Abstract
Recent studies have provided evidence that there are two possible systems for empathy: affective empathy (AE) and cognitive empathy (CE). Neuroimaging paradigms have proven that the insular cortex is involved in empathy processing, particularly in AE. However, these observations do not provide causal evidence for the role of the insula in empathy. Although impairments in empathy have been described following insular damage in a few case studies, it is not clear whether insular cortex is involved in CE and whether these two systems are impaired independently or laterally in patients with insular gliomas. In this study, we assessed 17 patients with an insular glioma, 17 patients with a noninsular glioma, and 30 healthy controls using a method that combined a self-report empathy questionnaire with the emotion recognition task, assessment of empathy for others’ pain, and the emotional perspective-taking paradigm. We found that patients with an insular glioma had lower scores for empathic concern and perspective taking than did either healthy controls or lesion controls. The patients’ abilities to recognize facial emotions, perceive others’ pain, and understand the emotional perspectives of others were also significantly impaired. Furthermore, we did not observe a laterality effect on either AE or CE among those with insular lesions. These findings revealed that both AE and CE are impaired in patients with an insular glioma and that the insular cortex may be a central neuroanatomical structure in both the AE and CE systems.
Similar content being viewed by others
The insula is an anatomically and functionally complex structure that is located deep in sylvian fissure. It is hidden by the operculum, which consists of the frontal, temporal and parietal lobes (Sanai, Polley, & Berger, 2010; Tanriover, Rhoton, Kawashima, Ulm, & Yasuda, 2004). The deep anatomical location of the insular cortex and its intricate structures has resulted in a lack of studies concerning its role in humans. Anatomically, the insular cortex is a highly connected region with a complicated network of afferent and efferent projections that connects adjacent and distant cortical regions (e.g., the amygdala, thalamus, orbitofrontal cortex, temporo-limbic regions, cingulate cortex, basal ganglia, and brain stem) (Bernhardt, Klimecki, Leiberg, & Singer, 2014; Martino, Brogna, Robles, Vergani, & Duffau, 2010; Martino, Vergani, Robles, & Duffau, 2010). Functionally, connective and functional data support the theory that the insular cortex serves in a hub-like manner to integrate sensorimotor information (Craig, 2009; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004), social emotional and cognitive processes (Jones, Ward, & Critchley, 2010), as well as the core network in empathy (Fan, Duncan, de Greck, & Northoff, 2011).
Empathy, a multidimensional psychological construct that requires the ability not only to share the emotions of others but also to infer and understand their feelings, motivations, and actions, is vital to our emotional experience and social interaction (Bernhardt & Singer, 2012; Decety, 2015; Engen & Singer, 2013; Melloni, Lopez, & Ibanez, 2014). Despite the extensive history of behavioral psychology and philosophy research, the definition of empathy differs from study to study and has no universally accepted definition (Batson, 2009; Eisenberg, 2000). A large number of studies on empathy stress that the construct is multifaceted, but it is composed of at least two important components (Decety & Jackson, 2004; Decety & Ickes, 2009; Decety, Michalska, & Kinzler, 2012; Eres, Decety, Louis, & Molenberghs, 2015; O’Brien, Konrath, Gruhn, & Hagen, 2013): an affective component, which is defined as the ability to experience an appropriate emotional response of another’s state (O’Brien et al., 2013), and a cognitive component, which is related to our capacity to predict and understand another’s mental state using cognitive processes (Decety et al., 2012). When we empathize, we vicariously experience the emotional state of the other person while realizing that what we are feeling is not our own affective state but that of another person. Affective empathy (AE) involves the sharing the emotional state, empathic concern and personal distress of others, whereas cognitive empathy (CE) includes perspective taking, the fantasy scale and, to a certain extent the theory of mind (Decety & Jackson, 2004; Decety et al., 2012; Moore, Dev, Jeste, Dziobek, & Eyler, 2015; Shamay-Tsoory, Aharon-Peretz, & Perry, 2009). More concisely, the neural mechanisms of the affective component of empathy, particularly the perception of pain in others and feeling the emotions of others, have been well studied in healthy participants (Bos, Montoya, Hermans, Keysers, & van Honk, 2015). Meanwhile, CE has been conceptualized as involving the primary neuropsychological process that includes the ability to cognitively assume the perspective of others and the theory of mind (Shamay-Tsoory, 2011; Shamay-Tsoory et al., 2009). In addition, affective and cognitive empathy are considered to have overlapping, but nonidentical, neural bases. AE is more related to unconscious processes, and CE is more related to the conscious processes, including the distinction between self and others (Gonzalez-Liencres, Shamay-Tsoory, & Brune, 2013; Mattan, Rotshtein, & Quinn, 2016).
The insula has been mostly associated with AE (Cox et al., 2012; Eslinger, 1998; Gu, Liu, Van Dam, Hof, & Fan, 2013; Jones et al., 2010; Lamm, Batson, & Decety, 2007; Lamm & Singer, 2010; Nummenmaa, Hirvonen, Parkkola, & Hietanen, 2008; Shamay-Tsoory, Tomer, Goldsher, Berger, & Aharon-Peretz, 2004; Zaki, Weber, Bolger, & Ochsner, 2009). Several studies have assessed AE in healthy participants through functional neuroimaging, using both subjective and objective measures. Banissy, et al. (2012) used the interpersonal reactivity index (IRI) to determine that AE positively correlates with the volume of the insula. A recent meta-analysis of 32 functional magnetic resonance imaging (fMRI) studies of empathy for pain also suggested that sharing pain in others most strongly activated the insular cortex (particularly the anterior insula) (Lamm, Decety, & Singer, 2011). Furthermore, a recent case study reported an impaired perception of others’ pain in patients with isolated anterior insular lesions (Gu et al., 2012; Wang et al., 2014). Sharing the emotions of others is another component of AE. Previous neuroimaging studies have shown that regions that are known to be involved in the perception of emotions in others include the insula, the amygdale, the inferior frontal gyrus (IFG), the limbic regions, the right fusiform cortex, and the superior temporal sulcus (STS) (Banks, Eddy, Angstadt, Nathan, & Phan, 2007; Paulus, Müller-Pinzler, Jansen, Gazzola, & Krach, 2015; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008). Calder et al. (2000) described a case wherein a patient presented with an impairment in the experience and recognition of the emotion of disgust following a left hemisphere infarction that involved the insula and the basal ganglia . Boucher et al. (2015) presented a study that examined social information processing in patients for whom the insula was partially or completely removed following epilepsy surgery. In doing so, they found the patients were impaired in recognizing facial expressions (Boucher et al., 2015). Leigh et al. (2013) suggested that the impairment of AE is associated with empathy network infarcts in patients with acute right hemisphere ischemic stroke, particularly in the temporal pole and anterior insular infarcts . These studies have provided evidence that the insula contributes to AE processes.
Nevertheless, there have been limited reports of a role for the insula in CE. For example, neuroimaging research proposes that CE is supported by activation of the IFG, the anterior midcingulate cortex (aMCC), the anterior insula (AI), the medial prefrontal cortex (MPFC), and the temporo-parietal junction (Gonzalez-Liencres et al., 2013). In addition, emotional perspective taking, which is the most important paradigm in assessing the CE system (Smith et al., 2015), and the process of understanding and inferring another person’s perspective appear to depend upon higher cognitive functions (Decety & Jackson, 2004). Neuroimaging studies have implicated that the process was associated with functional connectivity between parts of the MPFC, the AI, the STS and the brainstem (Gallagher & Frith, 2003). This evidence indicates that the insula may be associated with CE.
Existing laboratory measures of IRI self-report empathy questionnaire, emotion recognition task, perception of others’ pain, and emotional perspective taking have already been applied to the evaluation of empathy (Davis, 1983; Derntl et al., 2010; Singer et al., 2004). The IRI is the only published scale that allows for a multidimensional assessment of empathy and includes four subscales (two affective scales and two cognitive scales). The two affective scales were the Empathic Concern and Personal Distress scales, and the two cognitive scales were the Perspective-Taking and Fantasy scales (Davis, 1983). Nevertheless, the IRI and other self-report questionnaires of empathic function likely do not fully represent actual empathic abilities because of the limited ecological validity that is inherent to questionnaires (Dziobek et al., 2008). The gap between real-life interactions and written descriptions of such is self-evident. In contrast, some paradigms have been developed to study empathy using brain imaging, which allows for the assessment of concrete situations, such as allowing a participant to witness others’ facial expression or receiving a needling. Hence, the combination of empathy scales and empathy paradigms greatly improves their ecological validity.
The present study
In the present study, we primarily investigated the influences of AE and CE in a group of homogeneous patients with a glioma relatively limited to the insular cortex using AE and CE paradigms and the IRI self-report questionnaire. More specifically, our study sought to extend the previous findings that the insula is involved in the affective component of empathy (Nummenmaa et al., 2008; Cox et al., 2012; Gu, Liu, Van Dam, Hof, & Fan, 2013). In doing so, we included both components of empathy in our study. Gu et al. (2012) revealed that pain perception was impaired in patients with anterior insular cortex lesions. But they did not fully evaluate empathy because the perception of others’ pain is only one component of AE. Leigh et al. (2013) revealed that insular lesions impaired AE. However, in this study, the cohort was heterogeneous in the range of brain lesions and they did not assess patients with left hemisphere lesion.
In our study, we enrolled insula patients for evaluation within one week after seizure symptom onset, because there is evidence that long-term epilepsy impaired emotion recognition is a component of the neuropsychological profile that is associated with epilepsy (Jiang et al., 2014; Lippe & Lassonde, 2004; Realmuto et al., 2015). In addition, we included a group of lesion controls to control the influence of intracranial hypertension and mental change that was caused by the disease. Boucher et al. (2015) suggested that insular lesion patients had an impaired ability to recognize facial expressions and had low scores on perspective taking. Yet, this study could not exclude the possibility that performance on these tasks was already impaired prior to surgery as a result of the long-term effect of epilepsy on brain function (Realmuto et al., 2015). Finally, we are the first to attempt to explore the difference between AE and CE in left versus right insular lesions, although a meta-analysis of recent fMRI studies on empathy suggest that the right insula shows activation in response to tasks of AE and the left insula shows activation in response to tasks of both forms (Fan et al., 2011).
In the present study, we simultaneously assessed both components of empathy in insula patients through a method that combines affective and CE paradigms with IRI self-report questionnaire. Based on previous behavioral, functional and anatomical data, we hypothesized that (1) the insula patient group would show deficits in both AE and CE, and (2) that the two systems for empathy would demonstrate no obvious difference between AE and CE in left versus right insular lesions.
Material and method
Participants
Thirty-four patients with a localized glioma that was limited to either the insula (i.e., insula patients; N = 17) or a posterior, noninsula area (i.e., noninsular glioma controls; N = 17) were evaluated in the neurosurgery clinic of the Anhui Provincial Hospital Affiliated to Anhui Medical University from September 2014 to March 2016 were considered for participation in the present study. Additionally, 30 right-handed healthy controls were matched with the patients for age, gender, education, and ethnicity (Table 1). All participants had normal color vision and no previous or current neurological or psychiatric diseases. Patients were excluded if they failed to recognize frank hemiplegia and suffered from language deficits, anosognosia, or motor limitations that could have affected their performance in the neuropsychological tasks in the present experiment. Tasks (ER, EPT, EOP, and neuropsychological assessment) were performed in a random order. Picture stimulus, questions, and instructions were programmed into E-Prime (version 1.1), which was presented on a regular PC or notebook. Every participant was instructed to perform several exercises before the tasks. Each participant was given 200 Chinese yuan (about $30) at the end of the experiment as financial compensation. All patients were examined using MRI scans before the experiment. They were identified and contacted on the basis of this imaging data.
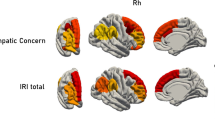
A professional neurosurgeon who was blinded to the study’s hypotheses and the neuropsychological data performed the anatomical classification on the basis of acute or recent MRIs. We used standard atlases to determine the localization of tumors and utilized the MRIcron program to further transcribe MRI images of lesions to the appropriate slice (http://people.cas.sc.edu/rorden/mricron/index.html) (Damasio & Damasio, 1989). Next, we identified and entered the lesions for each glioma patient onto templates derived from a digital MRI region of a normal control (ch2.nii), which was created by Christopher Rorden and provided for use with his MRIcron program. In each glioma case, lesions that were evident on the MRI were transcribed onto corresponding sections of the template to create a region of interest file. This file was then used to measure the location (in MNI coordinates) and region of individual lesions and to create within-group overlaps of multiple lesions using MRIcron (Fig. 1). Besides, we took advantage of 3-D measurements to estimate tumor volume (Dempsey, Condon, & Hadley, 2005; Warren, Patronas, Aikin, Albert, & Balis, 2001). For 3-D estimation of tumor volume, the digitized images were transferred to the Philips MRI Workstation. All 3-D measurements were performed on the MRI Workstation by one investigator. The tumor on digitized postcontrast T1-weighted images was outlined in three planes by the user with a paintbrush technique. Then, we list the average volumes of insular gliomas and posterior gliomas in the Table 1. This study was approved by the hospital’s ethics committee.
(a) Locations and overlap of brain tumors determined for the 17 insular glioma patients. (In the online figure, blue regions represent location and overlap of the left insular gliomas, red regions represent the right.) (b) Locations and overlap of brain lesions determined for the 17 posterior glioma patients. (In the online figure, blue regions represent location and overlap of the right posterior gliomas, red regions represent the left.) (c) Preoperative contrast-enhanced T1-weighted axial MRI scans from representative cases of the study sample. (Arrows show the right insular glioma)
Experimental measures
Neuropsychological examinations
All participants completed the Beijing version of the Montreal Cognitive Assessment (MoCA). The NEO-Five-Factor Inventory (Costa & McCrea, 1992) was used to assess general personality traits and Raven’s Progressive Matrices (Arthur & Day, 1994) was applied to estimate basic intelligence. We used the Toronto Alexithymia Scale (TAS-20; Taylor, 1994) to explore the alexithymia of patients. The Self-Rating Depression Scale (Zung, 1965; translated into Chinese) was administered to obtain a measure of depressive symptoms for the participants.
Affective and cognitive empathy
We administered the IRI (Davis, 1983) to evaluate empathy multidimensionally. The IRI is a 28-item self-report questionnaire that measures both components of empathy. So far, the IRI is the only published scale that allows for a multidimensional assessment of empathy. We use the interpersonal reactivity index–China (IRI-C) to assess multidimensional empathy (Zhang, Dong, & Wang, 2010), and this questionnaire includes four subscales (two affective scales and two cognitive scales). The two affective scales were the Empathic Concern (EC) scale and the Personal Distress (PD) scale. The EC scale assesses the respondents’ feelings of warmth, compassion, and concern for others (‘I often have tender, concerned feelings for people less fortunate than me’). The PD scale evaluates self-oriented feelings of anxiety and discomfort that results from tense interpersonal settings (Being in a tense emotional situation scares me). The two cognitive scales were the Perspective-Taking (PT) scale and the Fantasy (FS) scale. The PT scale measures the tendency to spontaneously adopt the psychological point of view of others (I sometimes try to understand my friends better by imagining how things look from their perspective), and the FS scale evaluates the tendency to imaginatively transpose oneself into fictional circumstances (When I am reading an interesting story or novel, I imagine how would I feel if the events in the story were happening to me).
Each subscale was rated on scales that varied from 0 to 4; all participants selected the best response for each item. To measure AE, we used the mean score of the EC and the PD subscales, whereas we used the mean score of the PT and the FS subscales to assess CE.
Emotion recognition task (ER)
We created a computerized task to assess the ability to recognize facial expressions of emotions. The test consists of 30 photographs of human faces displayed five basic emotions (happy, anger, fear, sadness, and disgust) and a neutral state (Britton, Taylor, Sudheimer, & Liberzon, 2006; Wang & Markham, 1999). Two words were displayed on either side of each stimulus, with one word as the correct emotion that was expressed and the other was a distracter of different emotions. Stimuli were presented for 6,000 ms and were immediately followed by a fixation cross (2,000–10,000 ms) at the center of the screen. The participants were instructed to respond as quickly and accurately as possible. The number of correct responses and reaction times for each emotion response were recorded separately.
Perception of others’ pain (empathy for others’ pain, EOP)
A series of 30 digital color pictures showing another individual’s left or right hand or foot in painful or nonpainful situations was exhibited on the computer screen (Gu et al., 2012). Participants were asked to judge whether the person in the condition was suffering from pain or not. Stimuli were presented for 6,000 ms and were immediately followed by a fixation cross (2,000–10,000 ms) at the center of the screen. Participants were instructed to respond as quickly and accurately as possible. The number of correct responses and reaction times for each emotional responses were recorded separately.
Emotional perspective taking (EPT)
For EPT trials, participants viewed 30 items in total. These items described scenes that showed two people involved in social interaction, thereby portraying five basic emotions or neutral (Derntl et al., 2010). The face of one person in the situation was masked and participants were instructed to infer the corresponding emotion of the masked face. These scenes were shown for 3 s and immediately afterward presented with two emotional (or neutral) faces (5,000 ms), the words described emotion were displayed under the picture. Participants were instructed to choose the best option that characterized the emotion of the masked face (fear, anger, sadness, disgust, happiness, or neutral) as quickly and accurately as possible and were immediately followed by a fixation cross (2,000–10,000 ms) at the center of the screen. The number of correct responses and reaction times for each emotional response were recorded separately.
Statistical method
All statistical analyses were performed using SPSS 16.0, and the level of significance was set at p = .05 after correction. We utilized the mean values (the standard deviations) that denoted the results that were normally distributed and used medians (along with the 75th–25th percentile range) to express the results that were not normally distributed. The differences in scores and reaction times of experimental tasks (ER, EPT, and EOP), neuropsychological examination performance (except MoCA scores), and IRI subscales (except PD scores) were assessed using analyses of variance (ANOVAs) as the results that were normally distributed. Post-hoc (S-N-K) comparisons were used to analyze group differences. Independent-sample t test was chosen as the means to compare tumor volumes. Comparisons for MoCA and PD scores were assessed with nonparametric tests (Kruskal–Wallis) and Mann–Whitney tests for three independent samples because the results were nonnormal distributions. Spearman correlations were used to test the relationships between scores of empathy (affective and cognitive empathy) and the performance in tasks and neuropsychological examinations.
Results
Neuropsychological functioning
An ANOVA revealed no significant difference among the groups with regard to age (F = 0.057, n.s.), education (F = 0.419, n.s.), NEO-Five-Factor Inventory (F = 0.043, n.s.), Raven’s Progressive Matrices (F = 0.991, n.s.), and Self-Rating Depression Scale (F = 0.888, n.s.). We observed no significant differences in MoCA scores (H = 3.482, n.s.) using nonparametric tests (Kruskal–Wallis); however, the TAS-20 scores of the patients with an insular glioma were lower than those of the others controls using post-hoc comparisons (F = 31.651, p < .001). See Table 1 and Tables S1 and S2 of Appendix A in the supplementary material.
AE and CE scales
The results from each group on the empathy subscales are reported in Table 2. Patients with an insular glioma were significantly different from the other group of patients on the EC and PT subscales (F = 8.151, p = .001; and F = 4.166, p = .020, respectively); however, there were no significant differences for the PD and FS subscales (H = 2.042, n.s.; and F = 0.520, n.s., respectively ). As we previously mentioned, we used the mean scores on the EC and PD subscales to assess AE, whereas the mean scores on the PT and the FS subscales were used to assess CE.
Significant group differences were apparent for both the AE and CE scales. As is shown in Table 2, for each of these outcomes, post-hoc comparisons showed that the insula patient group was impaired in both CE and AE, as compared to both the healthy controls and patients with posterior gliomas. The healthy and patient control groups did not differ from each other. See details in the Tables S1 and S1 of Appendix B in the supplementary material.
Emotion recognition, perception of others’ pain, and emotional perspective taking
The means, standard deviations, one-way ANOVA results, and post-hoc analyses of the group differences in these tasks are reported in Table 3. Analysis of correct responses on the experimental tasks demonstrated that the insula patient group was significantly different from the other two groups in all three tasks (ER, F = 78.893, p < .001; EOP, F = 18.496, p < .001; EPT, F = 15.252, p < .001). Post-hoc analyses revealed that patients with insular glioma were impaired in all tasks, as compared to both the healthy and patient control groups (Fig. 2). The posterior-glioma and healthy control groups did not differ from each other. For reaction times, the only difference was in perception of others’ pain task, which showed that the insula and control patient groups were significantly different from the healthy control group. See the details in Table S3 of Appendix B in the supplementary material.
Mean performance from each group on the emotion recognition test, perception of others’ pain, and emotional perspective taking. Patients with insular gliomas were impaired in all tasks when compared to both the healthy controls (HC) and patients with posterior glioma (PC). Error bars represent standard errors of the means. ** p < .01, * p < .05
The relationship between EPT and CE, ER/EOP, and AE
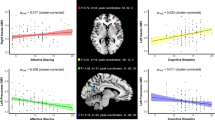
In the insula patient group, our analysis revealed a significant correlation between the EC score (a subscale for AE) and the percentage correct of ER (r = .645, p = .005; Fig. 3A.1), and the total AE score was also positively correlated with the percentage correct of ER (r = .847, p < .001; Fig. 3A.2). The score on the PD subscale did not correlate with the percentage correct of ER (r = .377, n.s.), and the results indicated a significant correlation between the overall AE score and the percentage correct of EOP (r = .670, p = .003; Fig. 3B.2), and the score of PD subscale positively correlated with the percent correct of EOP (r = .501, p = .038; Fig. 3B.1). However, the EC score did not correlate with the percent correct of EOP (r = .321, n.s.). Moreover, the same analysis was conducted between the overall CE score (PT and FS subscales) and the percentage correct of EPT. The total CE score significantly correlated with the percentage correct of EPT (r = .703, p = .002; Fig. 3C.2); however, only the score on the PT subscale was significantly correlated with the percentage correct of EPT (r = .562, p = .019; Fig. 3C.1), whereas the score on the FS subscale was not (r = .147, n.s.). See all of the correlation results in Tables S1–S3 of Appendix C in the supplementary material.
Scatter plots. (a) Correlations of scores on the (1) EC subscale and (2) full AE scale with the percentages correct of emotion recognition (ER) in the group of patients with insular glioma (r = .847, p < .001; and r = .645, p = .005, respectively). (b) Correlations of scores on the (1) PD subscale and (2) full AE scale with the percentages correct of perception of others’ pain (EOP) in the group of patients with insular glioma (r = .670, p = .003; and r = .501, p = .038, respectively). (c) Correlations of scores on the (1) PT subscale and (2) full CE scale with the percentages correct of emotional perspective taking (EPT) in the group of insula patients (r = .703, p = .002; and r = .562, p = .019, respectively)
Affective and cognitive indices
For each participant, the overall affective and cognitive indices were calculated. To compute each index, we computed z scores for the tasks and calculated the mean scores of the affective (AE scale, ER, and EOP) and the cognitive (CE scale and EPT) measures. We then used these cognitive and affective indices calculated using z scores to examine the performance of the insula group and of the control groups across tasks. Our results indicated that patients with insular lesions were not only impaired according to the affective index (p < .050), but also according to the cognitive index (p < .050), when compared to the posterior glioma and healthy control groups. Furthermore, we divided the insular-lesion group into two subgroups according to the laterality of their lesions (the right vs. left insula). This analysis indicated no significant difference between the subgroups on both the affective index (p = .820) and the cognitive index (p = .250). See the details in Table S1 of Appendix D in the supplementary material.
Discussion
In this case–control study, we investigated the central neuroanatomic structure underlying empathic behavior in insula patients, posterior glioma patients and healthy participants. We assessed empathic abilities with paradigms (emotion recognition, empathy for others’ pain, and emotional perspective taking) tapping the two core components of this human competency: AE and CE. Additionally, we collected self-report data from empathy questionnaires. The present study provides neuropsychological evidence of (a) both AE and CE being impaired in patients with insular gliomas, when compared to either a lesion control group or healthy volunteers matched on sex, age, education, and ethnicity, and (b) no obvious laterality effects on AE or CE in insular lesions.
Evaluation of AE
AE involves the set of feelings elicited in response to the affective state of others, which can carry feelings of warmth or concern for others (empathic concern) or a set of self-oriented feelings induced by an agent (personal distress; Gleichgerrcht et al., 2013). The emphasis of AE is representatively focused on experiencing the emotional states of others consciously, which implies a self–other distinction (Eres et al., 2015; Mattan et al., 2016), as well as an understanding of the origin of emotional experience (Bernhardt & Singer, 2012). AE differs from emotion contagion, which responds automatically to another person’s emotional state but not necessarily self-other distinction (de Waal, 2012; Dimberg & Thunberg, 2012; Mattan et al., 2016). Emotion contagion may depend on brain regions that are activated in correlation with recognition of others’ emotions through facial expression and prosody (Leigh et al., 2013). That is to say, it is difficult to imagine that other people feel certain emotions in specific circumstances or learn to attribute their own feelings to other people without recognizing those emotions in other people (Hillis, 2014). AE is also different from personal distress or empathic concern, as the latter reflects an internal state of emotion and discomfort that stems from the concern for another person but not necessarily a sharing of their emotions (Bernhardt & Singer, 2012; Decety & Cowell, 2014). What needs to be specifically stressed is that we do not indicate that these processes (e.g., emotion contagion or empathic concern) are unrelated to AE, rather AE can be deemed to be an umbrella term that encompasses multiple processes (Eres et al., 2015).
We used the IRI, a widely used self-report, multidimensional measure of dispositional empathy (Davis, 1983). More specifically, we used the EC and PD subscales of the IRI to measure AE (Davis, 1983; Rankin et al., 2006). The EC scale taps the respondents’ feelings of warmth, compassion and concern for others; the PD scale assesses self-oriented feelings of anxiety and discomfort that result from tense interpersonal settings (Davis, 1983). Several studies support the construct validity of these subscales as indices of AE. Specifically, EC scores are correlated with measures of emotionality, concern for others and affective empathy (de Corte et al., 2007). In our study, insula patients had significantly lower scores in the AE scale as well as EC subscale. In the behavioral paradigms research, furthermore, the insula is a key structure in the perception of oneself and others’ pain (Engen & Singer, 2013; Mazzola et al., 2010). Multiple imaging studies have shown that sharing pain in others most strongly activated the insular cortex (particularly the AI) (Lamm et al., 2011; Sheng, Liu, Li, Fang, & Han, 2014). Such lesion studies also confirmed that the AI plays a critical role in empathetic pain processing (Gu et al., 2012). For ER, previous neuroimaging studies have revealed that the insula is involved in the perception of emotions (Bernhardt & Singer, 2012; Jabbi, Swart, & Keysers, 2007; Lamm & Singer, 2010). Subsequent research from patients for whom the insula was removed found that the patients’ ability to recognize facial expressions of fear, happiness, and surprise was impaired (Boucher et al., 2015). Consistent with previous studies, we obtained data in patients with an insular glioma and corrected defects of previous studies. The results revealed that patients with an insular glioma showed deficits in performance on both empathy tasks of the ER and perception of others’ pain in our present research. Furthermore, our study shows that the ER and EOP performance was positively correlated with their AE scale. Based on previous studies that have depicted the conception of AE and the model of AE systems that operate in an exclusive manner (Eslinger, 1998; Shamay-Tsoory et al., 2009), we can conclude that insular gliomas damaged the AE, as they result in lower EC scores and poorer performance on the ER and EOP tasks. Because insular gliomas damage normal insular structures, it is possible that the insular cortex is the neuroanatomic structure of the AE system.
Assessment of CE
CE, the ability to infer and understand the feelings of others without necessarily implying that the experiencer is in an affective state himself (Shamay-Tsoory, 2011; Walter, 2012). For instance, there can be purely cognitive understanding that somebody is sad, without any emotional effect on the observer (Walter, 2012). This process involves perspective taking and theory of mind, and has been revealed to be dependent upon cognitive processes (Decety & Jackson, 2004; Decety, 2011; Shamay-Tsoory, 2011; Shamay-Tsoory et al., 2004; Walter, 2012). Perspective taking can be further subdivided into affective perspective taking (i.e., perspective taking to understand affect) and cognitive perspective taking (perspective taking to understand cognition) (Decety, & Jackson, 2004; Johnstone, Cohen, Bryant, Glass, & Christ, 2015; Lamm & Majdandzic, 2015; Sebastian et al., 2012). Furthermore, neuroimaging and psychological research show that theory of mind and CE are also different. Theory of mind refers to the ability to understand the mental states of others (Martin & Santos, 2016). Mental states not only include beliefs, desires, or intentions but also emotions and affective states. Mentalizing about emotional states of others is called affective theory of mind, which is more or less synonymous with cognitive empathy (Kanske, Böckler, & Singer, 2015; Reniers, Corcoran, Drake, Shryane, & Vollm, 2011; Walter, 2012).
In accord with the presumed role of the insula in subjective feelings and previous neuroimaging studies that reported insular activation during the observation and experience of emotions, the insula is typically related to AE. However, some studies have reported that the insula may also be involved in CE processes. For example, Fan et al. (2011) and Gonzalez-Liencres et al. (2013) revealed that CE is also supported by activation of the insula. We also found evidence that patients with insular resection had lower scores in the PT subscale, which denoted the tendency to take the mental perspective of others and composed of items that are associated with the social competence, other-oriented sensitivity and CE (Boucher et al., 2015; Shamay-Tsoory et al., 2009). To date, these results do not yet provide causal evidence for the role of the insula in CE. We are the first to evaluate CE in a comprehensive manner, wherein we observe lower scores in CE scale (as well as PT subscale) and significantly poorer performance on the EPT in those insula patients, which also positively correlated with CE scale. Shamay-Tsoory et al. suggested that the two systems of empathy were dissociation in their study (Shamay-Tsoory et al., 2009), that is to say, AE impairment may not affect CE. Thus, our results suggested that the damaged insula impaired the CE, on the basis of the self-report empathy questionnaire and a cognitive empathy paradigm.
Laterality effect of the insular gliomas
We also attempted to explore the laterality effects on two systems of empathy among our insula patient group. Our results revealed no obvious differences between AE and CE in left versus right insular lesions. However, Fan et al. (2011) found that the right insula was more likely to be activated in affective forms of empathy (with the left insula being involved in both forms) through meta-analysis. There is no unified conclusion about laterality impact on empathy from insular lesion studies. In a previous case study, Borg et al. (2013) described a patient with an ischemic lesion that involved the left posterior insula who was unable to recognize facial expressions of disgust; however, a recent study has shown that lesions in the right hemisphere cortices, including the insula, are associated with impaired recognition of emotions (Yuvaraj, Murugappan, Norlinah, Sundaraj, & Khairiyah, 2013). Recent reports have revealed that other structures can also be involved in ER, including the anterior temporal and amygdala lesions (Bach, Hurlemann, & Dolan, 2013; Boucher et al., 2015). We hypothesize that other structures may compensate for the functioning of empathy when the insula was impaired. Thus, our results cannot achieve the laterality effects on AE and CE in the insula group. Moreover, due to the large distinction between the numbers of left (N = 10) and right (N = 7) insular gliomas, further studies involving a lager number of patients with insular lesions are necessary to evaluate the course at the group level.
Measurement of neuropsychological functioning
Our TAS-20 results indicate that the insula patients had significantly lower scores than did a lesion control group and healthy volunteers. This evidence suggested that the insula abnormalities might be a neurophysiological factor leading to alexithymia. Alexithymia is a symptom characterized by impaired the ability to identify and communicate one’s emotional state (Aleman, 2005). Recent studies suggested that alexithymia might due to the impairment of “interoception,” referring to one’s sensitivity to the internal state of one’s own body (Gu, Hof, Friston, & Fan, 2013; Seth, 2013). Neuroimaging studies (i.e. voxel-based morphometry) found that alexithymic individuals had reduced gray matter volumes of the AI (Borsci et al., 2009; Ihme et al., 2013), and reduced covariance networks including fronto-insula (Bernhardt, Valk, et al., 2014). In accordance with the presence of AI abnormalities in alexithymia, and its role in interoception (Craig, 2009), Herbert et al. found that interoceptive awareness negatively related with the emotional symptoms of alexithymia (Herbert, Herbert, & Pollatos, 2011). Recent research that impaired AI might be involved in producing alexithymia and associated with elevated levels of alexithymia (Brewer, Cook, Cardi, Treasure, & Bird, 2015; Hogeveen, Bird, Chau, Krueger, & Grafman, 2016). Consistent with previous studies, our results of patients with an insular glioma indicated that the insular cortex lesions may lead to alexithymia. However, we did not find that the insula patients were damaged in their basic cognitive and intelligence functions when tested by MoCA and Raven’s Standard Progressive Matrices. A previous case study reported that a patient demonstrated significant personality changes immediately after an isolated right AI infarction (Cho et al., 2012), whereas our results with the NEO-Five-Factor Inventory suggest that the personality of insula patients do not change than for control groups. The present study also revealed no significant differences in AE and CE between the lesion and healthy control groups, thereby implying that intracranial hypertension and mentality changes that are caused by disease may not have an effect on empathy. This further demonstrated the reliability of the results of the insula patient group.
Limitation
One limitation of our study is the fact that some gliomas infiltrated the operculo-insula rather than purely the insular cortex. Prominent subcortical fiber bundles, including the uncinate and the arcuate fascicle, connect the insula to the fronto-orbital and temporopolar and temporomesial regions. Damage to the opercula or fiber might have impacted the performance on empathy scales in some patients (Martino et al., 2015; Oishi et al., 2015). Furthermore, a larger sample size would have allowed for comparisons according to the particular location of the insular-cortex lesions (anterior vs. posterior insular cortex), which could be of special interest given the functional segregation within the insular cortex revealed by functional imaging and intracerebral electrical stimulation studies (Kurth, Zilles, Fox, Laird, & Eickhoff, 2010). Another limitation is that lesion patients would be likely to have known insight problems, particularly in the right-hemisphere-lesioned patients (Besharati et al., 2016; Heilman, 2014). The patients who were enrolled in our research performed a physical examination strictly, including speaking, movement, anosognosia, and so on. We had already excluded the patients who failed to speak, move, or recognize frank hemiplegia before the self-report and empathy testing. Moreover, the result of correlational analysis in the present study in the healthy and lesion control groups are almost consistent with the insula patients, suggesting self-report may apply to individuals who have lesions as well. Although we had already gone through a very rigorous process of selection in these participants, anyway, we could not completely exclude those patients with anosognosia, particularly in the right hemisphere lesioned patients. Patients with anosognosia might fail to acknowledge deficits in social cognition and might influence the negative finding (using self-report) that patients with noninsular gliomas (Besharati et al., 2014). The third limitation is we were unable to demonstrate a dissociation between emotional empathy and emotional contagion, which much more commonly impaired in people with insular lesions; that is, we were not able to distinguish between neurologically normal participants and patients in emotional contagion, either using the self-report assessment or emotional face recognition task (Leigh et al., 2013). Future studies may use the autonomic responses to assess emotional contagion, such as skin conductance response and heart rate changes, when presented with emotional scenes or stories (Balconi & Bortolotti, 2012). Finally, ER deficits were more frequent in insula patients than in either of the control groups. The lack of specific emotion analysis is also a weakness of our study.
Conclusion
In summary, by simultaneously measuring both the self-report empathy questionnaire results and empathy paradigms in the homogeneous, well-matched cohort of insular-glioma patients and demographically matched healthy volunteers and lesion control participants, we confirmed previous results associating AE impairment with exposure to insular damage. We also identified impaired CE in patients with insular lesions. Finally, we could not identify obvious laterality effects on AE or CE and deficits in intelligence and personality in insular lesions. Although not providing mechanistic evidence and the study’s limitations, these results are consistent with (1) insular-glioma patients having impaired CE other than AE, and (2) insula contributing to both AE and CE processing. In the past few years, we have been attempting to better understand the neural basis of empathy. Identifying critical subcomponents and brain network interactions that are involved in empathy contributes to understanding of the generation of this multifaceted experience at the crucial intersection of human emotional and social behavior. Therefore, such studies may also guide the development of clinical and subclinical groups that are associated with deficient empathic ability, such as individuals with conduct disorder, autism spectrum disorder, and alexithymia (Bird et al., 2010; Caria & de Falco, 2015; Fan, Chen, Chen, Decety, & Cheng, 2013; Silani et al., 2008; Wang et al., 2014). Clinicians should recognize this important social disability and appropriate counseling should be provided to guardians. Additionally, innovative treatments to improve empathy, such as intranasal oxytocin should be evaluated in individuals with insular-cortex lesions (Hurlemann et al., 2010; Young & Barrett, 2015).
References
Aleman, A. (2005). Feelings you can’t imagine: Towards a cognitive neuroscience of alexithymia. Trends in Cognitive Sciences, 9, 553–555. doi:10.1016/j.tics.2005.10.002
Arthur, W., & Day, D. V. (1994). Development of a short form for the Raven Advanced Progressive Matrices Test. Educational and Psychological Measurement, 54, 394–403.
Bach, D. R., Hurlemann, R., & Dolan, R. J. (2013). Unimpaired discrimination of fearful prosody after amygdala lesion. Neuropsychologia, 51, 2070–2074. doi:10.1016/j.neuropsychologia.2013.07.005
Balconi, M., & Bortolotti, A. (2012). Empathy in cooperative versus non-cooperative situations: The contribution of self-report measures and autonomic responses. Applied Psychophysiology and Biofeedback, 37, 161–169. doi:10.1007/s10484-012-9188-z
Banissy, M. J., Kanai, R., Walsh, V., & Rees, G. (2012). Inter-individual differences in empathy are reflected in human brain structure. NeuroImage, 62, 2034–2039. doi:10.1016/j.neuroimage.2012.05.081
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan, P. J., & Phan, K. L. (2007). Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2, 303–312. doi:10.1093/scan/nsm029
Batson, C. D. (2009). These things called empathy. In J. Decety & W. J. Ickes (Eds.), The social neuroscience of empathy (pp. 16–31). Cambridge: MIT Press.
Bernhardt, B. C., Klimecki, O. M., Leiberg, S., & Singer, T. (2014). Structural covariance networks of the dorsal anterior insula predict females’ individual differences in empathic responding. Cerebral Cortex, 24, 2189–2198. doi:10.1093/cercor/bht072
Bernhardt, B. C., & Singer, T. (2012). The neural basis of empathy. Annual Review of Neuroscience, 35, 1–23. doi:10.1146/annurev-neuro-062111-150536
Bernhardt, B. C., Valk, S. L., Silani, G., Bird, G., Frith, U., & Singer, T. (2014). Selective disruption of sociocognitive structural brain networks in autism and alexithymia. Cerebral Cortex, 24, 3258–3267. doi:10.1093/cercor/bht182
Besharati, S., Forkel, S. J., Kopelman, M., Solms, M., Jenkinson, P. M., & Fotopoulou, A. (2014). The affective modulation of motor awareness in anosognosia for hemiplegia: Behavioural and lesion evidence. Cortex, 61, 127–140. doi:10.1016/j.cortex.2014.08.016
Besharati, S., Forkel, S. J., Kopelman, M., Solms, M., Jenkinson, P. M., & Fotopoulou, A. (2016). Mentalizing the body: Spatial and social cognition in anosognosia for hemiplegia. Brain, 139, 971–985. doi:10.1093/brain/awv390
Bird, G., Silani, G., Brindley, R., White, S., Frith, U., & Singer, T. (2010). Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain, 133, 1515–1525. doi:10.1093/brain/awq060
Borg, C., Bedoin, N., Peyron, R., Bogey, S., Laurent, B., & Thomas-Anterion, C. (2013). Impaired emotional processing in a patient with a left posterior insula-SII lesion. Neurocase, 19, 592–603. doi:10.1080/13554794.2012.713491
Borsci, G., Boccardi, M., Rossi, R., Rossi, G., Perez, J., Bonetti, M., & Frisoni, G. B. (2009). Alexithymia in healthy women: A brain morphology study. Journal of Affective Disorders, 114, 208–215. doi:10.1016/j.jad.2008.07.013
Bos, P. A., Montoya, E. R., Hermans, E. J., Keysers, C., & van Honk, J. (2015). Oxytocin reduces neural activity in the pain circuitry when seeing pain in others. NeuroImage, 113, 217–224. doi:10.1016/j.neuroimage.2015.03.049
Boucher, O., Rouleau, I., Lassonde, M., Lepore, F., Bouthillier, A., & Nguyen, D. K. (2015). Social information processing following resection of the insular cortex. Neuropsychologia, 71, 1–10. doi:10.1016/j.neuropsychologia.2015.03.008
Brewer, R., Cook, R., Cardi, V., Treasure, J., & Bird, G. (2015). Emotion recognition deficits in eating disorders are explained by co-occurring alexithymia. Royal Society Open Science, 2, 140382. doi:10.1098/rsos.140382
Britton, J. C., Taylor, S. F., Sudheimer, K. D., & Liberzon, I. (2006). Facial expressions and complex IAPS pictures: Common and differential networks. NeuroImage, 31, 906–919. doi:10.1016/j.neuroimage.2005.12.050
Calder, A. J., Keane, J., Manes, F., Antoun, N., & Young, A. W. (2000). Impaired recognition and experience of disgust following brain injury. Nature Neuroscience, 3, 1077–1078. doi:10.1038/80586
Caria, A., & de Falco, S. (2015). Anterior insular cortex regulation in autism spectrum disorders. Frontiers in Behavioral Neuroscience, 9, 38. doi:10.3389/fnbeh.2015.00038
Cho, H. J., Kim, S. J., Hwang, S. J., Jo, M. K., Kim, H. J., Seeley, W. W., & Kim, E. J. (2012). Social-emotional dysfunction after isolated right anterior insular infarction. Journal of Neurology, 259, 764–767. doi:10.1007/s00415-011-6246-z
Costa, P. T., & McCrea, R. R. (1992). Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor Inventory (NEO-FFI). Odessa, Fla. (P.O. Box 998, Odessa 33556): Psychological Assessment Resources.
Cox, C. L., Uddin, L. Q., Di Martino, A., Castellanos, F. X., Milham, M. P., & Kelly, C. (2012). The balance between feeling and knowing: Affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Social Cognitive and Affective Neuroscience, 7, 727–737. doi:10.1093/scan/nsr051
Craig, A. D. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70. doi:10.1038/nrn2555
Critchley, H. D., Wiens, S., Rotshtein, P., Ohman, A., & Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7, 189–195. doi:10.1038/nn1176
Damasio, H., & Damasio, A. R. (1989). Lesion analysis in neuropsychology. New York: Oxford University Press.
Davis, M. H. (1983). Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44, 113–126. doi:10.1037/0022-3514.44.1.113
de Corte, K., Buysse, A., Verhofstadt, L. L., Roeyers, H., Ponnet, K., & Davis, M. H. (2007). Measuring empathic tendencies: Reliability and validity of the Dutch version of the interpersonal reactivity index. Psychologica Belgica, 47, 235–260.
de Waal, F. B. M. (2012). The antiquity of empathy. Science, 336, 874–876. doi:10.1126/science.1220999
Decety, J. (2011). Dissecting the neural mechanisms mediating empathy. Emotion Review, 3, 92–108.
Decety, J. (2015). The neural pathways, development and functions of empathy. Current Opinion in Behavioral Sciences, 3, 1–6. doi:10.1016/j.cobeha.2014.12.001
Decety, J., & Cowell, J. M. (2014). Friends or foes: Is empathy necessary for moral behavior? Perspectives in Psychological Science, 9, 525–537. doi:10.1177/1745691614545130
Decety, J., & Ickes, W. J. (2009). The social neuroscience of empathy. Cambridge: MIT Press.
Decety, J., & Jackson, P. L. (2004). The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews, 3, 71–100. doi:10.1177/1534582304267187
Decety, J., Michalska, K. J., & Kinzler, K. D. (2012). The contribution of emotion and cognition to moral sensitivity: A neurodevelopmental study. Cerebral Cortex, 22, 209–220. doi:10.1093/cercor/bhr111
Dempsey, M. F., Condon, B. R., & Hadley, D. M. (2005). Measurement of tumor “size” in recurrent malignant glioma: 1D, 2D, or 3D? American Journal of Neuroradiology, 26, 770–776.
Derntl, B., Finkelmeyer, A., Eickhoff, S., Kellermann, T., Falkenberg, D. I., Schneider, F., & Habel, U. (2010). Multidimensional assessment of empathic abilities: Neural correlates and gender differences. Psychoneuroendocrinology, 35, 67–82. doi:10.1016/j.psyneuen.2009.10.006
Dimberg, U., & Thunberg, M. (2012). Empathy, emotional contagion, and rapid facial reactions to angry and happy facial expressions. PsyCH Journal, 1, 118–127. doi:10.1002/pchj.4
Dziobek, I., Rogers, K., Fleck, S., Bahnemann, M., Heekeren, H. R., Wolf, O. T., & Convit, A. (2008). Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET). Journal of Autism and Developmental Disorders, 38, 464–473. doi:10.1007/s10803-007-0486-x
Eisenberg, N. (2000). Emotion, regulation, and moral development. Annual Review of Psychology, 51, 665–697. doi:10.1146/annurev.psych.51.1.665
Engen, H. G., & Singer, T. (2013). Empathy circuits. Current Opinion in Neurobiology, 23, 275–282. doi:10.1016/j.conb.2012.11.003
Eres, R., Decety, J., Louis, W. R., & Molenberghs, P. (2015). Individual differences in local gray matter density are associated with differences in affective and cognitive empathy. NeuroImage, 117, 305–310. doi:10.1016/j.neuroimage.2015.05.038
Eslinger, P. J. (1998). Neurological and neuropsychological bases of empathy. European Neurology, 39, 193–199.
Fan, Y. T., Chen, C., Chen, S. C., Decety, J., & Cheng, Y. (2013). Empathic arousal and social understanding in individuals with autism: Evidence from fMRI and ERP measurements. Social Cognitive and Affective Neuroscience, 9, 1203–1213. doi:10.1093/scan/nst101
Fan, Y., Duncan, N. W., de Greck, M., & Northoff, G. (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews, 35, 903–911. doi:10.1016/j.neubiorev.2010.10.009
Gallagher, H. L., & Frith, C. D. (2003). Functional imaging of “theory of mind.”. Trends in Cognitive Sciences, 7, 77–83. doi:10.1016/S1364-6613(02)00025-6
Gleichgerrcht, E., Torralva, T., Rattazzi, A., Marenco, V., Roca, M., & Manes, F. (2013). Selective impairment of cognitive empathy for moral judgment in adults with high functioning autism. Social Cognitive and Affective Neuroscience, 8, 780–788. doi:10.1093/scan/nss067
Gonzalez-Liencres, C., Shamay-Tsoory, S. G., & Brune, M. (2013). Towards a neuroscience of empathy: Ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neuroscience & Biobehavioral Reviews, 37, 1537–1548. doi:10.1016/j.neubiorev.2013.05.001
Gu, X., Gao, Z., Wang, X., Liu, X., Knight, R. T., Hof, P. R., & Fan, J. (2012). Anterior insular cortex is necessary for empathetic pain perception. Brain, 135, 2726–2735. doi:10.1093/brain/aws199
Gu, X., Hof, P. R., Friston, K. J., & Fan, J. (2013). Anterior insular cortex and emotional awareness. Journal of Comparative Neurology, 521, 3371–3388. doi:10.1002/cne.23368
Gu, X., Liu, X., Van Dam, N. T., Hof, P. R., & Fan, J. (2013). Cognition–emotion integration in the anterior insular cortex. Cerebral Cortex, 23, 20–27. doi:10.1093/cercor/bhr367
Heilman, K. M. (2014). Possible mechanisms of anosognosia of hemiplegia. Cortex, 61, 30–42. doi:10.1016/j.cortex.2014.06.007
Herbert, B. M., Herbert, C., & Pollatos, O. (2011). On the relationship between interoceptive awareness and alexithymia: Is interoceptive awareness related to emotional awareness? Journal of Personality, 79, 1149–1175. doi:10.1111/j.1467-6494.2011.00717.x
Hillis, A. E. (2014). Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain, 137, 981–997. doi:10.1093/brain/awt317
Hogeveen, J., Bird, G., Chau, A., Krueger, F., & Grafman, J. (2016). Acquired alexithymia following damage to the anterior insula. Neuropsychologia, 82, 142–148. doi:10.1016/j.neuropsychologia.2016.01.021
Hurlemann, R., Patin, A., Onur, O. A., Cohen, M. X., Baumgartner, T., Metzler, S., & Kendrick, K. M. (2010). Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal of Neuroscience, 30, 4999–5007. doi:10.1523/JNEUROSCI.5538-09.2010
Ihme, K., Dannlowski, U., Lichev, V., Stuhrmann, A., Grotegerd, D., Rosenberg, N., & Suslow, T. (2013). Alexithymia is related to differences in gray matter volume: A voxel-based morphometry study. Brain Research, 1491, 60–67. doi:10.1016/j.brainres.2012.10.044
Jabbi, M., Swart, M., & Keysers, C. (2007). Empathy for positive and negative emotions in the gustatory cortex. NeuroImage, 34, 1744–1753. doi:10.1016/j.neuroimage.2006.10.032
Jiang, Y., Hu, Y., Wang, Y., Zhou, N., Zhu, L., & Wang, K. (2014). Empathy and emotion recognition in patients with idiopathic generalized epilepsy. Epilepsy and Behavior, 37, 139–144. doi:10.1016/j.yebeh.2014.06.005
Johnstone, B., Cohen, D., Bryant, K. R., Glass, B., & Christ, S. E. (2015). Functional and structural indices of empathy: Evidence for self-orientation as a neuropsychological foundation of empathy. Neuropsychology, 29, 463–472. doi:10.1037/neu0000155
Jones, C. L., Ward, J., & Critchley, H. D. (2010). The neuropsychological impact of insular cortex lesions. Journal of Neurology, Neurosurgery & Psychiatry, 81, 611–618. doi:10.1136/jnnp.2009.193672
Kanske, P., Böckler, A., & Singer, T. (2015). Models, mechanisms and moderators dissociating empathy and theory of mind. Current Topics in Behavioral Neuroscience. doi:10.1007/7854_2015_412. Advance online publication.
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R., & Eickhoff, S. B. (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function, 214, 519–534. doi:10.1007/s00429-010-0255-z
Lamm, C., Batson, C. D., & Decety, J. (2007). The neural substrate of human empathy: Effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience, 19, 42–58. doi:10.1162/jocn.2007.19.1.42
Lamm, C., Decety, J., & Singer, T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54, 2492–2502. doi:10.1016/j.neuroimage.2010.10.014
Lamm, C., & Majdandzic, J. (2015). The role of shared neural activations, mirror neurons, and morality in empathy—a critical comment. Neuroscience Research, 90, 15–24. doi:10.1016/j.neures.2014.10.008
Lamm, C., & Singer, T. (2010). The role of anterior insular cortex in social emotions. Brain Structure and Function, 214, 579–591. doi:10.1007/s00429-010-0251-3
Leigh, R., Oishi, K., Hsu, J., Lindquist, M., Gottesman, R. F., Jarso, S., & Hillis, A. E. (2013). Acute lesions that impair affective empathy. Brain, 136, 2539–2549. doi:10.1093/brain/awt177
Lippe, S., & Lassonde, M. (2004). Neuropsychological profile of intractable partial epilepsies; in French. Review of Neurology, 160, S144–S153.
Martin, A., & Santos, L. R. (2016). What cognitive representations support primate theory of mind? Trends in Cognitive Sciences, 20, 375–382. doi:10.1016/j.tics.2016.03.005
Martino, J., Brogna, C., Robles, S. G., Vergani, F., & Duffau, H. (2010). Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex, 46, 691–699. doi:10.1016/j.cortex.2009.07.015
Martino, J., Mato, D., de Lucas, E. M., García-Porrero, J. A., Gabarrós, A., Fernández-Coello, A., & Vázquez-Barquero, A. (2015). Subcortical anatomy as an anatomical and functional landmark in insulo-opercular gliomas: Implications for surgical approach to the insular region. Journal of Neurosurgery, 123, 1081–1092. doi:10.3171/2014.11.jns141992
Martino, J., Vergani, F., Robles, S. G., & Duffau, H. (2010). New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: Implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery, 66, 4–12. doi:10.1227/01.NEU.0000348564.28415.FA
Mattan, B. D., Rotshtein, P., & Quinn, K. A. (2016). Empathy and visual perspective-taking performance. Cognitive Neuroscience, 7, 1–12. doi:10.1080/17588928.2015.1085372. Advance online publication.
Mazzola, V., Latorre, V., Petito, A., Gentili, N., Fazio, L., Popolizio, T., & Bondolfi, G. (2010). Affective response to a loved one’s pain: Insula activity as a function of individual differences. PLoS ONE, 5, e15268. doi:10.1371/journal.pone.0015268
Melloni, M., Lopez, V., & Ibanez, A. (2014). Empathy and contextual social cognition. Cognitive, Affective, & Behavioral Neuroscience, 14, 407–425. doi:10.3758/s13415-013-0205-3
Moore, R. C., Dev, S. I., Jeste, D. V., Dziobek, I., & Eyler, L. T. (2015). Distinct neural correlates of emotional and cognitive empathy in older adults. Psychiatry Research, 232, 42–50. doi:10.1016/j.pscychresns.2014.10.016
Nummenmaa, L., Hirvonen, J., Parkkola, R., & Hietanen, J. K. (2008). Is emotional contagion special? an fMRI study on neural systems for affective and cognitive empathy. NeuroImage, 43, 571–580.
O’Brien, E., Konrath, S. H., Gruhn, D., & Hagen, A. L. (2013). Empathic concern and perspective taking: Linear and quadratic effects of age across the adult life span. Journals of Gerontology, 68B, 168–P175. doi:10.1093/geronb/gbs055
Oishi, K., Faria, A. V., Hsu, J., Tippett, D., Mori, S., & Hillis, A. E. (2015). Critical role of the right uncinate fasciculus in emotional empathy. Annals of Neurology, 77, 68–74. doi:10.1002/ana.24300
Paulus, F. M., Müller-Pinzler, L., Jansen, A., Gazzola, V., & Krach, S. (2015). Mentalizing and the role of the posterior superior temporal sulcus in sharing others’ embarrassment. Cerebral Cortex, 25, 2065–2075. doi:10.1093/cercor/bhu011
Rankin, K. P., Gorno-Tempini, M. L., Allison, S. C., Stanley, C. M., Glenn, S., Weiner, M. W., & Miller, B. L. (2006). Structural anatomy of empathy in neurodegenerative disease. Brain, 129, 2945–2956. doi:10.1093/brain/awl254
Realmuto, S., Zummo, L., Cerami, C., Agro, L., Dodich, A., Canessa, N., & Daniele, O. (2015). Social cognition dysfunctions in patients with epilepsy: Evidence from patients with temporal lobe and idiopathic generalized epilepsies. Epilepsy and Behavior, 47, 98–103. doi:10.1016/j.yebeh.2015.04.048
Reniers, R. L., Corcoran, R., Drake, R., Shryane, N. M., & Vollm, B. A. (2011). The QCAE: A questionnaire of cognitive and affective empathy. Journal of Personality Assessment, 93, 84–95. doi:10.1080/00223891.2010.528484
Sanai, N., Polley, M. Y., & Berger, M. S. (2010). Insular glioma resection: Assessment of patient morbidity, survival, and tumor progression. Journal of Neurosurgery, 112, 1–9. doi:10.3171/2009.6.JNS0952
Sebastian, C. L., Fontaine, N. M., Bird, G., Blakemore, S. J., Brito, S. A., McCrory, E. J., & Viding, E. (2012). Neural processing associated with cognitive and affective Theory of Mind in adolescents and adults. Social Cognitive and Affective Neuroscience, 7, 53–63. doi:10.1093/scan/nsr023
Seth, A. K. (2013). Interoceptive inference, emotion, and the embodied self. Trends in Cognitive Sciences, 17, 565–573. doi:10.1016/j.tics.2013.09.007
Shamay-Tsoory, S. G. (2011). The neural bases for empathy. The Neuroscientist, 17, 18–24. doi:10.1177/1073858410379268
Shamay-Tsoory, S. G., Aharon-Peretz, J., & Perry, D. (2009). Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132, 617–627. doi:10.1093/brain/awn279
Shamay-Tsoory, S. G., Tomer, R., Goldsher, D., Berger, B. D., & Aharon-Peretz, J. (2004). Impairment in cognitive and affective empathy in patients with brain lesions: Anatomical and cognitive correlates. Journal of Clinical and Experimental Neuropsychology, 26, 1113–1127. doi:10.1080/13803390490515531
Sheng, F., Liu, Q., Li, H., Fang, F., & Han, S. (2014). Task modulations of racial bias in neural responses to others’ suffering. NeuroImage, 88, 263–270. doi:10.1016/j.neuroimage.2013.10.017
Silani, G., Bird, G., Brindley, R., Singer, T., Frith, C., & Frith, U. (2008). Levels of emotional awareness and autism: An fMRI study. Social Neuroscience, 3, 97–112. doi:10.1080/17470910701577020
Singer, T., Seymour, B., O’Doherty, J., Kaube, H., Dolan, R. J., & Frith, C. D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303, 1157–1162. doi:10.1126/science.1093535
Smith, M. J., Schroeder, M. P., Abram, S. V., Goldman, M. B., Parrish, T. B., Wang, X., & Breiter, H. C. (2015). Alterations in brain activation during cognitive empathy are related to social functioning in schizophrenia. Schizophrenia Bulletin, 41, 211–222. doi:10.1093/schbul/sbu023
Tanriover, N., Rhoton, A. L., Jr., Kawashima, M., Ulm, A. J., & Yasuda, A. (2004). Microsurgical anatomy of the insula and the sylvian fissure. Journal of Neurosurgery, 100, 891–922. doi:10.3171/jns.2004.100.5.0891
Taylor, G. J. (1994). The alexithymia construct: Conceptualization, validation, and relationship with basic dimensions of personality. New Trends in Experimental & Clinical Psychiatry, 10, 61–74.
Wager, T. D., Davidson, M. L., Hughes, B. L., Lindquist, M. A., & Ochsner, K. N. (2008). Prefrontal–subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–1050. doi:10.1016/j.neuron.2008.09.006
Walter, H. (2012). Social cognitive neuroscience of empathy: Concepts, circuits, and genes. Emotion Review, 4, 9–17. doi:10.1177/1754073911421379
Wang, L., & Markham, R. (1999). The development of a series of photographs of Chinese facial expressions of emotion. Journal of Cross-Cultural Psychology, 30, 397–410. doi:10.1177/0022022199030004001
Wang, Q., Zhang, Z., Dong, F., Chen, L., Zheng, L., Guo, X., & Li, J. (2014). Anterior insula GABA levels correlate with emotional aspects of empathy: A proton magnetic resonance spectroscopy study. PLoS ONE, 9, e113845. doi:10.1371/journal.pone.0113845
Wang, X., Gu, X., Fan, J., Wang, S., Zhao, F., Hof, P. R., & Gao, Z. (2014). Recovery of empathetic function following resection of insular gliomas. Journal of Neuro-Oncology, 117, 269–277. doi:10.1007/s11060-014-1380-y
Warren, K. E., Patronas, N., Aikin, A. A., Albert, P. S., & Balis, F. M. (2001). Comparison of one-, two-, and three-dimensional measurements of childhood brain tumors. Journal of the National Cancer Institute, 93, 1401–1405.
Young, L. J., & Barrett, C. E. (2015). Neuroscience: Can oxytocin treat autism? Science, 347, 825–826. doi:10.1126/science.aaa8120
Yuvaraj, R., Murugappan, M., Norlinah, M. I., Sundaraj, K., & Khairiyah, M. (2013). Review of emotion recognition in stroke patients. Dementia and Geriatric Cognitive Disorders, 36, 179–196. doi:10.1159/000353440
Zaki, J., Weber, J., Bolger, N., & Ochsner, K. (2009). The neural bases of empathic accuracy. Proceedings of the National Academy of Science, 106, 11382–11387. doi:10.1073/pnas.0902666106
Zhang, F.-F., Dong, Y., & Wang, K. (2010). Reliability and validity of the Chinese version of the interpersonal reactivity index-C. Chinese Journal of Clinical Psychology, 2, 155–157.
Zung, W. W. (1965). A self-rating depression scale. Archives of General Psychiatry, 12, 63–70.
Author note
None of the authors have any conflicts of interest to disclose. We are grateful to the participants involved in our study. This study was supported by a Science and Technology Project grant from Anhui Province (No:1506c085017), the National Natural Science Foundation of China (31230032, 31171083, and 31471071), the Fundamental Research Fund for the Central Universities of China (WK2070000033), the 100 Talents Programme of the Chinese Academy of Sciences (KJ2070000018), and Hefei Science Center, CAS “User with Potential” (2015HSC-UP017).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 154 kb)
Rights and permissions
About this article
Cite this article
Chen, P., Wang, G., Ma, R. et al. Multidimensional assessment of empathic abilities in patients with insular glioma. Cogn Affect Behav Neurosci 16, 962–975 (2016). https://doi.org/10.3758/s13415-016-0445-0
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-016-0445-0