Abstract
Emotions affect many aspects of cognition (attention, decision-making, problem solving, conflict resolution, task switching, social cognition, etc.), but the cortical areas or networks through which these effects are achieved are still debatable. In the present study, the effect of emotion on cognition was studied in healthy young individuals (n = 56). Emotions were induced using high-arousing negative, positive, and low-arousing neutral pictures from the International Affective Picture System (IAPS). Sternberg’s verbal working memory task was administered at baseline and after each emotion exposure, while high-density EEG was recorded. Cortical sources were calculated using sLORETA in the 500-ms window (for every 100 ms bin) before the response and were compared with baseline. Though the number of correct responses were comparable, reaction times after emotion exposure reduced significantly. Source analysis revealed significant deactivation of default mode network (DMN) areas as well as early deactivation of decision-making areas during Sternberg’s task performed after both the negative and positive emotions. This early deactivation, much before the response was made, when compared with baseline suggests that tasks performed under high-arousing emotional states may help in making decisions earlier or faster. We conclude that the exposure to high-arousing emotional stimuli improves verbal working memory by helping in directing the attentional resources toward the task, thus decreasing the decision-making time and further suppressing the DMN areas.
Similar content being viewed by others
An individual’s emotional status affects many aspects of his or her behavior in everyday situations, such as attention, decision-making, problem-solving ability, conflict resolution, task switching, and social cognition. This interaction between emotional and cognitive behavior has long drawn the attention of researchers. In the literature, two approaches have been discussed to explain how emotions influence cognition. The first approach states that because the understanding of emotions is crucial for survival, the processing of emotions is fast, automatic, unintentional, and unconscious, and thus cannot be avoided or interrupted in its course (Schneider & Shiffrin, 1977). Many visual search paradigms have proved this theory, where an emotionally salient stimulus was shown to be detected faster and more accurately than the nonemotional stimulus (e.g., Öhman, Lundqvist, &Esteves, 2001b). This faster and efficient processing was seen to be mediated by the amygdala, a core area of limbic system. Whalen et al. (1998), in an fMRI study, showed that facial stimuli with emotional expressions are perceived even in the absence of awareness, and the amygdala is the core area mediating this unconscious processing. Such studies further proved that the processing of emotional stimuli is attention independent (Morris, Öhman, & Dolan, 1999; Öhman, Flykt, & Esteves, 2001a; Vuilleumier et al., 2001). Although the exact mechanism of this rapid and automatic processing is still unknown, studies have suggested that direct subcortical pathways that reach the amygdala independently of the typical cortical connections subserving various sensorial modalities are implicated in this automatic processing of emotion (Vuilleumier, 2005). The second approach states that emotion processing is not automatic, but rather dependent on attentional resources (Pessoa, 2005). An MRI study by Pessoa, McKenna, Gutierrez, and Ungerleider (2002) showed that emotional facial expressions cause activation in many areas related to emotion processing including the amygdala, but as the task demand increases, and more attentional resources are being used in the task, this activation pattern was not seen. These findings suggest that the emotional stimuli do capture attention, but only if enough attentional resources are available, proving that emotion processing is not attention independent.
One major reason for the presence of these discrepancies in the literature regarding the nature of emotion–cognition interaction could be that various researchers have employed various tasks to study these interactions, and the difficulty level of these tasks can influence the results of the studies. Also, in most of the studies, emotions have been used as distractors, where they have been presented during the task, thus masking the effect of task processing with emotion-related processing.
To overcome the limitations of the existing literature in the present study, emotions were used to induce an emotional state using standardized pictures from the International Affective Picture System (IAPS) before the cognitive function task.
The objective of the study was to identify the brain sources responsible for deciding the salience of the response during a task performed under the influence of negative, positive and neutral emotions. This was studied in healthy young individuals by assessing their performance in a Sternberg’s verbal working memory task (Sternberg, 1966) performed after the emotion exposure. Performance in a response-based task can be measured by assessing the number of correct scores and the corresponding reaction times obtained. To understand the cortical areas or networks influencing the performance in the task performed after emotion exposure, a time window of 500 ms just before the response (retrieval period) was selected for source localization. The cortical sources were calculated in this period and compared with the respective period of the task performed at baseline to elucidate the cortical areas or networks through which emotions influence cognition.
Method
Participants
Sixty right-handed (as assessed by Edinburgh’s Handedness Inventory) healthy young subjects, of both genders, with normal or corrected-to-normal vision, were selected for the study. The data of four subjects were discarded because of EEG artifact. Results are reported for 56 subjects (28 males, Mage = 26.1 ± 3.0 years; 28 females, Mage = 27.3 ± 3.0 years). Upon arrival at the laboratory, all participants were familiarized with the experimental procedure, and informed consent was given. To rule out the effects of cyclical hormonal changes on the perception of emotion and cognition, experiments with female participants were performed when they were in the follicular phase of the menstrual cycle. The study protocol was approved by the Institute Ethical Committee.
Emotional stimuli
Emotional pictures were selected from International Affective Picture system (Lang, Bradley & Cuthbert, 2008) on the basis of already available valence and arousal ratings for both the male and female population. High-arousing (5.3–6.7) emotional pictures of negative valence (1.3–2.7), positive valence (6.2–7.6), and low-arousing (2.5–3.9) neutral valence (4.2–5.6) pictures were chosen for the study separately for female (307 pictures) and male (285 pictures) participants.
Sternberg’s verbal working memory task
A chunk of six random English letters was presented at the center of the screen for 2 s for the subject to encode. After that, in place of letters, a fixation mark “+” appeared on the screen for 2 s. Then, a probe letter appeared on the screen to which the subjects responded using a response pad, where a key press of “1” was taken as “yes” if the probe letter was present, and a key press of “2” was taken as “No” if the probe letter was not present in the earlier presented chunk. Subjects were instructed to respond correctly and as quickly as possible. Twenty such trials were administered to each subject (see Fig. 1). If a subject failed to respond after the presentation of probe letter, the trial automatically terminated after 4 s. Feedback was given to the subject after every trial in terms of accuracy and response time (ms). This helped in keeping the subject’s attention on the task if they made too many incorrect responses.
All the picture presentation paradigms and the cognitive function tasks were designed using E-Prime 2.0 software on a stimulus presentation computer. The tasks were presented on a 19-inch computer screen with a frame refresh rate of 60 Hz. The screen was placed 1.5 m in front of the viewer, resulting in a picture presentation with a visual angle of 15° horizontally and 11° vertically.
Procedure
The experiment started with the baseline task performance on Sternberg’s task. Each participant was then exposed to all the three emotions sequentially, each followed by assessment on Sternberg’s task. In each emotional block, 20 randomly selected IAPS pictures of a particular emotional category were presented for 5,000 ms each, with no interstimulus interval, thus making total exposure time 100 s for each emotional block. At the end of the experiment, the subjects rated the pictures seen during the experiment in a pseudorandom order on the basis of a computer-based self-assessment manikin (SAM) scale of valence and arousal. The order of emotional picture block presentations was kept random, with the neutral block always appearing in the middle. A 2-minute resting period was given between each subsequent block presentation.
EEG recording
EEG was recorded for all the tasks and picture presentation periods using Electrical GeodesicsTM 128-electrode nets digitized at a rate of 500 Hz. Recording was done with common vertex (Cz) as a recording reference, and impedance was kept below 50 kΩ as recommended by the manufacturers for high input impedance amplifiers. All channels were preprocessed online by means of 0.1–Hz high-pass and 100-Hz low-pass filtering.
EEG preprocessing
EEG data processing was done off-line using Net Station 4.5.4 and EEGLAB (http://www.sccn.ucsd.edu/eeglab/, Salk Institute, La Jolla, CA; Delorme & Makeig 2004). The EEG signal was band-pass filtered at 0.5–100 Hz, with a notch filter at 50 Hz. Segments of 500 ms were then cut just before the response tag for each of the trials. For removing artifacts like eye movement, eye blinks, and bad channels, Netstation’s semi–automated artifact-detection algorithm was used. Bad channels were interpolated with spherical spline interpolation. Further, data were exported to the EEGLAB platform where average referencing was done to remove the reference site bias. Independent component analysis (ICA) was performed using the default RUNICA algorithm to remove artifacts like ECG, power line noise, and respiratory artifacts. These steps of preprocessing the data were performed for all tasks performed at baseline and after emotion presentation.
Source localization analysis
Based on the average response time and the standard deviation across all the subjects for all the four tasks (including baseline task), a window of 500 ms before response (i.e., retrieval period) was selected. This 500-ms period was further broken down into 100-ms epochs to calculate cortical sources. For each subject and each task, a segment of 500-ms duration before the response tag was selected for analysis.
Standardized low-resolution brain electromagnetic tomography (sLORETA; Pascual-Marqui, 2002) was used to calculate the intracortical electrical sources that generated the scalp-recorded activity. sLORETA is a distributed source localization technique that solves the inverse problem without assuming an a priori number of underlying sources, and computes electric neural activity as standardized current density (unit: amperes per square meter, A/m2). The probabilistic location of gray matter in the average MRI atlas of the Montreal Neurologic Institute (MNI) Atlas was used to constrain the location of 6,239 source voxels. Three orthogonal dipole moments (x, y, z) were defined and solved for each of the source voxels. The source space was restricted to 6,239 cortical voxels (5 mm3) that were each assigned to a gyrus and corresponding Brodmann areas using the MNI152 template.
Statistics
Both accuracy scores and correct response reaction times obtained in tasks performed after each emotion exposure were compared with their respective baseline values. Kolmogorov–Smirnov test was used to check the normality and data for both the parameters was found to be non-normal. Friedman test was used to compare both the parameters with their baseline, followed by Dunn’s post hoc test. Statistical significance was kept at p < .05.
For source localization, statistical comparisons were made for each of the 100-ms time windows of a 500 ms retrieval period by comparing them with their respective baselines. To find out the difference of cortical source localization between conditions, a voxel-by-voxel independent t test was used, based upon sLORETA current source density power. sLORETA applies a statistical nonparametric mapping method (SnPM; Holmes, Blair, Watson, & Ford, 1996) for statistical analysis of current source density. SnPM uses a nonparametric permutation/randomization procedure (i.e., based on the Fisher’s permutation method, with the threshold set at the 5% probability level), comparing the mean source power in each voxel and the distribution in the permuted values. By evaluating the empirical probability distribution of the “maximal-statistics” in the null hypothesis, permutation and randomization tests have demonstrated effectiveness in controlling Type I error in neuroimaging studies (Nichols & Holmes, 2002). For the present study, sLORETA used 5,000 data randomizations to determine the critical probability threshold values for the actually observed t test values with correction for multiple comparisons across all voxels, without the need to rely on Gaussianity. The levels of significance were corrected for multiple comparisons. For these comparisons, significance was determined at p < .05.
Results
Behavioral results
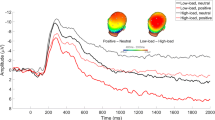
Accuracy scores obtained in the tasks performed after each emotion exposure showed no statistically significant difference when compared with their respective baselines, while correct response reaction times improved significantly for all the tasks performed after emotion exposure. When compared between emotional conditions, neither accuracy scores nor reaction times showed any statistically significant difference (see Fig. 2).
Source localization results
Sternberg’s task post negative emotion exposure
In the task performed after negative exposure, only decrease in the activation of source areas was observed in all the time windows studied before response was made, when compared with respective baseline. In 400–500 ms time window before response, decrease in activity was observed in the parahippocampal gyrus and fusiform gyrus. A significant decrease in activity was observed 300–400 ms before the response in the precuneus, superior parietal lobule, and middle frontal gyrus. In the 200–300 ms time window before response, only the precuneus showed significant decrease in activity. No significant changes were observed in the 0–200 ms time window before response (see Fig. 3a).
Sternberg’s task post neutral emotion exposure
For the task performed after the neutral exposure, only higher activation was observed in the source areas when compared with baseline. No significant change was observed in the 300–500 ms before response was made. A significantly higher activation was observed in the 200–300 ms time window before response in the middle temporal gyrus. In the 100–200 ms before response along with the middle temporal gyrus, significantly higher activation was observed in the middle frontal gyrus, middle occipital gyrus, and inferior temporal gyrus. Later, in the 0–100 ms time window before response, higher activation was again seen in the middle temporal gyrus and middle occipital gyrus (see Fig. 3b).
Sternberg’s task post positive emotion exposure
Similar to the task performed after negative exposure, in positive exposure task also, only lower activation in the source areas was observed when compared with baseline. In the 400–500 ms time window before response, significant lower activation was observed in the cuneus. In the 300–400 ms time window before response, significantly lower activation was observed in the posterior cingulate gyrus, medial frontal gyrus, postcentral gyrus, middle frontal gyrus, superior frontal gyrus, and precentral gyrus. In the 200–300 ms before response, along with middle frontal gyrus, significantly lower activation was observed for the middle temporal gyrus. In the 100–200 ms before response, significantly lower activation was observed in the middle frontal gyrus and inferior temporal gyrus. No significant change was observed for the 0–100 ms time window (see Fig. 3c).
Discussion
The present study was designed to elucidate the brain areas that govern the performance in a verbal working memory task after emotion exposure. For this purpose emotions were induced in the laboratory settings using standardized IAPS pictures in 56 young healthy subjects of both genders. Since emotional reactivity to an emotional stimuli is known to have gender-specific differences, IAPS has defined different picture sets for both male and female populations. To avoid confounding the results of the present study, instead of choosing similar pictures for both of the population, pictures were selected in a predefined range of valence and arousal from the original IAPS data set defined for both male and female populations.
For studying the effect of emotion on verbal working memory, subjects were asked to perform Sternberg’s task at baseline and after each emotion exposure. The cortical sources responsible for generating the responses in the task after emotion exposure were studied in the 500-ms time window before response (retrieval period) for every 100-ms bin using source current density analysis.
Neural correlates of Sternberg’s task performance after emotion exposure
The Sternberg’s task performed after negative emotion exposure, when compared with baseline task, showed a decrease in activity in the parahippocampal gyrus and fusiform gyrus during 400–500 ms before response was made. For the Sternberg’s task performed after positive emotion exposure, cuneus showed significant decrease in activity in the same time window. Cuneus has been shown to serve as a hub between default-mode network (DMN) type areas and the frontoparietal network. In an fMRI study by Sreenivas, Boehm, and Linden (2012) it was reported that along with many other areas, the cuneus showed deactivation in response to emotional faces. This study showed that DMN areas show deactivation during an emotional categorization task also. In the present study, this emotional processing related deactivation in the cuneus was seen for tasks after positive emotion only and not after negative and neutral conditions.
The DMN (Gusnard & Raichle, 2001; Raichle et al., 2001) is a set of cortical regions identified by reduced activity during many externally oriented tasks (Buckner, Andrews-Hanna, & Schacter, 2008; Shulman et al., 1997). DMN regions also exhibit coherent fluctuations during the resting state (Greicius, Krasnow, Reiss, & Menon, 2003). The task-based fMRI studies have shown that medial temporal lobe subregions such as the hippocampus and parahippocampal gyrus can be dynamically coupled and uncoupled with the DMN during the memory encoding phase or retrieval phase, suggesting that the interaction between medial temporal lobe subregions and DMN is context-dependent rather than static retrieval (Huijbers, Pennartz, Cabeza, & Daselaar, 2011; McCormick, Moscovitch, Protzner, Huber, & McAndrews, 2010; Vannini et al., 2010). A study by Ward et al. (2014) clearly demonstrated that the medial temporal lobe is connected to DMN inversely during the encoding phase through the hippocampus, while at rest it is connected to DMN directly through the parahippocampal gyrus. This suggests that the parahippocampal gyrus is functionally coupled to DMN at rest. A decrease in activity in the parahippocampal gyrus during the negative condition task further shows that subjects were attentive to the task. For Sternberg’s task after the neutral condition, no significant change was observed for a period of 300–500 ms before the response was made.
In the 300–400-ms time window before the response, a significant decrease in activity was observed in the precuneus during the task after negative emotion exposure. Precuneus is a posterior medial portion of the parietal lobe. Studies have reported a central role of the precuneus in a wide variety of highly integrated tasks including episodic memory retrieval (Lundstrom et al., 2003), reflective self-awareness (Kjaer, Nowak, & Lou, 2002), and motor imagery (Hanakawa et al., 2003). The precuneus, along with the superior parietal lobule, has been considered as a high-order visual processing area that is generally involved in controlling spatial aspects of motor behavior (Connolly et al., 2000; Grafton, Fagg, Woods, & Arbib, 1996; Grefkes, Ritzl, Zilles, & Fink, 2004; Seitz & Binkofski, 2003). The precuneus has also been known to show high activation in the resting state and is a part of DMN (Raichle et al., 2001). Along with the precuneus, a decrease in activation was also observed in the superior parietal lobule. The superior parietal lobule has been shown to be activated during motor imagery (Johnson et al., 2002; Stephan et al., 1995) and has links with the mirror neuron system (Buccino et al., 2004). Middle frontal gyrus also showed significant decrease in activity during this time window. The middle frontal gyrus acts as a link between the dorsal and ventral attentional network by acting as a “circuit breaker” (Corbetta, Patel, & Shulman, 2008; Fox, Corbetta, Snyder, Vincent, & Raichle, 2006), thus disrupting the ongoing processes in the dorsal network and reorienting the attention toward a task-relevant novel stimuli. This has been further emphasized in an fMRI study by Japee, Holiday, Satyshur, Mukai, and Ungerleider (2015), where they showed that the middle frontal gyrus exerts control over both the attentional systems and helps in reorienting attention. The middle frontal gyrus is also involved in planning and decision-making. Decreased activity in all these areas suggests that subjects were attending to the task, and probably decision-making has already happened earlier than the baseline condition, and that is why no significant activation was seen in the middle frontal gyrus.
After positive emotion exposure during the task, decrease in activation was observed in DMN areas such as the posterior cingulate gyrus, medial frontal gyrus, and postcentral gyrus, which is necessary for better performance in task condition. A significant decrease was also observed in the middle frontal gyrus and superior frontal gyrus. The superior frontal gyrus has been known to be involved in a wide variety of cognitive and motor control tasks. The superior frontal gyrus has been divided into subregions, and each of these regions has been assigned different functions. The posterior region is involved in motor tasks (Chouinard & Paus, 2010; Martino et al., 2011; Nachev, Kennard, & Husain, 2008), the lateral part is involved in execution within working memory (Boisgueheneuc et al., 2006; Owen, 2000; Owen et al., 1998; Petrides, 2000) and attention (Corbetta et al., 2008; Fox et al., 2006), and the medial part shows deactivation during cognition-related processing, and also has been ascribed to be a part of DMN (Buckner et al., 2008; Greicius et al., 2003; Raichle et al., 2001). The precentral gyrus also showed a decrease in activity. This shows that before making a response during the task after the positive emotion condition, a significant reduction in the DMN areas was also observed. The decision-making area also showed a decrease in activity, suggesting that, under the influence of emotion, decision-making is faster.
In the 200–300-ms time window before the response, Sternberg’s task after the negative emotion exposure showed a decrease in activity in the precuneus, whereas after the positive condition, a decrease in activity in the middle temporal gyrus, an area known to be involved in the experience of emotion, was observed (Esslen, Pascual-Marqui, Hell, Kochi, & Lehmann, 2004; Kensinger & Schacter, 2006). The middle temporal gyrus showed an increase in activity for the neutral condition during this time period.
Sternberg’s task after the positive emotion further showed a decrease in activity in the middle frontal gyrus. In the 100–200 ms time window before the response, in the task condition after the positive emotions, a significant decrease was seen for activation in the middle frontal gyrus and the inferior temporal gyrus. The inferior temporal gyrus (Gerlach et al., 2002) is involved in high-level integration of information and helps with the integration of visual elements, thus giving meaning to the object being seen into a perceptual whole. Along with these two areas, the neutral condition showed an increase in activity in the middle temporal gyrus and the middle occipital gyrus during this time window. The middle occipital gyrus (Knauff, Mulack, Kassubek, Salih, & Greenlee, 2002) has a role in visual mental imagery. This suggests that during the neutral condition task, picture-related processing was happening even in the retrieval period. The negative Sternberg’s task showed no significant difference from 0-200 ms before the response was made. This suggests that by this time, high order visual processing, decision-making, or stimulus understanding has already taken place. Just before the response was made (between 0–100 ms), a significant increase in activation was observed in the Sternberg’s task after the neutral emotion only in the middle temporal and middle occipital gyrus, and not in the other two conditions.
Overall, the results of source analysis of Sternberg’s task after the negative and positive emotional conditions, when compared with the baseline task obtained from the 500-ms time period before the response, revealed a significant decrease in activation of DMN areas. On the other hand, in the neutral condition task the areas of emotion experience, decision-making, and integration of information stayed active during the retrieval phase. The results of behavioral analysis revealed that reaction times obtained in the tasks performed after emotion exposure were always better, as the subjects performed faster when compared with baseline. The source analysis further revealed that this performance improvement is probably mediated through the deactivation of DMN areas, which is expected to improve performance in a cognitive task (Raichle et al., 2001). After the neutral picture exposure no such DMN suppression was observed, while the performance improved. Also, Sternberg’s task performed after both the negative and positive emotions showed a significant decrease in activation of the decision-making area before the response was made, compared with baseline, while after the neutral emotion exposure, the decision-making area stayed active, suggesting that the task performed under high-arousing emotional states may help in making decisions earlier or faster.
Conclusion
The results of the present study suggest that exposure to high-arousing emotional stimuli improves verbal working memory. The high-arousing emotional stimuli may be helping in directing the attentional resources toward the task by decreasing the decision-making time and suppressing the DMN areas, thus making faster correct responses in the task at hand.
References
Boisgueheneuc, F. D., Levy, R., Volle, E., Seassau, M., Duffau, H., Kinkingnehun, S., … Dubois, B. (2006). Functions of the left superior frontal gyrus in humans: A lesion study. Brain, 129(12), 3315–3328.
Buccino, G., Vogt, S., Ritzl, A., Fink, G. R., Zilles, K., Freund, H. J., & Rizzolatti, G. (2004). Neural circuits underlying imitation learning of hand actions: An event-related fMRI study. Neuron, 42(2), 323–334.
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network. Annals of the New York Academy of Sciences, 1124(1), 1–38.
Chouinard, P. A., & Paus, T. (2010). What have we learned from “perturbing” the human cortical motor system with transcranial magnetic stimulation?. Frontiers in Human Neuroscience, 4, 173.
Connolly, J. D., Goodale, M. A., Desouza, J. F., Menon, R. S., Vilis, T., Medical Research Council Group for Action and Perception. (2000). A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. Journal of Neurophysiology, 84(3), 1645–1655.
Corbetta, M., Patel, G., & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324.
Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21.
Esslen, M., Pascual-Marqui, R. D., Hell, D., Kochi, K., & Lehmann, D. (2004). Brain areas and time course of emotional processing. NeuroImage, 21(4), 1189–1203.
Fox, M. D., Corbetta, M., Snyder, A. Z., Vincent, J. L., & Raichle, M. E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences, 103(26), 10046–10051.
Gerlach, C., Aaside, C. T., Humphreys, G. W., Gade, A., Paulson, O. B., & Law, I. (2002). Brain activity related to integrative processes in visual object recognition: Bottom-up integration and the modulatory influence of stored knowledge. Neuropsychologia, 40(8), 1254–1267.
Grafton, S. T., Fagg, A. H., Woods, R. P., & Arbib, M. A. (1996). Functional anatomy of pointing and grasping in humans. Cerebral Cortex, 6(2), 226–237.
Grefkes, C., Ritzl, A., Zilles, K., & Fink, G. R. (2004). Human medial intraparietal cortex subserves visuomotor coordinate transformation. NeuroImage, 23(4), 1494–1506.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, 100(1), 253–258.
Gusnard, D. A., & Raichle, M. E. (2001). Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience, 2(10), 685.
Hanakawa, T., Immisch, I., Toma, K., Dimyan, M. A., Van Gelderen, P., & Hallett, M. (2003). Functional properties of brain areas associated with motor execution and imagery. Journal of Neurophysiology, 89(2), 989–1002.
Holmes, A. P., Blair, R. C., Watson, J. D. G., & Ford, I. (1996). Nonparametric analysis of statistic images from functional mapping experiments. Journal of Cerebral Blood Flow & Metabolism, 16(1), 7–22.
Huijbers, W., Pennartz, C. M., Cabeza, R., & Daselaar, S. M. (2011). The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLOS ONE, 6(4), e17463.
Japee, S., Holiday, K., Satyshur, M. D., Mukai, I., & Ungerleider, L. G. (2015). A role of right middle frontal gyrus in reorienting of attention: A case study. Frontiers in Systems Neuroscience, 9, 23.
Johnson, S. H., Rotte, M., Grafton, S. T., Hinrichs, H., Gazzaniga, M. S., & Heinze, H. J. (2002). Selective activation of a parietofrontal circuit during implicitly imagined prehension. NeuroImage, 17(4), 1693–1704.
Kensinger, E. A., & Schacter, D. L. (2006). Processing emotional pictures and words: Effects of valence and arousal. Cognitive, Affective, & Behavioral Neuroscience, 6(2), 110–126.
Kjaer, T. W., Nowak, M., & Lou, H. C. (2002). Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage, 17(2), 1080–1086.
Knauff, M., Mulack, T., Kassubek, J., Salih, H. R., & Greenlee, M. W. (2002). Spatial imagery in deductive reasoning: A functional MRI study. Cognitive Brain Research, 13(2), 203–212.
Lang, P. J, Bradley, M. M., & Cuthbert, B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual (Technical Report). Gainesville: National Institute of Mental Health Center for the Study of Emotion and Attention.
Lundstrom, B. N., Petersson, K. M., Andersson, J., Johansson, M., Fransson, P., & Ingvar, M. (2003). Isolating the retrieval of imagined pictures during episodic memory: Activation of the left precuneus and left prefrontal cortex. NeuroImage, 20(4), 1934–1943.
Martino, J., Gabarrós, A., Deus, J., Juncadella, M., Acebes, J. J., Torres, A., & Pujol, J. (2011). Intrasurgical mapping of complex motor function in the superior frontal gyrus. Neuroscience, 179, 131–142.
McCormick, C., Moscovitch, M., Protzner, A. B., Huber, C. G., & McAndrews, M. P. (2010). Hippocampal–neocortical networks differ during encoding and retrieval of relational memory: functional and effective connectivity analyses. Neuropsychologia, 48(11), 3272–3281.
Morris, J. S., Öhman, A., & Dolan, R. J. (1999). A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences, 96(4), 1680–1685.
Nachev, P., Kennard, C., & Husain, M. (2008). Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience, 9(11), 856.
Nichols, T. E., & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping, 15(1), 1–25.
Öhman, A., Flykt, A., & Esteves, F. (2001a). Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General, 130(3), 466.
Öhman, A., Lundqvist, D., & Esteves, F. (2001b). The face in the crowd revisited: A threat advantage with schematic stimuli. Journal of Personality and Social Psychology, 80(3), 381.
Owen, A. M. (2000). The role of the lateral frontal cortex in mnemonic processing: The contribution of functional neuroimaging. Experimental Brain Research, 133(1), 33–43.
Owen, A. M., Stern, C. E., Look, R. B., Tracey, I., Rosen, B. R., & Petrides, M. (1998). Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proceedings of the National Academy of Sciences, 95(13), 7721–7726.
Pascual-Marqui, R. D. (2002). Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods and Findings in Experimental and Clinical Pharmacology, 24(Suppl. D), 5–12.
Pessoa, L. (2005). To what extent are emotional visual stimuli processed without attention and awareness?. Current Opinion in Neurobiology, 15(2), 188–196.
Pessoa, L., McKenna, M., Gutierrez, E., & Ungerleider, L. G. (2002). Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences, 99(17), 11458–11463.
Petrides, M. (2000). The role of the mid-dorsolateral prefrontal cortex in working memory. Experimental Brain Research, 133(1), 44–54.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682.
Schneider, W., & Shiffrin, R. M. (1977). Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review, 84(1), 1.
Seitz, R. J., & Binkofski, F. (2003). Modular organization of parietal lobe functions as revealed by functional activation studies. Advances in Neurology, 93, 281.
Shulman, G. L., Fiez, J. A., Corbetta, M., Buckner, R. L., Miezin, F. M., Raichle, M. E., & Petersen, S. E. (1997). Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience, 9(5), 648–663.
Sreenivas, S., Boehm, S. G., & Linden, D. E. J. (2012). Emotional faces and the default mode network. Neuroscience Letters, 506(2), 229–234.
Stephan, K. M., Fink, G. R., Passingham, R. E., Silbersweig, D., Ceballos-Baumann, A. O., Frith, C. D., & Frackowiak, R. S. (1995). Functional anatomy of the mental representation of upper extremity movements in healthy subjects. Journal of Neurophysiology, 73(1), 373–386.
Sternberg, S. (1966). High-speed scanning in human memory. Science, 153(3736), 652–654.
Vannini, P., O’Brien, J., O’Keefe, K., Pihlajamäki, M., Laviolette, P., & Sperling, R. A. (2010). What goes down must come up: Role of the posteromedial cortices in encoding and retrieval. Cerebral Cortex, 21(1), 22–34.
Vuilleumier, P. (2005). How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9(12), 585-594.
Vuilleumier, P., Armony, J. L., Driver, J., & Dolan, R. J. (2001). Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron, 30(3), 829–841.
Ward, A. M., Schultz, A. P., Huijbers, W., Van Dijk, K. R., Hedden, T., & Sperling, R. A. (2014). The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Human Brain Mapping, 35(3), 1061–1073.
Whalen, P. J., Rauch, S. L., Etcoff, N. L., McInerney, S. C., Lee, M. B., & Jenike, M. A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18(1), 411–418.
Acknowledgements
We thank all the subjects who participated in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Rights and permissions
About this article
Cite this article
Pegwal, N., Pal, A. & Sharma, R. Deactivation of default-mode network and early suppression of decision-making areas during retrieval period by high-arousing emotions improves performance in verbal working memory task. Cogn Affect Behav Neurosci 19, 231–238 (2019). https://doi.org/10.3758/s13415-018-00661-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-018-00661-4