy, 44, 249–261. doi:10.1016/j.beth.2012.11.001
Lubow, R. E., & Moore, A. U. (1959). Latent inhibition: The effect of nonreinforced pre-exposure to the conditional stimulus. Journal of Comparative and Physiological Psychology, 52, 415–419. doi:10.1037/h0046700
Luque, D., Cobos, P. L., & López, F. J. (2008). Interference between cues requires a causal scenario: Favorable evidence for causal reasoning models in learning processes. Learning and Motivation, 39, 196–208. doi:10.1016/j.lmot.2007.10.001
Luque, D., Morís, J., Cobos, P. L., & López, F. J. (2009). Interference between cues of the same outcome in a non-causally framed scenario. Behavioural Processes, 81, 328–332. doi:10.1016/j.beproc.2008.11.009
Luque, D., Morís, J., & Cobos, P. L. (2010). Spontaneous recovery from interference between cues but not from backward blocking. Behavioural Processes, 84, 521–525. doi:10.1016/j.beproc.2009.12.016
Matute, H., & Pineño, O. (1998). Stimulus competition in the absence of compound conditioning. Animal Learning & Behavior, 26, 3–14. doi:10.3758/BF0319915
Miguez, G., Cham, H. X., & Miller, R. R. (2012). Spontaneous recovery and ABC renewal from retroactive cue interference. Learning & Behavior, 40, 42–53. doi:10.3758/s13420-011-0044-4
Miller, R. R., & Escobar, M. (2002). Associative interference between cues and between outcomes presented together and presented apart: An integration. Behavioural Processes, 57(2–3), 163–185. doi:10.1016/S0376-6357(02)00012-8
Miller, R. R., & Laborda, M. A. (2011). Preventing recovery from extinction and relapse: A product of current retrieval cues and memory strengths. Current Directions in Psychological Science, 20, 325–329. doi:10.1177/0963721411418466
Miller, R. R., & Matzel, L. D. (1989). Contingency and relative associative strength. In S. B. Klein & R. R. Mowrer (Eds.), Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory (pp. 61–84). Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.
Nelson, J. B. (2002). Context specificity of excitation and inhibition in ambiguous stimuli. Learning and Motivation, 33, 284–310. doi:10.1006/lmot.2001.1112
Neumann, D. L. (2006). The effects of physical context changes and multiple extinction contexts on two forms of renewal in a conditioned suppression task with humans. Learning and Motivation, 37, 149–175. doi:10.1016/j.lmot.2005.06.004
Neumann, D. L., Lipp, O. V., & Cory, S. E. (2007). Conducting extinction in multiple contexts does not necessarily attenuate the renewal of shock expectancy in a fear-conditioning procedure with humans. Behaviour Research and Therapy, 45, 385–394. doi:10.1016/j.brat.2006.02.001
Ortega-Castro, N., & Vadillo, M. A. (2013). Retrieval-induced forgetting and interference between cues: Training a cue-outcome association attenuates retrieval by alternative cues. Behavioural Processes, 94, 19–25. doi:10.1016/j.beproc.2012.11.010
Pavlov, I. P. (1927). Conditioned reflexes (G.V. Anrep, Ed. & Trans.). London: Oxford University Press.
Pineño, O., & Miller, R. R. (2004). Signaling a change in cue-outcome relations in human associative learning. Learning & Behavior, 32, 360–375. doi:10.3758/BF03196034
Pineño, O., Ortega, N., & Matute, H. (2000). The relative activation of associations modulates interference between elementally trained cues. Learning and Motivation, 31, 128–152. doi:10.1006/lmot.1999.1047
Rosas, J. M., Aguilera, J. E. C., Álvarez, M. M. R., & Abad, M. J. F. (2006a). Revision of retrieval theory of forgetting: What does make information context-specific? International Journal of Psychology & Psychological Therapy, 6, 147–166.
Rosas, J. M., García-Gutiérrez, A., & Callejas-Aguilera, J. (2006b). Effects of context change upon retrieval of first and second-learned information in human predictive learning. Psicológica, 27, 35–56.
Rosas, J. M., Vila, N. J., Lugo, M., & López, L. (2001). Combined effect of context change and retention interval on interference in causality judgments. Journal of Experimental Psychology: Animal Behavior Processes, 27, 153–164. doi:10.1037/0097-7403.27.2.153
Stout, S. C., & Miller, R. R. (2007). Sometimes-competing retrieval (SOCR): A formalization of the comparator hypothesis. Psychological Review, 114, 759–783. doi:10.1037/0033-295X.114.3.759
Vadillo, M. A., Orgaz, C., & Matute, H. (2008). Overshadowed cues have reduced ability to retroactively interfere with other cues. Learning and Motivation, 39, 313–322. doi:10.1016/j.lmot.2008.05.005
Vansteenwegen, D., Vervliet, B., Iberico, C., Baeyens, F., Van den Bergh, O., & Hermans, D. (2007). The repeated confrontation with videotapes of spiders in multiple contexts attenuates renewal of fear in spider-anxious students. Behaviour Research and Therapy, 45, 1169–1179. doi:10.1016/j.brat.2006.08.023
Author Note
This research was supported by National Institute of Mental Health Grant 33881. The authors thank Vincent DeNinis, Sara O’Hara, Cody W. Polack, and Julia S. Soares for their comments on an earlier version of th manuscript. Gonzalo Miguez was supported by the Fulbright Program and the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT-Chile).
Questions concerning this research should be addressed to Ralph R. Miller, Department of Psychology, SUNY-Binghamton, Binghamton, NY 13902-6000; e-mail: rmiller@binghamton.edu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miguez, G., Laborda, M.A. & Miller, R.R. Enhancement and reduction of associative retroactive cue interference by training in multiple contexts. Learn Behav 42, 318–329 (2014). https://doi.org/10.3758/s13420-014-0149-7
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-014-0149-7