Abstract
The present study attempts to better identify the neurophysiological changes occurring during flow experience and how this can be related to the mobilization of attentional resources. Self-reports of flow (using a flow feelings scale) and attention (using thought probes), autonomic activity (heart rate, heart rate variability, and breathing rate), and cerebral oxygenation (using near-infrared spectroscopy) in two regions of the frontoparietal attention network (right lateral frontal cortex and right inferior parietal lobe) were measured during the practice of two simple video games (Tetris and Pong) played at different difficulty conditions (easy, optimal, hard, or self-selected). Our results indicated that an optimal level of difficulty, compared with an easy or hard level of difficulty led to greater flow feelings and a higher concentration of oxygenated hemoglobin in the regions of the frontoparietal network. The self-selected, named autonomy condition did not lead to more flow feelings than the optimal condition; however, the autonomy condition led to greater sympathetic activity (reduced heart rate variability and greater breathing rate) and higher activation of the frontoparietal regions. Our study suggests that flow feelings are highly connected to the mobilization of attentional resources, and all the more in a condition that promotes individuals’ choice and autonomy.
Similar content being viewed by others
It is crucial to understand what happens in the brain when individuals perform an activity and feel at their best. The concept of flow has been introduced to describe this typical psychological state occurring when a person produces an optimal performance in a task and experiences feeling of enjoyment (Csziksentmihalyi, 1975, 1990). Another central aspect of the flow state is the subjective experience of heightened concentration and absorption in the ongoing performance (Csziksentmihalyi, 1990; Moneta & Csziksentmihalyi, 1996). However, the relation of flow with attention has not been the subject of a specific investigation. Moreover, the flow state has also the particularity to be defined as a peculiar state of attention as it would requires no mental effort (Ullén, de Manzano, Theorell, & Harmat, 2010) in contrast to most attentive states that typically require mental effort (see Kahneman, 1973; Posner, 1990). So, it is still unknown if flow really mobilizes the physiological and neural resources commonly related to attention. Using psychological, physiological, and neurophysiological indexes of attention during a task performance, the present study attempts to answer this question.
The term of sustained attention is used to describe the situation where heightened concentration is maintained when performing a specific task. Traditionally, such attentive state has been linked to the mobilization of resources (Kahneman, 1973). This engagement of resources can be seen at a physiological level as sustained attention during a task performance typically leading to a state of high arousal, suggesting a positive sympathovagal balance (Pribham & McGuiness, 1975). In other words, the autonomic system is altered in a way that the sympathetic branch is activated and the parasympathetic branch is down-regulated during sustained attention, which can be observed through many peripheral indices such as cardiac or respiratory measures (e.g., Richards, 1987; Zanstra, Schellekens, Schaap, & Kooistra, 2006). At a neural level, sustained attention in a task also mobilizes resources as it is related to the activation of a large network of cortical structures, called the frontoparietal attention network. This network includes regions that are mainly located in lateral parts of the frontal and parietal cortices, such as the dorsolateral prefrontal cortex, the inferior frontal gyrus, the inferior parietal lobe, or the intraparietal sulcus. Also, it has been frequently reported that this frontoparietal attentional network has a right lateralized dominance (Cabeza & Nyberg, 2000; Coull, 1998; Lim et al., 2010; Ogg et al., 2008; Paus et al., 1997). Interestingly, this network has often been shown to work in an anticorrelated fashion with the default mode network (Fox, Zhang, Snyder, & Raichle, 2009; Greicius, Krasnow, Reiss, & Menon, 2003). The default mode network, also composed of frontal and parietal structures but located closer to the medial line, is thus rather activated during attentional lapses, when individuals are mind wandering and have self-preoccupations (Esterman, Rosenberg, & Koonan, 2014; Gusnard, Akbudak, Shulman, & Raichle, 2001).
Previous experimental studies attempting to characterize the state of flow have provided preliminary evidence as to whether the flow state corresponds to the physiological and neurophysiological markers of the attentive states. Physiological studies have shown an effect of flow on the autonomic system, showing for example a larger respiratory depth and an increase in low-frequency/high-frequency rate in heart rate variability (HRV), which suggest a positive sympathovagal balance (de Manzano, Theorell, Harmat, & Ullén, 2010). Other studies have suggested a positive sympathovagal balance by noticing a general decrease in HRV (Chanel, Rebetez & Bétrancourt, 2011; Keller, Bless, Blomann, & Kleinböhl, 2011). This deviation of the sympathovagal balance seemed mostly driven by the activation of the sympathetic branch as studies have shown increased heart rate (HR), (de Manzano et al., 2010; Gaggioli, Cipresso, Serino, & Riva, 2013) during flow. The deviation to a positive sympathetic predominance is common during effortful attentive states (Richards, 1987; Zanstra et al., 2006).
Only few studies have examined brain activity during flow. In a functional magnetic resonance imaging (fMRI) study, flow was associated with increased activation in the lateral frontal cortex (inferior frontal gyrus) and decreased activation in the medial prefrontal cortex (Ulrich, Keller, Hoenig, Waller, & Grön, 2014). In another fMRI study, Klasen, Weber, Kircher, Mathiak, and Mathiak (2012) examining flow by monitoring the brain activity associated with some defined aspects of flow that could be objectively measured during a video game (balance between ability and challenge, concentration and focus, clear goals, control, and feedback of action results). Observing concentration and focus by comparing active and passive phases of the game indicated that focus was associated with activations in the cerebellum, occipital lobe, precuneus, and motor areas, but also with deactivations in the intraparietal sulcus and the medial frontal cortex. However, only the activations in the parietal lobe, motor areas, and cerebellum were related to the other observable aspects of flow (Klasen et al., 2012). Monitoring the changes in oxygenated hemoglobin (O2Hb) concentrations in the prefrontal cortex (PFC) using near-infrared spectroscopy (NIRS), Yoshida et al. (2014) showed that the flow condition (optimal level of difficulty) led to an increased activity (elevated O2Hb) in lateral areas of the PFC, whereas a general PFC deactivation was reported during the easy condition. These results fit well with the hypothesis of mobilization of attentional resources during flow as the lateral PFC is part of the attentional network. However, another NIRS study monitoring cerebral oxygenation changes in the PFC did not observe any changes during flow (Harmat et al., 2015).
The present study attempts to better understand how flow is related to the mobilization of attentional resources. A secondary objective was to test a new experimental induction of flow. Previous flow experiments have typically exploited the skills–challenge balance aspect to induce flow (Moller, Meier, & Wall, 2010). Simple video games were often used because the difficulty level can be easily manipulated. Typically, participants report greater flow feelings in an optimal level of difficulty (i.e., a difficulty that is slightly more difficult than their actual level) compared with an easy or a hard condition (Chanel et al., 2011; Harmat et al., 2015; Keller & Bless, 2008; Keller et al., 2011; Keller & Blomann, 2008; Rheinberg & Vollmeyer, 2003; Yoshida et al., 2014). Using a self-selected level of difficulty could be a good opportunity to include another condition of flow in this paradigm. This condition would provide participants with choice and autonomy, and this factor has been suggested to be an important determinant of flow (Moller et al., 2010) since flow is an autotelic experience typically occurring in contexts where individuals have a high degree of self-determination over their actions (Kowal & Fortier, 1999). Therefore, an “autonomy condition” was added to the traditional easy, optimal, and hard conditions where the level of the game was not imposed but self-selected. In order to improve the reliability of our findings, we also assessed the effects of these manipulations in two different video games: Tetris, which has been traditionally used in previous studies, and Pong, another simple video game that also provide an easy way to manipulate the difficulty level.
Cardiac and respiratory measurements were monitored continuously to quantify the contribution of the sympathetic and the parasympathetic systems during flow. We used NIRS to monitor brain activity during the games. Changes in O2Hb derived from the NIRS optical signal have been shown in relation to the mobilization of attentional resources (Durantin, Dehais, & Delorme, 2015; Harrivel, Weissman, Noll, & Peltier, 2013; Stevenson, Russell, & Helton, 2011). Given that this noninvasive technique is limited to monitor brain regions located at the cerebral surface (depth sensitivity ~20 mm), (Haeussinger et al., 2011), we selected regions of interest (ROIs) that are accessible by NIRS and that are main regions of the frontoparietal attention network (i.e., right dorsolateral PFC and right inferior parietal lobe).
Method
Subjects
Twenty adult volunteers (seven females; three left-handed; age: 26.45 ± 4.83 years) without a history of brain injury were included in the experiment. All participants gave their written informed consent and were free to withdraw from the experiment at any time. The protocol of the study was approved by the Scientific Council of the Sports Science faculty of the Université de Nice Sophia Antipolis and was performed in accordance with the ethical standards of the declaration of Helsinki and American Psychological Association.
General procedure
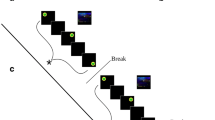
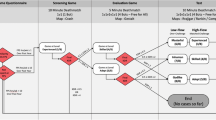
Participants were first equipped with the NIRS head cap and the belt collecting cardiac and respiratory measures. The experiment consisted of playing two simple video games (Tetris and Pong). Tetris is a popular game where falling pieces of various geometrical shapes have to be arranged to form lines. Pong is another popular game where a short line representing a racket has to be moved on an axis to intercept a moving ball. In both games, the participants played by pressing keyboard buttons with their right-hand fingers. The order of the games was counterbalanced across participants. For each game, the participant started with a level determination phase followed by four different playing sessions (easy, optimal, hard, and autonomy). E-Prime software (Version 2.0, Psychology Software Tools, Sharpsburg, USA) was used to present the four playing sessions in a randomized order and to ensure the correct timing of the events of the study. Each playing session started with 1-minute rest period. Then, the participants were told which level they would play (depending on the experimental condition) and played at the specific level of difficulty for 3 minutes. At the end of this time, the session was terminated, and the participant had to fill out a psychological questionnaire assessing flow feelings and the subjective difficulty of the task in this last session. Figure 1 provides an illustration of the protocol and photographs of the setup).
Level determination procedure
For the level determination phase in Tetris, the number of lines for 30 pieces was recorded at five different difficulty levels by changing the falling speed of the pieces. A linear regression of the scores obtained at these five levels was used to infer the “optimal” level, defined as the level where four lines would be made with 30 pieces (Chanel et al., 2011). For the “easy” condition, the minimal level of the game was used. For the “hard” condition, the falling speed determined in the “optimal” level was doubled.
Given that participants played against a virtual opponent in the Pong game, we used both the ball speed and velocity of the virtual opponent to manipulate game difficulty. Velocity of the virtual opponent was, however, always adjusted in response of the ball speed so that the virtual opponent was always able to cope with the ball speed. As such, ball speed was the only critical factor to manipulate task difficulty. The participants played 20 balls against the computer in five different difficulty levels. A linear regression was used to infer the “optimal” level, defined as the level where the participant would lose by 4 points against the virtual opponent. This challenging situation where task difficulty slightly exceeds individuals’ skills is associated with the greatest flow feelings (Ceja & Navarro, 2012). The minimal and the maximal ball speed of the game were used to create the “easy” and “hard” conditions, respectively.
The experimenter vocally announced the speed levels when changing the difficulty levels in the determination phases so that the participants could have a sense of the difficulty levels. At the end of each game determination phase, participants were asked to indicate the level of difficulty at which they would prefer to play. This level was used as the difficulty level in the “autonomy” conditions. Table 1 presents the actual level played by each participant in the two games.
Measures
Experienced difficulty
In order to ensure that the manipulation was successful, experienced difficulty was measured at the end of each playing session in each condition. As done in previous studies (e.g., Engeser & Rheinberg, 2008), a single item was used to evaluate participants’ perceptions of the level of difficulty using a Likert scale from 1 (too easy) to 7 (too hard).
Subjective flow experience
At the end of each playing session, participants answered the Flow Short Scale (Engeser & Rheinberg, 2008; Rheinberg, Volmmeyer, & Engeser, 2003). The French translations of the nine following items were used: 1. I felt just the right amount of challenge; 2. My thoughts/activities did run fluidly and smoothly; 3. I didn’t notice time passing; 4. I had no difficulty concentrating; 5. My mind was completely clear; 6. I was totally absorbed in what I was doing; 7. The right thoughts/movements did occur of their own accord; 8. I knew what I had to do each step of the way; 9. I felt that I had everything under control (Cronbach α = 0.685). One item of the original scale was not used (“I am completely lost in thoughts”) because we had difficulty to find a valid translation in French.
Attentional focalization
To accurately evaluate attentional focalization during the task, we used the random probes procedure (e.g., Christoff et al., 2009; Smallwood, Beach, Schooler, & Handy, 2008). At three moments during each playing times, a sound probe arrived at a random time point ranging from 40 to 80 s after the beginning of the playing session or after the last probe. The participants were asked to indicate whether or not their attention was focused on the game immediately before the sound probe. If focused on the game, participants had to say “on-task,” and if thinking about something else, they had to say “off-task.”
Physiological measures
All physiological measures (HR, HRV, and BR) were collected using the BioHarness thoracic belt (BH3, Zephyr Technology, Annapolis, USA). The electrocardiographic signal was sampled at 2000 Hz through conductive silver fabric electrodes that are relayed to the transmitter. HR (beats per minute, BPM) were automatically derived from the ECG signal by the transmitter signal. The breathing rate (cycle per minute, CPM) was measured at 25 Hz by monitoring thoracic expansion using a strain gauge sensor. It should be noted that the reliability of the BioHarness has been adequately demonstrated for such cardiac (Kim, Roberge, Powell, Shafer, & Williams, 2013) and respiratory (Hailstone & Kilding, 2011) measurements. After removing extreme values using the outlier labelling rule, an average score was computed for HR and BR in epochs corresponding to the different playing sessions of each individual. HRV derived from the root mean square of successive differences (RMSSD) of beat-to-beat interval was calculated on these same epochs. RMSSD is associated with short-term, rapid changes in heart rate and can therefore be reliably assessed in a short duration (Laborde, Mosley, & Thayer, 2017; Munoz et al., 2015). RMSSD has been previously found to be highly associated to the vagal component of HRV (e.g., Otzenberger et al., 1998; Stein, Bosner, Kleiger, & Conger, 1995). HR, RMSSD-HRV, and BR were then simply averaged (with no additional filtering and artifact reduction techniques) from the values provided by the internal algorithm of the BioHarness.
Cerebral oxygenation measure
Cerebral hemodynamics were measured using a continuous-wave eight-channel NIRS system (Oxymon Mk III, Artinis Medical Systems, Zetten, The Netherlands). Optodes were placed into thermoplastic shells and mounted on a head cap (Easycap, Herrsching, Germany) to facilitate the positioning of the optodes on the head. To maintain pressure, elastic bands inserted on the probe holders were used to push the probes into the holders. The area measured between the emitter and detector probes was defined as a channel. NIRS sensitivity to gray matter depends on the source-detector distance and head region (Quaresima, Bisconti, & Ferrari, 2012; Strangman, Zhang, & Li, 2014). Since penetration of light is elevated in the frontal region because of the absence of hair, the distance between the source and detector was fixed at 4.0 cm for frontal channels in order to maximize the NIRS sensitivity to gray matter. However, in order to increase the penetration of NIRS light into cortical tissue and ensure a sufficient amount of NIRS light reaching the detector (around 15% according to Strangman et al., 2014), the distance was fixed at 3.0 cm for parietal channels. The positioning of the probes was arranged to cover a large part of the right dorsolateral frontal cortex and a large part of the inferior parietal lobe. Figure 2 presents the localization of each channel on their stereotaxic coordinates recorded after digitalization (Patriot, Polhemus, Colchester, VT, USA). The concentrations (in μm) of oxyhemoglobin (O2Hb) were provided with an age-specific differential path length factor obtained using the modified Beer–Lambert equation (Obrig & Villringer, 2003). Data were sampled at 10 Hz and acquired with Oxysoft (Version 3.0.43, Artinis Medical Systems, Zetten, The Netherlands). After data collection, noisy channels were removed based on the variance level. Specifically, the channel variances were z transformed, and the channels with a variance above one standard deviation were removed (Fekete, Rubin, Carlson, & Mujica-Parodi, 2011). On remaining channels, the NIRS signals was denoised using the motion artifact reduction algorithm (Scholkmann, Spichtig, Muehlemann, & Wolf, 2010) and filtered with a low-pass Butterworth filter (0.1 Hz cutoff) to remove peripheral influences (Huppert, Diamond, Franceschini, & Boas, 2009). Data were averaged for each playing session of each game and normalized to express the magnitude of changes from the baseline period preceding the playing period (representing 0 μm).
Statistical analysis
The normality of the distribution of all dependent variables was determined by visual inspection and Shapiro–Wilk tests. Mixed models were conducted to take into account the nonindependence of the repeated measures grouped within participants. It should be noted that this approach is highly recommended for repeated-measures analysis (Baayen, Davidson, & Bates, 2008). If the distribution of the dependent variables did not follow a normal distribution, a generalized mixed model was used. The mixed model included fixed factors representing the type of game, the type of playing sessions, and the interaction between these two factors. For the mixed model on O2Hb, a channel factor with its interactions were also entered as fixed factors. The mixed models were also controlled for time effect by entering variables representing game order, playing session order, and time of measurements within the playing sessions (not reported in the Results section). The intercept was defined as a random factor that could vary for each participant. Effects were considered significant at an alpha level of .05. Pairwise comparisons with Sidak adjustments were carried out to explore the effects of the fixed factors. For exploratory purposes, Pearson’s correlations between self-reports and physiological and cerebral hemodynamics measures were also performed using data obtained for each condition in each game for each participant (4 × 2 × 20 = 160 data points). To explore specific patterns within the frontoparietal attention network, we also correlated each individual channel with self-reports of flow and attentional focalization.
Results
Psychological measures
Concerning experienced difficulty, the mixed model revealed a main effect of condition, F(3, 1251) = 1366.514, p < .001, indicating that for both games, the easy condition was perceived as less difficult than optimal, which was perceived as less difficult than autonomy, which was also perceived as less difficult than the hard condition (see Fig. 3). The model also revealed an interaction between condition and game. In Tetris, the significant effect of condition, F(3, 6160) = 1,068.346, p < .001, indicated that while autonomy and optimal conditions led to similar experienced difficulty, the easy condition led to less experienced difficulty than the other conditions, and hard led to greater experienced difficulty than the other conditions (see Fig. 3). In Pong, the significant effect of condition, F(3, 6160) = 578.946, p < .001, indicated that the easy condition was perceived as less difficult than optimal, which was perceived as less difficult than autonomy, which was also perceived as less difficult than hard (see Fig. 3).
Perceived difficulty (a), flow experience (d), and attention (e) as a function of the condition (easy, hard, optimal, or autonomy). Results of perceived difficulty are also shown separately for Tetris (b) and Pong (c) because a significant interaction indicated a different pattern in the two games. *p < .05. **p < .01. Error bars represent standard errors. (Color figure online)
Concerning subjective flow experiences, the mixed model revealed a main effect of condition, F(3, 1310) = 14.091, p < .001, indicating that for both games combined, the optimal and autonomy conditions showed similar flow feelings scores, which were greater than the flow feelings reported in the easy and hard conditions (see Fig. 3).
For attentional focalization, a generalized mixed model with a logistic function was used to deal with the binary nature of the data. The model revealed a main effect of condition, F(3, 4660) = 32.215, p < .001, indicating that for both games, attentional focusing was less important in easy than in the three other conditions (see Fig. 3).
Physiological parameters
The mixed model on HR revealed a main effect of condition, F(3, 1180) = 6.592, p < .001. For both games, HR was significantly lower in the easy and significantly higher in the hard than in the other conditions (see Fig. 4).
Heart rate (HR) (a), heart rate variability (HRV) (b), and breathing rate (BR) (e) measured by condition. Results of HRV are also showed separately for Tetris (c) and Pong (d) because a significant interaction indicated a different pattern in the two games. *p < .05. ** p < .01. Error bars represent standard errors. (Color figure online)
For HRV, the mixed model revealed a main effect of condition, F(3, 994) = 9.105, p < .001, indicating that for both games, the easy condition led to higher HRV than in the other conditions. HRV was smaller in autonomy than in the other conditions (see Fig. 4). The model also revealed an interaction between condition and game, F(3, 942) = 3.430, p = .020. In Tetris, the significant effect of condition, F(3, 463) = 3.102, p < .036, indicated that the autonomy condition led to lower HRV than in the other conditions (see Fig. 4). In Pong, the significant effect of condition, F(3, 590) = 9.080, p < .001, indicated that the easy condition showed higher HRV than all the other conditions (see Fig. 4).
For BR, the mixed model revealed a main effect of game, F(1, 1289) = 8.960, p = .003, indicating a higher value for Pong (M = 18.553, SE = 0.535) than for Tetris (M = 17.756, SE = 0.535). A main effect of condition was also found, F(3, 1287) = 32.333, p < .001, indicating that BR was smaller in the easy than in the other three conditions. BR was also smaller in the optimal than in the autonomy condition (see Fig. 4).
Brain hemodynamic parameters
After exclusion of noisy channels (19% of the data), the mixed model showed a main effect of game, with a higher O2Hb concentration in Tetris than in Pong, F(1, 2948) = 3.901, p = .048. A channel effect was found, indicating regional variations in O2Hb, F(7, 2064) = 7.295, p < .001. A condition effect was also revealed, F(3, 3056) = 15.457, p < .001, with higher O2Hb concentrations during the autonomy condition than in all other conditions, and higher concentrations during the optimal condition than in the easy condition. Importantly, the interaction between channel and condition was also found, F(21, 3056) = 2.785, p < .001, indicating regional variations in the condition effect. Figure 5 illustrates the spatial localization of O2Hb concentration changes as a function of the condition and displays the significant pairwise comparisons. As observed, while a condition effect was present on most channels (all channels except Channel 7), the pattern of the condition effect was not homogeneous. In most cases (Channels 1, 2, 3, 5, 6 and 8), an inverted-U pattern could be seen as a function of the level of difficulty, with the highest concentrations in the autonomy condition and the lowest concentrations for the easy and hard conditions. A different pattern was seen on the most medial channel of the frontal area (Channel 4) as the relation between O2Hb and the level of difficulty was instead linear there, with the lowest value in the easy condition and the highest value in the hard condition.
Effects of the experimental conditions (easy, optimal, autonomy and hard) on oxyhemoglobin (O2Hb) concentration changes to the resting periods preceding the playing sessions (representing 0 μm), in all measured channels and combined games. Average signal in all areas measured (a) and in each individual channels (b). *p < .05. **p < .01. ***p < .001. Error bars represent standard errors. In the lower panel, oxyhemoglobin concentrations are then projected on the cortical surface depending on the spatial location of each individual NIRS channel (c). (Color figure online)
Correlation between psychological and physiological parameters
The correlation matrix (see Table 2) indicated that flow feelings were strongly associated to self-reports of attention. Experienced difficulty was positively associated with self-reports of attention, and with breathing rate. Self-reports of attention were also positively correlated with breathing rate. Both flow feelings and attention were also positively associated with the average O2Hb concentration of all channels of the right frontoparietal areas. As indicated by Table 3, flow feelings were specifically correlated to all channels of the frontal area at the exception of the most medial channel (Channel 4) and correlated to the two most inferior/lateral channels of the parietal area. Attentional focalization was only correlated to the two most lateral channels of the frontal area.
Discussion
In the present study, we examined the neurophysiological responses associated with the flow state in two video games in order to examine if these responses are linked to the mobilization of attentional resources. Having two different game tasks in the same protocol allowed us to distinguish general effect from task-specific effects. In addition, while flow is always manipulated through the variation of the difficulty level, which is problematic, as each difficulty level is associated with a different amount of information processing, we also wanted to compare this standard manipulation of flow with another type of flow induction, that is, by providing participants with autonomy in the task.
The results on experienced difficulty confirmed that our manipulation of the level of difficulty was effective, as participants perceived the easy condition as easier and the hard condition as harder than the optimal condition. As participants often chose a more challenging difficulty level in the Pong game in the autonomy condition than in the optimal condition (see Table 1), they perceived the autonomy condition as slightly more difficult than the optimal condition in this game. On the other hand, the majority of participants chose the same level detected as optimal condition level to play in autonomy conditions (11 in Tetris, 13 in Pong), and there was no significant difference between the level of difficulty played in the optimal and autonomy conditions, what characterized a workload not very different in both conditions. Importantly, the optimal and autonomy conditions induced greater flow feelings than the easy and hard conditions. This result is in agreement with the results of previous studies which showed that an optimal level of difficulty elicits greater flow feelings than an easy or hard level of playing difficulty (Chanel, et al., 2011; Keller & Bless, 2008; Keller et al., 2011; Keller & Blomann, 2008; Yoshida et al., 2014). It is interesting to note that this pattern was different for attentional focusing, as the optimal and autonomy conditions had greater attentional focus compared with the easy condition but were not different from the hard condition. Even if flow and attentional focusing are strongly related (see Table 2), a dissociation can thus be observed between flow and attentional focusing because, in spite of attentional focusing, the participants did not achieve flow in the hard condition. The observation that participants were focused on the task in the hard condition can, however, be related to a lack of precision of our measure. Hard conditions where the level of difficulty exceeds the player’s ability typically are known to elicit worries about the performance and ego-related concerns (Moneta & Csikszentmihalyi, 1996). Thus, it is possible that the participants indicated that they were thinking about the task when they experienced this type of thoughts and worries. Unfortunately, our measure was not precise enough to determine if participants thought about their negative performance in the task or if they stayed focused and tried to cope with the difficulty of the game during the hard condition.
In line with previous research, we wanted to examine how the autonomic system responded during flow. HR increase, an index of the prevalence of the sympathetic system (Thijssen et al., 2014), was significantly affected by the manipulation of the difficulty level, displaying a linear pattern with the difficulty of the task. While HR was similar in optimal and autonomy gaming conditions, the easy condition led to a lower HR and the hard condition led to a higher HR. As indicated by other studies (de Manzano et al., 2010; Harmatt et al., 2015; Peifer, Schulz, Schächinger, Baumann, & Antoni, 2014; Tozman, Magdas, MacDougall, & Volmeyer, 2015), this result could suggest that there was a sympathovagal balance controlling HR related to significantly higher flow in optimal and autonomy conditions. Concerning HRV (RMSSD), which predominantly reflects the vagal tone, or in other words, the activity of the parasympathetic system (Goldberger, Challapalli, Tung, Parker, & Kadish, 2001; van Ravenswaaij-Arts, Kollee, Hopman, Stoelinga, & van Geijn, 1993), it was higher on easy sessions than in all other conditions, and it was lower in the autonomy condition than in all other conditions. Previous findings have similarly observed a decrease in HRV during flow (e.g. Chanel et al., 2011; Keller et al., 2011; Tozman et al., 2015). Interestingly, it should be noted that a decrease in HRV has typically been found in conditions of mental effort and effortful attentive state (Hjortskov et al., 2004; Mukherjee, Yadav, Yung, Zajdel & Oken, 2011; Taelman, Vandeput, Vlemincx, Spaepen, & Van Huffel, 2011). As for BR, another index of the sympathetic system, it was lower on the easy than on the other conditions and it was higher on the autonomy compared with the optimal condition. It should be noted that if HR, HRV, and BR were not correlated with flow, BR were significantly associated with the attentional focus.
In sum, in the optimal and autonomy conditions, participants displayed a high level of flow feelings on the task, which was accompanied by a high level of attention compared with easy condition, by a medium-balance of HR, by a balanced level of BR in the optimal condition, and by an increase of BR and by a reduction of HRV in the autonomy condition. Accordingly, these results could suggest rather a medium vagal-sympathetic balance in the optimal-high-flow condition, and a positive sympathovagal balance in the autonomy-high-flow condition.
Several studies have shown that engagement of attentional resources is intimately linked to the positive sympathovagal balance (e.g., Zanstra et al., 2006). In fact, this close correspondence may be explained by the fact that attention and sympathetic activity share the same regulatory mechanism, relying both on the locus coeruleus and the noradrenergic pathway (Aston-Jones & Cohen, 2005; Sara & Bouret, 2012).
An important objective of our study was to examine cerebral activity in the regions of the frontoparietal attentional network. We monitored changes in O2Hb concentrations as they can be interpreted as changes in brain activity since O2Hb is presumed to increase as a metabolic adjustment in response to neural firing (Obrig & Villringer, 2003). The significant positive correlation we observed between the self-reported measure of attention and the average O2Hb concentration in the frontoparietal regions measured in this study indicated that this neurophysiological index was adequate to monitor the engagement of attentional resources. Nevertheless, when looking at correlations of individual channels (see Table 3), only the most lateral channels of the frontal area (right dlPFC) were significantly associated to self-reports of attentional focalization. Previous NIRS studies also reported significant activations in this area during the mobilization of attentional resources (Durantin et al., 2015; Harrivel et al., 2013; Stevenson et al., 2011). The different playing conditions of our study led to significant differences in the average O2Hb concentration of the frontoparietal regions recorded. It should also be noted that no interaction effect was found with the type of game, which suggests that the pattern of results obtained on this average O2Hb index was not task dependent. Specifically, we found that, in the optimal and hard conditions, the average O2Hb concentration was higher than in the easy condition, which suggests a higher activity of the frontoparietal attentional network in these conditions. It should be noted that no significant difference was found between the overall O2Hb concentrations in the optimal and hard conditions, suggesting that the frontoparietal attentional network was similarly active in these two conditions. However, whereas the average O2Hb concentration was of similar amplitude, the localization of the signal within the different regions suggested a different spatial pattern between these two conditions for the PFC area. As can be seen in Fig. 5, an inverted-U pattern could be seen in most channels with the level of difficulty, and this was particularly salient on the channels located in the lateral part of the frontoparietal network. In these channels, both the autonomy and optimal conditions led to higher activations than in the hard conditions. Because the lateral part of the frontoparietal network is often viewed as being primarily involved during top-down attention (Corbetta & Schulman, 2002) and sustained attention in a task (Esterman et al., 2014), these results, in fact, can be interpreted as an active engagement of attentional resources during flow. But the optimal and autonomy conditions not only led to strong activations in lateral PFC, these also led to a deactivation in medial PFC compared with the previous rest periods, in contrast to what happened in the hard condition. Given that the medial PFC has been frequently related to mind wandering and self-preoccupations occurring during a task (Esterman et al., 2014; Gusnard et al., 2001), it could support the hypothesis that the hard condition led participants to worry about their performance in the task. The deactivation during the optimal and autonomy difficulty conditions on medial PFC could explain the commonly related effortless attention (Ullén et al., 2010) as a lack of mind wandering and worries, as it can provide support to the hypothesis about a state of localized hypofrontality (Dietrich, 2003) during flow.
We also found that the autonomy condition led to greater average O2Hb concentration in the frontoparietal regions than did the easy and hard conditions but also that it also did in the optimal conditions. When comparing regional differences between the optimal and autonomy conditions, significant differences were observed in both frontal and parietal areas. Specifically, more activation was found in the lateral posterior part of the inferior frontal cortex and in the lateral part of the frontal cortex. These locations typically represent the core regions involved in the engagement of voluntary attention (Corbetta & Schulman, 2002). In sum, while no significant difference emerged between optimal and autonomy gaming conditions on the reports of flow feelings, these two conditions led to different results for several variables. It was related to greater activation of the frontoparietal attentional network concerning the use of an autonomy condition to induce a flow experience.
The results on the self-selected condition could be explained by the self-choice bringing an enhancement of importance on the task, firing an engagement of attentional resources. Perhaps with the autotelic parameter of flow arising through the autonomic free choices (e.g., Moller et al., 2010), and suggesting flow experiences for the participants in a different way compared with the condition that considered only an optimal skill-challenge balance.
Some limitations of the present study are worth mentioning. Firstly, the increasing learning effect throughout the playing sessions could have affected our manipulation. For example, the hard condition would be less hard if played as the last than as the first block. In addition, because some participants chose a different difficulty level in the autonomy condition than in the optimal difficulty condition, it is possible that some differences found could be explained by the workload and not by the level of autonomy. For this reason, we encourage further research with a more controlled manipulation of autonomy, in order to really establish the capacity of autonomy to induce flow. We also encourage future studies to use a frequency analysis of HRV in order to more accurately estimate the sympathovagal balance (Berntson et al., 1997; Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology, 1996). Last, the use of subjective measures of effort could also be beneficial to distinguish states of effortless from states of effortful attention.
In conclusion, our study shows flow is tightly linked to attention. We observed that flow feelings were highly correlated with self-reports of attention and with a neurophysiological index accounting for the activity of the neural attention network. Interestingly, we also found that even if flow is characterized as a state of effortless attention (Bruya, 2010; Ullén et al., 2010), the neurophysiological changes occurring during flow are very similar to those occurring during effortful attentive states, mostly in autonomy conditions. In line with the regional pattern of neural activations found, an experience of flow could possibly, due to the concurrent activation of the lateral frontoparietal attention network (Corbetta & Schulman, 2002) and deactivation of the medial PFC, suggest an absence of worry and self-concern about one’s performance.
Author note and acknowledgements
Marcelo Felipe de Sampaio Barros was supported by a scholarship from the CAPES Foundation of the Ministry of Education of Brazil, Brasília–DF 70040-020, Brazil (No. 99999.010663/2014-02). Rémi Radel was supported by a grant of the Agence Nationale de la Recherche (ANR–JCJC, 2013-069).We would like to thank Guillaume Chanel and Vitória M. Piai for their help and advices for the design of this study.
References
Aston-Jones, G., & Cohen, J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403–450. https://doi.org/10.1146/annurev.neuro.28.061604.135709
Baayen, R. H., Davidson, D. J., & Bates, D. M. (2008). Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language, 59(4), 390–412. https://doi.org/10.1016/j.jml.2007.12.005
Berntson, G. G., Bigger, J. T., Eckberg, D. L., Grossman, P., Kaufmann, P. G., Malik, M., & Molen, M. W. D. (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. https://doi.org/10.1111/j.1469-8986.1997.tb02140.x
Cabeza, R., & Nyberg, L. (2000). Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience, 12, 1–47. https://doi.org/10.1162/08989290051137585
Ceja, L., & Navarro, J. (2012). ‘Suddenly I get into the zone’: Examining discontinuities and nonlinear changes in flow experiences at work. Human Relations, 65(9), 1101–1127. https://doi.org/10.1177/0018726712447116
Chanel, G., Rebetez, C., Betrancourt, M., & Pun, T. (2011). Emotion assessment from physiological signals for adaptation of game difficulty. IEEE Transactions on Systems, Man, and Cybernetics—Part A: Systems and Humans, 41(6), 1052–1063. https://doi.org/10.1109/TSMCA.2011.2116000
Christoff, K., Gordon, A. M., Smallwood, J., Smith, R., &, Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8719–872. https://doi.org/10.1073/pnas.09002341064
Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–215. https://doi.org/10.1038/nrn755
Coull, J. T. (1998). Neural correlates of attention and arousal: Insights from electrophysiology, functional neuroimaging and psychopharmacology. Progress in Neurobiology, 55(4), 343–361. https://doi.org/10.1016/S0301-0082(98)00011-2
Csikszentmihalyi, M. (1975). Beyond boredom and anxiety. San Francisco, CA: Jossey-Bass.
Csikszentmihalyi, M. (1990). Flow: The psychology of optimal experience. New York, NY: Harper Perennial.
de Manzano, Ö., Theorell, T., Harmat, L., & Üllen, F. (2010). The psychophysiology of flow during piano playing. Emotion, 10(3), 301–311. https://doi.org/10.1037/a0018432
Dietrich, A. (2003). Functional neuroanatomy of altered states of consciousness: The transient hypofrontality hypothesis. Consciousness and Cognition, 12, 231–256. https://doi.org/10.1016/S1053-8100(02)00046-6
Durantin, G., Dehais, F., & Delorme, A. (2015). Characterization of mind wandering using fNIRS. Frontiers in Systems Neuroscience, 9, 45. https://doi.org/10.3389/fnsys.2015.00045
Engeser, S., & Rheinberg, F. (2008). Flow, performance and moderators of challenge-skill balance. Motivation and Emotion, 32(3), 158–172. https://doi.org/10.1007/s11031-008-9102-4
Esterman, M., Rosenberg, M. D., & Koonan, S. K. (2014). Intrinsic fluctuations in sustained attention and distractor processing. Journal of Neuroscience, 34(5), 1724–1730. https://doi.org/10.1523/JNEUROSCI.2658-13.2014
Fekete, T., Rubin, D., Carlson, J. M., & Mujica-Parodi, L. R. (2011). The NIRS analysis package: Noise reduction and statistical inference. PLOS ONE, 6(9), e24322. https://doi.org/10.1371/journal.pone.0024322
Fox, MD., Zhang, D., Snyder, A. Z., & Raichle, M. E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101(6), 3270–3283. https://doi.org/10.1152/jn.90777.2008
Gaggioli, A., Cipresso, P., Serino, S., Riva, G. (2013). Psychophysiological correlates of flow during daily activities. Studies in Health Technology and Informatics, 191, 65–9. https://doi.org/10.3233/978-1-61499-282-0-65
Goldberger, J. J., Challapalli, S., Tung, R., Parker, M. A., & Kadish, A. H., (2001). Relationship of heart rate variability to parasympathetic effect. American Heart Association: Clinical Investigation and Reports, 103, 1977–1983. https://doi.org/10.1161/01.CIR.103.15.1977
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258. https://doi.org/10.1073/pnas.0135058100
Gusnard, D. A., Akbudak, E., Shulman, G. L., & Raichle, M., E. (2001). Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(7), 4259–4264. https://doi.org/10.1073/pnas.071043098
Haeussinger, F. B, Heinzel, S., Hahn, T., Schecklmann, M., Ehlis, A.-C., & Falgatter, A.J. (2011). Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: Implications for optical neuroimaging. PLOS ONE, 6(10), e26377. https://doi.org/10.1371/journal.pone.0026377
Hailstone, J., & Kilding, A. E. (2011). Reliability and validity of the Zephyr™ BioHarness™ to measure respiratory responses to exercise. Measurement in Physical Education and Exercise Science, 15(4), 293–300. https://doi.org/10.1080/1091367X.2011.615671
Harmat, L., de Manzano, Ö., Theorell, T., Högman, L., Fischer, H., & Ullén, F. (2015). Physiological correlates of the flow experience during computer game playing. International Journal of Psychophysiology, 97, 1–7. https://doi.org/10.1016/j.ipsycho.2015.05.001
Harrivel, A. R., Weissman, D. H., Noll, D. C., & Peltier, S. J. (2013). Monitoring attentional state with fNIRS. Frontiers in Human Neuroscience, 7(861). https://doi.org/10.3389/fnhum.2013.00861
Hjortskov, N., Rissén, D., Blangsted, A. K., Fallentin, N., Lundberg, U., & Søgaard, K. (2004). The effect of mental stress on heart rate variability and blood pressure during computer work. European Journal of Applied Physiology 92, 84–89. https://doi.org/10.1007/s00421-004-1055-z
Huppert, T. J., Diamond, S. G., Franceschini, M. A., & Boas, D. A. (2009). HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Applied Optics, 48(10), 280–298. https://doi.org/10.1364/AO.48.00D280
Kahneman, D. (1973). Attention and effort. Upper Saddle River, NJ: Prentice Hall.
Keller, J., & Bless, H. (2008). Flow and regulatory compatibility: an experimental approach to the flow model of intrinsic motivation. Personality and Social Psychology Bulletin, 34(2), 196–209. doi:https://doi.org/10.1177/0146167207310026
Keller, J., Bless, H., Blomann, F., & Kleinböhl, D. (2011). Physiological aspects of flow experiences: Skills-demand-compatibility effects on heart rate variability and salivary cortisol. Journal of Experimental Social Psychology, 47, 849–852. https://doi.org/10.1016/j.jesp.2011.02.004
Keller, J., & Blomann, F. (2008). Locus of control and the flow experience: An experimental analysis. European Journal of Personality, 22(7) 589–607. https://doi.org/10.1002/per.692
Kim, J.-H., Roberge, R., Powell, J. B., Shafer, A. B., & Williams, W. J. (2013). Measurement accuracy of heart rate and respiratory rate during graded exercise and sustained exercise in the heat using the Zephyr BioHarnessTM. International Journal of Sports Medicine, 34(6), 497–501. https://doi.org/10.1055/s-0032-1327661
Klasen, M., Weber, R., Kircher, T. T., Mathiak, K. A., & Mathiak, K. (2012). Neural contributions to flow experience during video game playing. Social Cognitive and Affective Neuroscience. 7(4), 485–495. https://doi.org/10.1093/scan/nsr021
Kowal, J., & Fortier, M. S. (1999). Motivational determinants of flow: Contributions from self-determination theory. The Journal of Social Psychology, 139(3) 355–368. https://doi.org/10.1080/00224549909598391
Laborde, S., Mosley, E., & Thayer, J. F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research—Recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology, 8, 213. https://doi.org/10.3389/fpsyg.2017.00213
Lim, J., Wu, W., Wang, J., Detre, J.A, Dinges, D. F., & Rao, H. (2010). Imaging brain fatigue from sustained mental workload: An ASL perfusion study of the time-on-task effect. NeuroImage, 49(4), 3426–3435. https://doi.org/10.1016/j.neuroimage.2009.11.020
Moller, A. C., Meier, B. P., & Wall, R. D. (2010). Developing an experimental induction of flow: effortless action in the lab. In Bruya, B. Effortless attention: a new perspective in cognitive science of attention and action (pp. 191-204). The MIT Press. https://doi.org/10.7551/mitpress/9780262013840.003.0010
Moneta, G. B., & Csikszentmihalyi, M. (1996). The effect of perceived challenges and skills on the quality of subjective experience. Journal of Personality, 64, 275–310. https://doi.org/10.1111/j.1467-6494.1996.tb00512.x
Mukherjee, S., Yadav, R., Yung, I., Zajdel, D. P., & Oken, B. S. (2011). Sensitivity to mental effort and test–retest reliability of heart rate variability measures in healthy seniors. Clinical Neurophysiology, 122(10), 2059–2066. https://doi.org/10.1016/j.clinph.2011.02.032
Munoz, M. L., van Roon, A., Riese, H., Thio, C., Oostenbroek, E., Westrik, I., . . . Sneider, H. (2015). Validity of (ultra-)short recordings for heart rate variability measurements. PLOS ONE, 10(9), e0138921. https://doi.org/10.1371/journal.pone.0138921
Obrig, H., & Villringer, A. (2003). Beyond the visible—Imaging the human brain with light. Journal of Cerebral Blood Flow & Metabolism, 23(1), 1–18. https://doi.org/10.1097/01.WCB.0000043472.45775.29
Ogg, R. J., Zou, P., Allen, D. N., Hutchins, S. B., Dutkiewicz, R. M., & Mulhern, R. K. (2008). Neural correlates of a clinical continuous performance test. Magnetic Resonance Imaging, 26(4), 504–512. https://doi.org/10.1016/j.mri.2007.09.004
Otzenberger, H., Gronfier, C., Simon, C., Charloux, A., Ehrhart, J., Piquard, F., & Brandenberger, G. (1998). Dynamic heart rate variability: A tool for exploring sympathovagal balance continuously during sleep in men. American Journal of Physiology, 275(3, Pt. 2), H946–H950. https://doi.org/10.1152/ajpheart.1998.275.3.H946
Paus, T., Zatorre, R. J., Hofle, N. Caramanos, Z., Gotman, J., Petrides, M., & Evans, A. C. (1997). Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. Journal of Cognitive Neuroscience, 9(3), 392–408. https://doi.org/10.1162/jocn.1997.9.3.392
Peifer, C., Schulz, A., Schächinger, H., Baumann, N., & Antoni, C. H. (2014). The relation of flow-experience and physiological arousal under stress—Can u shape it?. Journal of Experimental Social Psychology, 53, 6269. https://doi.org/10.1016/j.jesp.2014.01.009
Posner, M. I. (1990). The attention system of the human brain. Annual Review of Neurosciences, 13, 25–42. https://doi.org/10.1146/annurev.ne.13.030190.000325
Pribram, K. H., & McGuinness, D. (1975). Arousal, activation, and effort in the control of attention. Psychological Review, 82(2), 116–149. https://doi.org/10.1037/h0076780
Quaresima, V., Bisconti, S., & Ferrari, M. (2012). A brief review on the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain and Language, 121(2), 79–89. doi:https://doi.org/10.1016/j.bandl.2011.03.009
Rheinberg, F., & Vollmeyer, R. (2003). Diagnosis of motivation and self-concept. Hogrefe, 261–279.
Rheinberg, F., Vollmeyer, R., & Engeser, S. (2003). Die Erfassung des Flow-Erlebens [The capture of the flow experience]. Retrieved from http://www.psych.uni-potsdam.de/people/rheinberg/messverfahren/flow-fks.pdf
Richards, J. E. (1987). Infant visual sustained attention and respiratory sinus arrhythmia. Child Development, 58(2), 488–496. https://doi.org/10.1111/j.1467-8624.1987.tb01396.x
Sara, S. J., & Bouret, S. (2012). Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron, 76(1), 130–141. https://doi.org/10.1016/j.neuron.2012.09.011
Scholkmann, F., Spichtig, S., Muehlemann, T., & Wolf, M. (2010). How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiological Measurement, 31(5), 649–662. https://doi.org/10.1088/0967-3334/31/5/004
Smallwood, J., Beach, E., Schooler, J. W., & Handy, T. C. (2008). Going AWOL in the brain: Mind wandering reduces cortical analysis of external events. Journal of Cognitive Neuroscience, 20(3), 458–469. https://doi.org/10.1162/jocn.2008.20037
Stevenson, H., Russell, P. N., & Helton, W. S. (2011). Search asymmetry, sustained attention, and response inhibition. Brain Cognition, 77(2), 215–22. https://doi.org/10.1016/j.bandc.2011.08.007
Strangman, G. E., Zhang, Q., & Li, Z. (2014). Scalp and skull influence on near infrared photon propagation in the Colin27 brain template. NeuroImage, 85, 136–149. https://doi.org/10.1016/j.neuroimage.2013.04.090
Taelman, J., Vandeput, S., Vlemincx, E., Spaepen, A., Van Huffel, S. (2011). Instantaneous changes in heart rate regulation due to mental load in simulated office work. European Journal of Applied Physiology, 111(7), 1497–1505. https://doi.org/10.1007/s00421-010-1776-0
Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology. (1996). Heart rate variability—Standards of measurement, physiological interpretation, and clinical use. European Heart Journal, 17, 254–381.
Thijssen, D. H., Atkinson, C. L., Ono, K., Sprung, V. S., Spence, A. L., Pugh, C. J., & Green, D. J. (2014). Sympathetic nervous system activation, arterial shear rate, and flow-mediated dilation. Journal of Applied Physiology, 116(10), 1300–1307. https://doi.org/10.1152/japplphysiol.00110.2014
Tozman, T., Magdas, E. S., MacDougall, H. G., & Vollmeyer, R. (2015). Understanding the psychophysiology of flow: A driving simulator experiment to investigate the relationship between flow and heart rate variability. Computers in Human Behavior, 52, 408–418. https://doi.org/10.1016/j.chb.2015.06.023
Ullén, F., de Manzano, Ö., Theorell, T., & Harmat, L. (2010). The physiology of effortless attention: Correlates of state flow and flow proneness. In B. Bruya (Ed.), Effortless attention: A new perspective in the cognitive science of attention and action (pp. 210–218). Cambridge, MA: MIT Press. doi:https://doi.org/10.7551/mitpress/9780262013840.003.0011
Ulrich, M., Keller, J., Hoenig, K., Walter, C., & Grön, G. (2014). Neural correlates of experimentally induced flow experiences. NeuroImage, 86, 194–202. doi:https://doi.org/10.1016/j.neuroimage.2013.08.019
van Ravenswaaij-Arts, C. M. A., Kollee, L. A. A., Hopman, J. C. W., Stoelinga, G. B. A, & van Geijn, H. P. (1993). Heart rate variability. Annals of Internal Medicine, 118, 436–447. https://doi.org/10.7326/0003-4819-118-6-199303150-00008
Yoshida, K., Sawamura, D., Inagaki, Y., Ogawa, K., Ikoma, K., & Sakai, S. (2014). Brain activity during the flow experience: A functional near-infrared spectroscopy study. Neuroscience Letters, 573, 30–34. https://doi.org/10.1016/j.neulet.2014.05.011
Zanstra, Y. J., Schellekens, J. M. H., Schaap, C., & Kooistra, L. (2006). Vagal and sympathetic activity in burnouts during a mentally demanding workday. Psychosomatic Medicine, 68(4), 583–590. https://doi.org/10.1097/01.psy.0000228012.38884.49
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 51 kb)
Rights and permissions
About this article
Cite this article
de Sampaio Barros, M.F., Araújo-Moreira, F.M., Trevelin, L.C. et al. Flow experience and the mobilization of attentional resources. Cogn Affect Behav Neurosci 18, 810–823 (2018). https://doi.org/10.3758/s13415-018-0606-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-018-0606-4