Abstract
Recent research has demonstrated the critical role of the feeling of familiarity in recognition memory. Various neuroimaging paradigms have been developed to identify the brain regions that sustain the processing of familiarity; however, there is still considerable controversy about the functional significance of each brain region implicated in familiarity-based retrieval. Here, we focused on the differences between paradigms that assess familiarity, with or without the encoding phase. We used the activation likelihood estimation (ALE) algorithm to conduct a whole-brain meta-analysis of neuroimaging studies that involved a familiarity task. Sixty-nine studies, performed in healthy subjects to determine the specific functions of the identified regions in familiarity processing, were finally selected. Distinct subanalyses were performed according to the experimental procedures used in the original studies. The ALE clusters that were highlighted revealed common activations for paradigms with and without encoding in the prefrontal cortex and in the parietal cortex. Additionally, supplementary activations related to specific familiarity (i.e., without the encoding phase) were observed in the limbic system (i.e., the amygdala, hippocampus, cingulate cortex, and insula) and in the associative sensory areas. The differences in the reported findings for different procedures are possibly due to differences in the concept of familiarity. To aid the exploration of the neural correlates of familiarity in future studies, the strengths and weaknesses of these experimental procedures are critically discussed.
Similar content being viewed by others
Familiarity processing is a crucial aspect of recognition, and it provides the feeling that an item has previously been encountered, independent of any recollection of the associated details. Numerous studies have been performed to evaluate recognition, but there is still considerable controversy about the functional significance of each brain region consistently implicated in familiarity-based retrieval (Spaniol et al., 2009). In the current study, we hypothesized that discrepancies in the results of brain imaging studies that have explored familiarity may be due to the heterogeneity of the experimental procedures used to assess this phenomenon. To overcome this limitation, we conducted a systematic literature search and completed an activation likelihood estimate (ALE) meta-analysis of the retained published neuroimaging data concerning familiarity processing.
Familiarity has been studied using two major approaches. The first one, based on the dual-process theory (Yonelinas, 1994), relies on the discrimination between familiarity and recollection. This approach is based on the postulate that recognition memory depends on two memory retrieval processes, namely, familiarity, which is the feeling that a stimulus has been encountered previously without the recall of contextual details, and recollection, which occurs when subjects can retrieve the details linked to the initial exposure, such as where or when the first encounter occurred (e.g., Eichenbaum, Yonelinas, & Ranganath, 2007; Migo, Mayes, & Montaldi, 2012; Vilberg & Rugg, 2008; Yonelinas, 2001; Yonelinas, Aly, Wang, & Koen, 2010). The second approach relies on the presentation of “personally” familiar stimuli (i.e., stimuli that the participant has personally experienced previously; Ramon, Dricot, & Rossion, 2010). These two approaches differ in that, in one case, familiarity is specifically generated for the test and the same stimuli can be used for any participant, while in the other case, familiarity is a consequence of natural exposure in daily life and is based on stimuli that refer to the participant’s personal life. To our knowledge, familiarity has always been studied according to one of these approaches, but no one study has attempted to compare results between the two approaches.
Various paradigms have been developed to test familiarity processing according to these two approaches. First, paradigms have been used to estimate the respective contributions of familiarity and recollection during recognition tasks. They are performed in two steps: (1) an encoding phase and (2) a test phase. During the test phase, the participants are asked to distinguish known stimuli (previously presented in the encoding phase) from new stimuli. Recognition is considered to be based on recollection or familiarity, according to the ability of the participants to recollect some specific aspects of the encoding conditions present when the stimulus was encountered (Yonelinas et al., 2010; Yonelinas, 2001). In Remember/Know (R/K) paradigms, participants are required to complete a subjective evaluation of familiarity;; that is, they are asked to provide a “Remember” answer if recognition is accompanied by a conscious recollection of information and a (Know” answer if they recognize a stimulus without being able to say when or where (Diana, Yonelinas, & Ranganath, 2007; Guillaume et al., 2007; Mayes, Montaldi, & Migo, 2007; Yonelinas, 2001). In source memory retrieval paradigms (also called associative or context recognition paradigms), recollection is assumed to reflect the ability to retrieve source information available at the time of encoding, while familiarity is the ability to recognize items that are not recollected (Libby, Yonelinas, Ranganath, & Ragland, 2013). Stimuli are presented in different contexts (such as various font colors or spatial positions) in context recognition paradigms or with another item in associative paradigms, and at the test the participants are asked to decide whether a stimulus is “old” (i.e., had been presented during the encoding phase) or not, and then to identify the context for each “old” stimulus (Cansino, Maquet, Dolan, & Rugg, 2002; Lefèbvre et al., 2010; Migo et al., 2012).
Alongside these paradigms that require qualitative assessments of familiarity, a second evaluation of familiarity uses a method in which participants are asked to rate the stimulus in terms of familiarity strength or confidence (Kim, 2010). This method assumes that familiarity varies in a continuous manner and can thus be assessed quantitatively (Daselaar, Fleck, & Cabeza, 2006; Montaldi, Spencer, Roberts, & Mayes, 2006; Ranganath et al., 2004; Yonelinas, Otten, Shaw, & Rugg, 2005). The familiarity strength studies also have an encoding phase followed by a test phase. During the test phase, the participants were asked to indicate whether they remember the item being presented during the encoding phase. In each trial, the old/new decision was followed by a confidence rating (low to high), yielding a familiarity strength scale. An advantage of such paradigms, particularly in the brain-imaging context, is that they potentially allow the identification of familiarity-related brain regions (i.e., those regions that are associated with confidence recognition levels; Daselaar et al., 2006; Ranganath et al., 2004; Yonelinas et al., 2005).
A third method to explore familiarity is based on stimuli that are specifically familiar for the participant (i.e., stimuli that refer to self-related emotional responses; Maddock, Garrett, & Buonocore, 2001; P. Qin et al., 2012). This experimental method involves the presentation of specific familiar stimuli without requiring an initial encoding or familiarization task (P. Qin et al., 2012). In these paradigms, which have a unique test phase, the participants are asked to detect familiar stimuli among unfamiliar stimuli. This task can be realized with famous stimuli (i.e., stimuli known by everyone) or with personally familiar stimuli (i.e., stimuli that are only familiar to the participant; Gobbini, Leibenluft, Santiago, & Haxby, 2004). This approach, which does not involve an encoding phase, is expected to enable the specific and objective study of familiarity, independent of recollection.
Numerous studies seeking to reveal the neural basis of familiarity have been performed using one of these different types of paradigms (Diana et al., 2007; Johnson, Suzuki, & Rugg, 2013; Skinner & Fernandes, 2007; Squire, Wixted, & Clark, 2007). On one hand, neuroimaging studies have been conducted using paradigms based on the dual-process theory. In a previous review, Eichenbaum et al. considered studies that examined the subregions of the medial temporal lobe (MTL) that were activated by recollection or familiarity (Eichenbaum et al., 2007). These authors found that among the 15 studies that have investigated familiarity contrasts, 13 produced evidence of the involvement of the perirhinal cortex (PRC), and that among the 19 studies that examined recollection contrasts, 16 reported hippocampal (HC) activation. Moreover, when familiarity was dissociated from recollection, several authors identified the PRC as critical for familiarity (Bowles et al., 2007; Brown & Aggleton, 2001; Eichenbaum et al., 2007; Kafkas & Migo, 2009; Ranganath et al., 2004; Ryals, Cleary, & Seger, 2013). However, while some imaging studies have found PRC activation for familiar stimuli (Kafkas & Montaldi, 2012; Ryals et al., 2013), others have found PRC deactivation (Brown & Aggleton, 2001; Eichenbaum et al., 2007; Ranganath et al., 2004; Ryals et al., 2013; Staresina et al., 2012). Another review that did not focus on MTL regions was conducted to explore the brain structures that were associated with familiarity and recollection (Skinner & Fernandes, 2007). This review revealed that familiarity relies on MTL regions but also involves the lateral prefrontal cortex (lPFC) and the parietal cortex (PC). When experiments that used confidence measures were considered, activities associated with increasing confidence in the feeling of familiarity were observed in the frontal lobe and the parietal lobe (Skinner & Fernandes, 2007; Yonelinas et al., 2005).
On the other hand, brain regions that sustain familiarity processing have been explored by using paradigms that use specific familiar stimuli. A recent meta-analysis of imaging studies that sought to differentiate self-related processing and personal familiarity (P. Qin et al., 2012; P. Qin & Northoff, 2011) revealed that specific familiar stimuli might be associated with the responses of the posterior cingulate cortex (PCC). These findings appear to be consistent with the suggestion that the PCC may mediate interactions between emotional and memory-related processes (Maddock, Garrett, & Buonocore, 2003). However, the meta-analysis presented by P. Qin et al. (2012) was only based on personally familiar (PF) stimuli and was conducted by combining several types of contrasts—PF versus unfamiliar (UF), PF versus self, PF versus baseline, and PF versus famous—in a unique analysis. Some regions that are involved in familiarity processing might not have been highlighted in this analysis due to the heterogeneity of the contrasts.
Different meta-analyses have previously been performed in order to highlight the brain networks involved in familiarity in an efficient and bias-free way. These studies sought to specify some of the disparate results in the literature about familiarity-based retrieval. For instance, Kim (2010) demonstrated the involvement of three distinct functional networks in episodic retrieval from the activations observed in Remember/Know paradigms. Hutchinson et al. (Hutchinson, Uncapher, & Wagner, 2009; Hutchinson et al., 2014) examined the roles of different subregions in the parietal cortex in episodic retrieval. In their study, Spaniol et al. (2009) aimed to compare the results from the experimental procedures that test subjective versus objective recollection. While these studies have focused on the differences in the results between the different paradigms based on familiarity/recollection dissociation, in our study, we focused on the differences between paradigms that used encoded stimuli versus specific familiar stimuli. Indeed, it has been postulated that the neural correlates of familiarity depend on how familiarity is operationalized (Frithsen & Miller, 2014). We conducted separate ALE whole-brain meta-analyses of published neuroimaging data, using the three major approaches that were previously described, in order to clearly link the use of a familiarity process with the recruitment of the subportions of the identified network: (1) paradigms based on the recollection/familiarity dissociation, such as qualitative paradigms (R/K paradigms and source memory retrieval paradigms); (2) paradigms based on familiarity strength; and (3) paradigms based on specific familiar stimuli. The neuroimaging data that were included in the analyses were exclusively obtained from contrasts between familiar versus unfamiliar stimuli during memory retrieval. Moreover, these analyses were performed while simultaneously considering all stimulus types (regardless of the sensory modality involved) to ensure that the obtained results were amodal and did not reflect the brain structures that primarily subserve stimulus-specific pathways. A major concern about meta-analyses is the extent to which they mix studies that are different in type. In the present work, we chose to combine the results of studies that we considered to be sufficiently similar to be combinable, according to the type of familiarity to which they refer. Nevertheless, to prevent additional heterogeneity, we compromised by excluding contrasts other than familiar versus unfamiliar contrasts, as well as data recorded during the encoding phase, and by separately analyzing data from paradigms based on familiarity strength. Considering the results of previous studies, we hypothesized that the feeling of familiarity emerges from the concomitant recruitment of the medial temporal, parietal, and prefrontal regions. We predicted that the familiarity related to personal experience, laboratory-based perceptual exposures, or quantitative assessment of the familiarity would result in distinct outcomes associated with different types of operationalization of familiarity across these paradigms. Specific familiarity and familiarity that is related to laboratory-based perceptual exposures mainly differ in that they refer or do not refer to the participant’s personal experience and self-related emotional responses. Thus, we predicted that the processing of specific familiar stimuli would mainly activate limbic structures. In contrast, we predicted that the processing of familiarity associated with laboratory exposures would mainly activate prefrontal and parietal cortex. Therefore, we expected to clarify the functional significance of each brain region consistently implicated in familiarity-based retrieval.
Material and method
Literature search and inclusion criteria
A systematic literature search was conducted on PubMed (U.S. National Library of Medicine) using the keywords [(fMRI or PET or BOLD) and familiar]. We chose wide first-line criteria to avoid excluding relevant studies. No publication dates were imposed, but the final search was performed in December 2013.
The inclusion criteria for this meta-analysis were as follows: (1) adult studies, (2) fMRI or PET studies, and (3) familiarity tasks used in the studies. As previously described, we defined familiarity as the feeling of having seen a stimulus independent of the ability to retrieve where or when the stimulus was encountered (Montaldi & Mayes, 2010; Yovel & Paller, 2004).
The following exclusion criteria were applied: (1) reviews and meta-analysis; (2) studies that did not report whole-brain analyses in standard reference spaces (i.e., Talairach or MNI spaces), encompassing studies that reported findings based on regions of interest (ROI; to avoid experimenter-imposed biases in the locations at which the effects were identified) (Bartra, McGuire, & Kable, 2013; Hardwick, Rottschy, Miall, & Eickhoff, 2013; Vilberg & Rugg, 2008); (3) studies that reported data recorded during the encoding phase (Skinner & Fernandes, 2007); (4) studies that did not report activation for the contrast examining familiarity versus unfamiliarity; (5) studies that did not report data from healthy participants; and (6) papers written in a language other than English or French. Additionally, data from the control groups of patient studies were included when these data were reported independently from the group comparisons. We chose to not include studies that provided contrasts only on familiarity versus recollection to prevent heterogeneity between the contrasts pooled within the same analysis and to avoid the risk of not highlighting activations of regions that would be involved in both familiarity and recollection.
For each study, the activation foci for the contrasts comparing familiarity with unfamiliarity were extracted. Finally, the following experimental characteristics were extracted: the number of subjects, the paradigm type, the acquisition system (PET or fMRI), stereotactic space, the contrasts tested, and the number of foci obtained for each contrast.
Statistical analyses
Data analyses were carried out using the activation likelihood estimation (ALE) algorithm (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012; Eickhoff et al., 2009; Turkeltaub et al., 2012), which allows for the computation of coordinate-based random-effects meta-analyses of neuroimaging data, and implemented in GingerALE 2.3 (http://www.brainmap.org/ale). In the ALE method (Turkeltaub, Eden, Jones, & Zeffiro, 2002), for a unidirectional contrast of interest (e.g., familiar vs. unfamiliar), each activation focus reported in the literature is modeled as the peak of a 3-D Gaussian probability distribution. The ALE value, calculated as the sum of these probabilities across studies, represents the probability that a voxel contains at least one of the activation foci. The cluster-size volume is calculated using the false discovery rate and the total volume above the threshold. The resulting minimum volume removes any cluster that is smaller than the allowed false positives, leaving clusters that should contain true positives. The numbers of participants in each included study were used to weight the contributions of each study to the parameter estimates. The analysis was corrected for multiple comparisons using a false-discovery rate (FDR) with a threshold value of q < 0.01. A minimum cluster size of 200 mm3 was applied.
The Talairach space (Talairach & Tournoux, 1988) was defined as the common stereotactic space for the meta-analysis, and the results that were reported in the MNI coordinates were converted into Talairach space using the icbm2tal transform (Lancaster et al., 2007) as implemented in GingerALE 2.3. The data were then grouped according to the contrasts and experimental paradigms employed.
Analyses were performed to specifically analyze the roles of the different components in the previously identified network as follows:
-
1.
Activations in response to “encoded” familiar stimuli (EF) versus UF stimuli,
-
2.
Activations correlated with the confidence levels in the feelings of familiarity in response to EF [(EF) corr.],
-
3.
Activations in response to specific familiar (SF) stimuli versus UF stimuli.
The resulting thresholded ALE maps were visualized on a flat-map representation overlaid on the ICBM-152a standardized brain atlas using Mango software, which is an anatomical image overlay program (http://ric.uthscsa.edu/mango//mango.html).
Results
Results of the systematic literature review
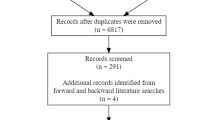
The systematic literature review identified 2,295 potential journal articles. According to the exclusion criteria, 1,723 studies were excluded based on their titles and abstracts. Of the remaining 572 studies, 504 articles were then excluded based on the aforementioned criteria. To accurately determine the effects of different procedures on the results, we did not include foci from contrasts that combined different procedures (e.g., contrasts that combined “natural familiarity” and “familiarity” as opposed to recollection). Finally, 69 studies fulfilling all criteria were included in the meta-analyses, as synthesized in Figure 1.
The 69 included studies were published in English and specifically tested familiarity, and each study was conducted with healthy subjects. These experiments grouped 996 participants and provided 771 foci. In five of these experiments, the data were extracted from adult control samples from patient comparative studies (schizophrenia = 2, neurodegenerative disease = 2, and autism = 1). Fifty-five studies used “paradigms with specific familiar stimuli” [(SF) > (UF)], nine used paradigms with “encoded stimuli” and a qualitative assessment [(EF) > (UF)], and five used paradigms with “encoded stimuli” and “confidence-level evaluations” [(EF) corr.]. The details of the 69 included studies are summarized in the Table 1.
Results of the analyses
The [(EF) > (UF)] analysis revealed selective activations in the prefrontal cortex (PFC) and in the PC. Selective analyses of the studies that reported [(EF) corr.] revealed activation in the left precentral gyrus. The contrast [(SF) > (UF)] revealed significant activations in the PFC and the PC as well as in the limbic system, the fusiform gyrus, the temporal gyrus, and the occipital gyrus (see Table 2 and Figure 2).
Activation likelihood estimates for the contrasts [(EF) > (UF)], ([(EF) corr.]) (A), and [(SF) > (UF)] (B) (threshold fixed at q < 0.01, FDR-corrected, with a cluster size > 200 mm3). Representative slices in the axial plane. L = left, R = right, PG = precentral gyrus, MFG = middle frontal gyrus, SFG = superior frontal gyrus, mFG = medial frontal gyrus, IPL = inferior parietal lobule, MTG = middle temporal gyrus, PCC = posterior cingulate cortex, A = amygdala, HC = hippocampus, FG = fusiform gyrus, CG = cingulate gyrus, mPFC = medial prefrontal cortex, IFG = inferior frontal gyrus, AG = angular gyrus. (Color figure online.)
Smaller minimum cluster size analyses
Numerous studies that have focused on the MTL have found PRC activations related to familiarity. To ensure that the use of a minimum cluster size of 200 mm3 did not result in the underestimation of smaller structures, such as the PRC, the previously described analyses were performed with a minimum cluster size of 100 mm3. Activations were observed in the MTL in the same regions as those highlighted by the analyses with a larger minimum cluster size (HC and amygdala); however, the results did not reveal greater activation of small MTL structures; specifically, no PRC activations were observed (cf. Table 3).
Discussion
In the present study, we aimed to clarify the brain regions that support familiarity processing. We proposed that the discrepancies in the available imaging findings were related to the heterogeneity of the experimental procedures used in previous studies to assess familiarity. To test this hypothesis, separate coordinate-based meta-analyses of functional imaging data were conducted for different familiarity paradigms.
Common activations for paradigms with and without encoding using a qualitative assessment [(EF) > (UF)] and [(SF) > (UF)]
The analyses revealed common activations for both paradigms with and without encoding phases in (1) the PFC (lateral, medial, and superior) and (2) the PC (BA 39/40). Although most researchers restricted their analyses to the MTL, others have reported PFC involvement in familiarity (e.g., Aly, Yonelinas, Kishiyama, & Knight, 2011; Henson, Rugg, Shallice, Josephs, & Dolan, 1999). Familiarity processing can be considered as a signal-detection/retrieval process that necessitates stages of memory assessment and decision (Yonelinas et al., 2010; Yonelinas, 2001). These two processes are known to involve the PFC (Aly et al., 2011; Henson et al., 1999; Kafkas & Montaldi, 2012; Ragland et al., 2012; Rugg, Fletcher, Frith, Frackowiak, & Dolan, 1996). In the current study, the left lPFC was predominantly found to be activated during familiarity processing. Previously, this region has been shown activated during context memory retrieval tasks and has been postulated to be implicated in cognitive control processes that guide access to relevant information from semantic memory (Badre & Wagner, 2007). Nevertheless, some authors have shown that patients with left PFC lesions display context memory deficits but not impaired recollective processing, measured by the R/K procedure (Duarte, Ranganath, & Knight, 2005). This has led to suggest that when the level of control, or effort, needed to perform a memory task increases, additional left PFC-mediated processes may be required (Skinner & Fernandes, 2007). Thus, lPFC activation would reflect that processing of stimuli that are identified as familiar may engage a more exhaustive search for details, which suggests more detailed processing of familiar stimuli (Wheeler & Buckner, 2004). In contrast, it has been suggested that the mPFC may belong to a system (in association with amygdala and ACC) that exerts emotion-driven influences on action selection (Ernst & Paulus, 2005; Müller, Cieslik, Laird, Fox, & Eickhoff, 2013; Ridderinkhof, Van den Wildenberg, Segalowitz, & Carter, 2004). Thus, both in the paradigms with and without encoding, activations within the lPFC (i.e., the inferior frontal gyrus) may be associated with the cognitive assessment of familiarity, whereas those within the medial PFC (mPFC) may reflect affective appraisal (Ernst & Paulus, 2005; Martínez-Selva, Sánchez-Navarro, Bechara, & Román, 2006; Ridderinkhof et al., 2004).
In the PC, the inferior parietal lobule (BA 39/40) was also found to be activated in our analyses of both specific familiarity and familiarity from encoding paradigms. Numerous functional neuroimaging studies have revealed that recollection and familiarity not only depend on the MTL and the PFC activities but also are consistently associated with activity in the lateral posterior PC, including the intraparietal sulcus (in the dorsal PC) and the inferior parietal lobule (in the ventral PC; Hutchinson et al., 2009; Skinner & Fernandes, 2007; Vilberg & Rugg, 2008). Based on the results of these studies, different models have been proposed to account for the role of PC in attention and memory, suggesting a dorsal/ventral dissociation (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; Frithsen & Miller, 2014). According to these models, dorsal parietal areas are involved in the top-down allocation of attention, while ventral parietal areas mediate bottom-up attention to retrieved contents (Spaniol et al., 2009). A dorsal/ventral dissociation has also been evidenced in memory retrieval, although the parietal regions implicated were revealed to be distinct from those associated with the attention process (Hutchinson et al., 2009; Nelson, McDermott, Wig, Schlaggar, & Petersen, 2013). Thus, dorsal PC has been proposed to contribute to familiarity-based judgements while regions in the ventral PC have been proposed to support the representation of recollected information (Ciaramelli, Grady, & Moscovitch, 2008; Vilberg & Rugg, 2008). Nevertheless, different studies showed that ventral PC activity could be associated with familiarity, as found in our study. This familiarity-related activity was notably observed when the R/K paradigm was used (Frithsen & Miller, 2014). In another study, activations in the ventral PC regions were observed when the studied items were correctly identified as well as when new, unstudied items were mistakenly judged to be old (Wheeler & Buckner, 2003). Ventral PC activity was therefore proposed to be sensitive to the subjects’ perception or decision that items had been previously experienced (Ciaramelli et al., 2008). Furthermore, a recent study showed that within the ventral PC, the angular gyrus and the temporoparietal junction differed substantially in their response characteristics, suggesting that the ventral PC cannot be considered to be a single functional unit (Hutchinson et al., 2009; Hutchinson et al., 2014). Thus, the temporoparietal junction and the supramarginal gyrus belong to the network involved in attention reorientation, while the angular gyrus is included in the “default network,” which is implicated in internally focused tasks (Hutchinson et al., 2014). The supramarginal gyrus/temporoparietal junction region has been found to be activated in our analyses of both specific familiarity and familiarity associated with encoding paradigms. On the contrary, the angular gyrus is only activated by specific familiarity contrasts, which corroborates its role in the tasks that are related to personal experiences. Our results confirmed the role of the ventral parietal areas in familiarity processing and the involvement of the angular gyrus in self-referential tasks. Nevertheless, further exploration may shed light on the relative contributions of parietal subregions to familiarity.
The assumption of a coactivation of the frontal and parietal areas in familiarity appears consistent with the results from previous event-related-potentials studies that have shown that responses recorded in the parietal and frontal locations are sensitive to familiarity (Gobbini & Haxby, 2007). While we expected that specific familiarity would mainly be supported by structures involved in emotional processing, cognitive and attentional processes appear also to be necessary in the specific familiarity processing. As a whole, we posit that PC activation in familiarity processing may reflect the integration of information and the orientation of attention to information that is specifically relevant to a given stimulus; PFC activation may reflect the affective appraisal and decision-making processes required to consider a stimulus as familiar.
Activations related to specific familiarity
While prefrontal and parietal regions are involved in the processing of familiar stimuli regardless of the paradigm (i.e., with or without an encoding phase), in paradigms that involve specific familiarity [(SF) > (UF)], activations were also observed in the limbic system (LS), which encompasses the amygdala, the HC, the cingulate cortex (ACC and PCC) and insula, the sensory cortices (temporal gyrus and occipital gyrus), and the fusiform gyrus.
LS is known to be involved in the integration of emotional states with cognition and behavior, consolidating memories, and forming emotions (Catani, Dell’acqua, & Thiebaut de Schotten, 2013). Because specific familiarity refers to the stimuli for which the participants have personal experience and that are associated with self-related emotional responses (Maddock et al., 2001; P. Qin et al., 2012), it appears obvious that familiarity with standardized stimuli that are encoded in laboratory settings does not constitute the same process that is involved in the complex and emotionally salient familiarity related to specific familiar stimuli. More specifically, the amygdala appears to have roles in emotional experience and in affective processing (Sabatinelli et al., 2011; White et al., 2008), and the insula has been demonstrated to be involved in the processing of subjective feelings (Singer, Critchley, & Preuschoff, 2009) and to play a prominent role in the detection of salient stimuli (Menon & Uddin, 2010). Within the LS, the amygdala and the insula both appear to support the emotional response that is experienced when perceiving specific familiar stimuli (Gobbini & Haxby, 2006).
Furthermore, activations were also observed in the anterior and posterior cingulate cortices. The cingulate cortices were shown to ensure the regulation of information flow between the limbic and prefrontal regions (Walton & Mars, 2007). The ACC has been described as the center of the brain’s self-regulatory system, integrating inputs from diverse sources to regulate responses and guide behavior (Bush, Luu, & Posner, 2000; Kelly et al., 2009). In our analysis, ACC activations were found in the rostral portions, which have been reported to be implicated in evaluative functions, including processing of conflict, response to errors, reasoning, and decision making, and in social cognitive functions, such as mentalizing and self-reflection (Ernst & Paulus, 2005; Fleck, Daselaar, Dobbins, & Cabeza, 2006; Frith, 2002; Kelly et al., 2009). Moreover, the PCC has been particularly highlighted by Qin et al. (P. Qin et al., 2012) in a study that sought to differentiate specific familiarity and self-related processing. In agreement with previous reports (Cloutier, Kelley, & Heatherton, 2011;. Gobbini & Haxby, 2006; Jurjanz et al., 2011; Maddock et al., 2003; P. Qin et al., 2012), the PCC appeared to be a key structure for specific familiarity in the present study.
The HC and PHC were also found to be activated by specific familiarity. While the HC and PHC are known to support recollection, their role in familiarity remains unclear (Daselaar et al., 2006; Song, Jeneson, & Squire, 2011; Squire et al., 2007; Wixted & Squire, 2011). Several authors have suggested that these structures are selectively involved in recollection and are insensitive to familiarity (e.g., Diana et al., 2007; Eichenbaum et al., 2007; Montaldi & Mayes, 2010; Ranganath & Ritchey, 2012; Yonelinas et al., 2005). However, the type of stimulus used in specific familiarity-related paradigms can be a strong source of variability. For example, when studies use famous faces, the images are often “iconic” pictures of celebrities (e.g., Che Guevera or Marilyn Monroe; Ramon, Caharel, & Rossion, 2011) that are likely to promote both familiarity and recollection processes. Therefore, the hypothesis of recollection-related activations during specific familiarity processing cannot be fully excluded. Nevertheless, if HC activation was only due to recollection, this could be observed in paradigms both with and without encoding. The HC activation limited to specific familiarity suggests a link with emotional content of familiar stimuli. This hypothesis is notably supported by the observation that memory performances are better for emotional events, when compared with neutral events (Bennion, Ford, Murray, & Kensinger, 2013; Buchanan, 2007). More accurately, amygdala activation has been shown to prioritize memories (Bennion et al., 2013), and this improvement in memory depends on interactions between the amygdala and the HC (Buchanan, 2007; Phelps, 2004).
Finally, activations were observed in the sensory cortices (temporal gyrus and occipital gyrus) and notably in the fusiform gyrus. The involvement of the fusiform gyrus in face perception has been demonstrated for many years (Kanwisher, McDermott, & Chun, 1997; Fairhall & Ishai, 2007; Haxby, Hoffman, & Gobbini, 2002). Nevertheless, Tarr and Gauthier (2000) collected evidence that the fusiform gyrus is associated with the processing of objects for which observers are experts. In order to determine whether the activation of the fusiform gyrus in our analysis is related to the processing of face stimuli, we conducted a new analysis in which only studies that used face stimuli were included (see results in Supplementary File 1). This “face stimuli” analysis did not reveal activation in the fusiform gyrus. This result implies that fusiform gyrus activation in our specific familiarity analysis is not due to the overrepresentation of experiments that used face stimuli. Greater activation of the sensory cortices and the fusiform gyrus may reflect a stronger perceptive processing of specific familiar stimuli, compared with unfamiliar stimuli (Ramon et al., 2011). The amygdala has been described as having a role in enhancing the perception of emotionally arousing stimuli (Maddock et al., 2003). Given its connection with the sensory cortices (Pourtois, Schettino, & Vuilleumier, 2013; Richter-Levin & Akirav, 2000), the amygdala may promote activations related to familiarity in the visual (occipital gyrus) and auditory (temporal gyrus) cortices (the majority of the studies included in this meta-analysis used visual or auditory stimuli), which accounts for the preferential perceptive processing of familiar stimuli (Skinner & Fernandes, 2007). Therefore, specific familiarity appears to be a result of a spatially distributed process that involves areas participating in cognitive, emotional, and sensory functions.
Absence of PRC activation
In contrast with a few previous neuroimaging studies, the PRC was not found to be activated in familiarity processing in the current meta-analysis. Although caution is required when interpreting negative findings, the absence of PRC activation could be explained by several factors. First, a confused nomenclature has burdened the PRC and its location is often confounded with that of its neighbors (Augustinack et al., 2013). Augustinack et al. recently performed a probabilistic mapping based on high-resolution ex vivo imaging to predict the location of the PRC in the human brain and concluded that the term “perirhinal cortex” should only be used to refer to Brodmann’s area 35. Second, PRC activations have primarily been observed in studies that have focused on the MTL or directly observed in analyses that were based on ROI strategies (i.e., studies that did not report whole-brain analyses and were thus excluded from our analyses). Reiteration of the meta-analysis by applying a smaller minimum cluster size (i.e., with a minimum cluster size of 100 mm3) did not result in the detection of PRC activation. Finally, many of the studies that have highlighted the involvement of the PRC in familiarity have found that PRC activation increases with the decreasing confidence (Eichenbaum et al., 2007; Montaldi et al., 2006; Skinner & Fernandes, 2007). The purpose of the present meta-analysis was to precisely determine which neural networks are recruited by familiarity processing, and, to this end, we only considered brain structures that were activated by familiar stimuli processing. In contrast, familiarity-associated PRC deactivation suggests that the PRC may play a role in the detection of novel objects (Augustinack et al., 2013; Brown & Aggleton, 2001). Further analyses regarding deactivation are required to clarify this point and determine the exact role of PRC in familiarity.
Differences in the concept of familiarity lead to differences in the cerebral networks that are recruited
On one hand, familiarity based on encoded stimuli appears as a cognitive process that involves PFC and parietal activations. On the other hand, specific familiarity appears to result from a complex interplay between cognitive, emotional, and sensory functions that are supported by different subnetworks. In the case of specific familiarity, different models based on patient behavioral data have been proposed for recognition of faces and people (Bruce & Young, 1986; Burton, Bruce, & Johnston, 1990). To reconcile these models, it has been hypothesized that familiarity may be a complex system based on (1) an objective component linked to the number of times the subject has been exposed to the stimulus, (2) a subjective component formed by the personally relevant and emotional experiences, and (3) a control component (Gainotti, 2007). Paradigms that are based on specific familiar stimuli account for these three components: PFC and parietal activations would reflect the objective and control components, and activations of the limbic structures would reflect the emotional processing that is particular to specific familiarity; the interaction between information from the limbic and the prefrontal regions would be ensured by the cingulated cortices (Ridderinkhof et al., 2004; Walton & Mars, 2007); and the activation between the amygdala and the sensory cortices would account for the preferential perceptive processing of familiar stimuli.
In contrast, because stimuli that are employed in encoding paradigms have not been personally experienced, these methods would only test familiarity in its objective and control components. Accordingly, the emotional component would not be integrated in the feeling of familiarity and would be considered as dissociated from familiarity per se. Some authors consider that emotional response to a familiar face should be dissociated from recognition of the familiar visual appearance (e.g., Gobbini & Haxby, 2006). Thus, encoded familiarity would be based on activations of PFC and PC, which have been assumed to be involved in the convergence of information to generate an integrated processing and in cognitive control and decision making. Paradigms that highlight cognitive processes related to familiarity are based on discrimination between familiarity and recollection. Nevertheless, these paradigms present some limitations, precisely a difficulty in accurately distinguishing between these two processes (Diana, Yonelinas, & Ranganath, 2008; Migo et al., 2012; Montaldi & Mayes, 2010; Vilberg & Rugg, 2008; Wais, 2008). For example, R/K paradigms, which are some of the most commonly used paradigms to assess familiarity in the cognitive neuroscience literature, are based on subjective responses, and it has been shown using post hoc tests that the “Know” responses are regularly associated with source recollection (Wais, 2008). In source memory retrieval paradigms, source or context recognition are known to be supported by familiarity when the item and its context are unitized during encoding, which occurs when the contextual information is encoded as a feature of the item (e.g., the item and its color; Diana et al., 2007; Elfman, Parks, & Yonelinas, 2008; Parks, Murray, Elfman, & Yonelinas, 2011). Another issue is that participants may fail to retrieve the source memory questions but may be able to retrieve task-irrelevant source information about the study episode (Kafkas & Migo, 2009; Song et al., 2011). Furthermore, it has been suggested that the processing of a studied stimulus could elicit a weak activation of the associated context, even when recollection fails, leading some authors to define a “contextual familiarity” (Addante, Ranganath, & Yonelinas, 2012). This context familiarity would contribute to the difficulty of accurately distinguishing between familiarity and recollection. It would have been particularly suitable to distinctly analyze R/K paradigms and source memory retrieval paradigms in order to test whether brain activations remain consistent within procedures with encoding, as shown in a recent study (Spaniol et al., 2009). Unfortunately, the number of studies was not sufficient for such an analysis. Although we could not confirm the result of Frithsen findings with our meta-analysis, our data agree with the conclusion that the neural correlates of familiarity depend on how it is operationalized (Frithsen & Miller, 2014).
It should be noted that several brain regions (i.e., the inferior parietal lobule, the MTL, the mPFC and the Pr/PCC region) that were identified in a previous meta-analysis focused on semantic processing (Binder, Desai, Graves, & Conant, 2009) were found to be activated during familiarity processing in our meta-analysis of specific familiarity. Various authors have suggested that familiarity can be compared to semantic information, whereas recollection refers to episodic information (Skinner & Fernandes, 2007; Yonelinas, 2002). However, this idea has been challenged (Waidergoren, Segalowicz, & Gilboa, 2012), and several reports suggest that familiarity processing cannot be reduced to the retrieval of semantic information. First, a recent study found that semantic information was associated with specific familiarity but that this association cannot be easily generated through large numbers of laboratory-based perceptual exposures (X. A. Qin, Koutstaal, & Engel, 2014). Accordingly, the close link between semantic knowledge retrieval and familiarity experience concerns specific familiarity alone. Second, the regions that were specifically found activated in familiarity, particularly in the LS and sensory cortices, are not associated with semantic processes. Thus, although familiarity is a process that is closely associated with semantic retrieval and, in some cases, automatically activates a large amount of semantic information (Rossion, Schiltz, Robaye, Pirenne, & Crommelinck, 2001), these are two different processes. It is notably possible that familiarity may occur without semantic information retrieval when a stimulus is perceived as having been encountered already; however, it is not possible to retrieve the associated information, neither the semantic information (i.e., timeless details such as the name of the person or the place) nor the episodic information (i.e., details linked to the previous exposures, such as where or when the first encounter occurred).
Finally, paradigms that report the correlations between BOLD intensities and confidence ratings are relevant for detecting the brain regions whose activations are correlated with the intensity of the feeling of familiarity. Considering that familiarity reflects the assessment of quantitative memory strength (Yonelinas et al., 2010; Yonelinas, 2001), familiarity may linearly increase as a function of perceived oldness. Although these paradigms are statistically less powerful than comparative methods, they should be useful for accurately discriminating familiarity from recollection (Daselaar et al., 2006; Ranganath et al., 2004; Yonelinas et al., 2005). Our meta-analysis highlighted that the region identified as significantly activated by these paradigms is the precentral gyrus in the lPFC. This suggests that a cognitive operation involved in the decision-making contributes to the confidence level in the feeling of familiarity. Nevertheless, the limited number of studies included may have led to this unique result. Further studies will be necessary to determine if activations in other regions could be correlated with the intensity of the feeling of familiarity.
The present quantitative review confirmed our hypothesis that the heterogeneity of the experimental procedures used to assess familiarity led to discrepancies in the results of brain imaging studies. Although we acknowledge that the number of studies included for the [(EF) > (UF)] and ([(EF) corr.]) contrasts was smaller than that for the [(SF) > (UF)] contrast, it appears obvious that familiarity with standardized stimuli that are encoded in laboratory settings does not constitute the same process that is involved in the complex and emotionally salient familiarity induced by specific familiar stimuli (Trinkler, King, Doeller, Rugg, & Burgess, 2009). The resulting heterogeneity between the procedures is possibly due to differences in opinion regarding the concept of familiarity, and we join the idea that a more consensual approach to familiarity appears to be essential in determining the neural correlates of familiarity (Maddock et al., 2001).
Conclusions
Currently, the known divergences in the findings of brain imaging studies that have investigated familiarity reflect the lack of a reliable assessment of this construct (Kafkas & Migo, 2009). Based on an ALE meta-analysis of 68 published functional studies that grouped 979 participants, we were able to unravel these apparently conflicting results. A long-range brain network was identified for specific familiarity, including the LS, the PFC, and the parietal and associative sensory regions. Thus, specific familiarity can be considered a metaprocess that results from the integration of different processes that could be associated with the different components that have been identified to support familiarity. However, in paradigms with encoding, only prefrontal and parietal activations were observed, suggesting that when considering familiarity from the dual-process theory, emotional response is dissociated from familiarity. Crucially, this work highlights the influence that procedure selection can have on the final results. We believe that a more consensual approach to familiarity would allow for the functional recruitment of the dedicated structures identified in this meta-analysis.
Abbreviations
- ACC:
-
anterior cingulate cortex
- EF:
-
encoded familiar
- F:
-
familiar
- HC:
-
hippocampus
- lPFC:
-
lateral prefrontal cortex
- LS:
-
limbic system
- mPFC:
-
medial prefrontal cortex
- MTL:
-
medial temporal lobe
- PC:
-
parietal cortex
- PCC:
-
posterior cingulate cortex
- PF:
-
personal familiar
- PFC:
-
prefrontal cortex
- PHC:
-
parahippocampal cortex
- PRC:
-
perirhinal cortex
- SF:
-
specific familiar
- UF:
-
unfamiliar
References
Addante, R. J., Ranganath, C., & Yonelinas, A. P. (2012). Examining ERP correlates of recognition memory: Evidence of accurate source recognition without recollection. NeuroImage, 62(1), 439–450. doi:10.1016/j.neuroimage.2012.04.031
Aly, M., Yonelinas, A. P., Kishiyama, M. M., & Knight, R. T. (2011). Damage to the lateral prefrontal cortex impairs familiarity but not recollection. Behavioural Brain Research, 225(1), 297–304. doi:10.1016/j.bbr.2011.07.043
Augustinack, J. C., Huber, K. E., Stevens, A. A., Roy, M., Frosch, M. P., Van der Kouwe, A. J. W., . . . Alzheimer’s Disease Neuroimaging Initiative. (2013). Predicting the location of human perirhinal cortex, Brodmann’s area 35, from MRI. NeuroImage, 64, 32-42. doi:10.1016/j.neuroimage.2012.08.071
Badre, D., & Wagner, A. D. (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45(13), 2883–2901. doi:10.1016/j.neuropsychologia.2007.06.015
Bartra, O., McGuire, J. T., & Kable, J. W. (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–427. doi:10.1016/j.neuroimage.2013.02.063
Bennion, K. A., Ford, J. H., Murray, B. D., & Kensinger, E. A. (2013). Oversimplification in the study of emotional memory. Journal of the International Neuropsychological Society: JINS, 19(9), 953–961. doi:10.1017/S1355617713000945
Binder, J. R., Desai, R. H., Graves, W. W., & Conant, L. L. (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–2796. doi:10.1093/cercor/bhp055
Bowles, B., Crupi, C., Mirsattari, S. M., Pigott, S. E., Parrent, A. G., Pruessner, J. C., . . . Köhler, S. (2007). Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 104(41), 16382-16387. doi:10.1073/pnas.0705273104
Brown, M. W., & Aggleton, J. P. (2001). Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews: Neuroscience, 2(1), 51–61. doi:10.1038/35049064
Bruce, V., & Young, A. (1986). Understanding face recognition. British Journal of Psychology, 77(Pt. 3), 305–327.
Buchanan, T. W. (2007). Retrieval of emotional memories. Psychological Bulletin, 133(5), 761–779. doi:10.1037/0033-2909.133.5.761
Burton, A. M., Bruce, V., & Johnston, R. A. (1990). Understanding face recognition with an interactive activation model. British Journal of Psychology, 81(Pt. 3), 361–380.
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222.
Cabeza, R., Ciaramelli, E., Olson, I. R., & Moscovitch, M. (2008). The parietal cortex and episodic memory: An attentional account. Nature Reviews: Neuroscience, 9(8), 613–625. doi:10.1038/nrn2459
Cansino, S., Maquet, P., Dolan, R. J., & Rugg, M. D. (2002). Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex, 12(10), 1048–1056.
Catani, M., Dell’acqua, F., & Thiebaut de Schotten, M. (2013). A revised limbic system model for memory, emotion and behaviour. Neuroscience and Biobehavioral Reviews, 37(8), 1724–1737. doi:10.1016/j.neubiorev.2013.07.001
Ciaramelli, E., Grady, C. L., & Moscovitch, M. (2008). Top-down and bottom-up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia, 46(7), 1828–1851. doi:10.1016/j.neuropsychologia.2008.03.022
Cloutier, J., Kelley, W. M., & Heatherton, T. F. (2011). The influence of perceptual and knowledge-based familiarity on the neural substrates of face perception. Social Neuroscience, 6(1), 63–75. doi:10.1080/17470911003693622
Daselaar, S. M., Fleck, M. S., & Cabeza, R. (2006). Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. Journal of Neurophysiology, 96(4), 1902–1911. doi:10.1152/jn.01029.2005
Diana, R. A., Yonelinas, A. P., & Ranganath, C. (2007). Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences, 11(9), 379–386. doi:10.1016/j.tics.2007.08.001
Diana, R. A., Yonelinas, A. P., & Ranganath, C. (2008). The effects of unitization on familiarity-based source memory: Testing a behavioral prediction derived from neuroimaging data. Journal of Experimental Psychology: Learning, Memory, and Cognition, 34(4), 730–740. doi:10.1037/0278-7393.34.4.730
Duarte, A., Ranganath, C., & Knight, R. T. (2005). Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(36), 8333–8337. doi:10.1523/JNEUROSCI.1392-05.2005
Eichenbaum, H., Yonelinas, A. P., & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30(1), 123–152. doi:10.1146/annurev.neuro.30.051606.094328
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., & Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. doi:10.1002/hbm.20718
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., & Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. NeuroImage, 59(3), 2349–2361. doi:10.1016/j.neuroimage.2011.09.017
Elfman, K. W., Parks, C. M., & Yonelinas, A. P. (2008). Testing a neurocomputational model of recollection, familiarity, and source recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition, 34(4), 752–768. doi:10.1037/0278-7393.34.4.752
Ernst, M., & Paulus, M. P. (2005). Neurobiology of decision making: A selective review from a neurocognitive and clinical perspective. Biological Psychiatry, 58(8), 597–604. doi:10.1016/j.biopsych.2005.06.004
Fairhall, S. L., & Ishai, A. (2007). Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex, 17(10), 2400–2406.
Fleck, M. S., Daselaar, S. M., Dobbins, I. G., & Cabeza, R. (2006). Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cerebral Cortex, 1623-1630. doi:10.1093/cercor/bhj097
Frith, C. (2002). Attention to action and awareness of other minds. Consciousness and Cognition, 11(4), 481–487.
Frithsen, A., & Miller, M. B. (2014). The posterior parietal cortex: Comparing remember/know and source memory tests of recollection and familiarity. Neuropsychologia, 61, 31–44. doi:10.1016/j.neuropsychologia.2014.06.011
Gainotti, G. (2007). Face familiarity feelings, the right temporal lobe and the possible underlying neural mechanisms. Brain Research Reviews, 56(1), 214–235. doi:10.1016/j.brainresrev.2007.07.009
Gobbini, M. I., & Haxby, J. V. (2006). Neural response to the visual familiarity of faces. Brain Research Bulletin, 71(1/3), 76–82. doi:10.1016/j.brainresbull.2006.08.003
Gobbini, M. I., & Haxby, J. V. (2007). Neural systems for recognition of familiar faces. Neuropsychologia, 45(1), 32–41. doi:10.1016/j.neuropsychologia.2006.04.015
Gobbini, I. M., Leibenluft, E., Santiago, N., & Haxby, J. V. (2004). Social and emotional attachment in the neural representation of faces. NeuroImage, 22(4), 1628–1635. doi:10.1016/j.neuroimage.2004.03.049
Guillaume, F., Guillem, F., Tiberghien, G., Martin, F., Ganeva, E., Germain, M., . . . Lalonde, P. (2007). Use of the process dissociation procedure to study the contextual effects on face recognition in schizophrenia: Familiarity, associative recollection and discriminative recollection. Psychiatry Research, 149(1/3), 105–119.
Hardwick, R. M., Rottschy, C., Miall, R. C., & Eickhoff, S. B. (2013). A quantitative meta-analysis and review of motor learning in the human brain. NeuroImage, 67, 283–297. doi:10.1016/j.neuroimage.2012.11.020
Haxby, J. V., Hoffman, E. A., & Gobbini, M. I. (2002). Human neural systems for face recognition and social communication. Biological Psychiatry, 51(1), 59–67.
Henson, R. N. A., Rugg, M. D., Shallice, T., Josephs, O., & Dolan, R. J. (1999). Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. The Journal of Neuroscience, 19(10), 3962–3972.
Hutchinson, J. B., Uncapher, M. R., & Wagner, A. D. (2009). Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn Memory, 16(6), 343–356. doi:10.1101/lm.919109
Hutchinson, J. B., Uncapher, M. R., Weiner, K. S., Bressler, D. W., Silver, M. A., Preston, A. R., & Wagner, A. D. (2014). Functional heterogeneity in posterior parietal cortex across attention and episodic memory retrieval. Cerebral Cortex, 24(1), 49–66. doi:10.1093/cercor/bhs278
Johnson, J. D., Suzuki, M., & Rugg, M. D. (2013). Recollection, familiarity, and content-sensitivity in lateral parietal cortex: A high-resolution fMRI study. Frontiers in Human Neuroscience, 7, 219. doi:10.3389/fnhum.2013.00219
Jurjanz, L., Donix, M., Amanatidis, E. C., Meyer, S., Poettrich, K., Huebner, T., . . . Holthoff, V. A. (2011). Visual personal familiarity in amnestic mild cognitive impairment. PLOS ONE, 6(5), e20030. doi:10.1371/journal.pone.0020030
Kafkas, A., & Migo, E. M. (2009). Familiarity and recollection in the medial temporal lobe. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(8), 2309–2311. doi:10.1523/JNEUROSCI.5874-08.2009
Kafkas, A., & Montaldi, D. (2012). Familiarity and recollection produce distinct eye movement, pupil and medial temporal lobe responses when memory strength is matched. Neuropsychologia, 50(13), 3080–3093. doi:10.1016/j.neuropsychologia.2012.08.001
Kanwisher, N., McDermott, J., & Chun, M. M. (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 17(11), 4302–4311.
Kelly, A. M. C., Di Martino, A., Uddin, L. Q., Shehzad, Z., Gee, D. G., Reiss, P. T., . . . Milham, M. P. (2009). Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex, 19(3), 640-657. doi:10.1093/cercor/bhn117
Kim, H. (2010). Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. NeuroImage, 50(4), 1648–1657. doi:10.1016/j.neuroimage.2010.01.051
Lancaster, J. L., Tordesillas-Gutiérrez, D., Martinez, M., Salinas, F., Evans, A., Zilles, K., . . . Fox, P. T. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping, 28(11), 1194-1205. doi:10.1002/hbm.20345
Lefèbvre, A.-A., Cellard, C., Tremblay, S., Achim, A., Rouleau, N., Maziade, M., & Roy, M.-A. (2010). Familiarity and recollection processes in patients with recent-onset schizophrenia and their unaffected parents. Psychiatry Research, 175(1/2), 15–21. doi:10.1016/j.psychres.2009.01.007
Libby, L. A., Yonelinas, A. P., Ranganath, C., & Ragland, J. D. (2013). Recollection and familiarity in schizophrenia: A quantitative review. Biological Psychiatry, 73, 944–950. doi:10.1016/j.biopsych.2012.10.027
Maddock, R., Garrett, A., & Buonocore, M. (2001). Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience, 104(3), 667–676. doi:10.1016/S0306-4522(01)00108-7
Maddock, R. J., Garrett, A. S., & Buonocore, M. H. (2003). Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping, 18(1), 30–41. doi:10.1002/hbm.10075
Martínez-Selva, J. M., Sánchez-Navarro, J. P., Bechara, A., & Román, F. (2006). Brain mechanisms involved in decision-making. Revista de Neurologia, 42(7), 411–418.
Mayes, A., Montaldi, D., & Migo, E. (2007). Associative memory and the medial temporal lobes. Trends in Cognitive Sciences, 11(3), 126–135. doi:10.1016/j.tics.2006.12.003
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5/6), 655–667. doi:10.1007/s00429-010-0262-0
Migo, E. M., Mayes, A. R., & Montaldi, D. (2012). Measuring recollection and familiarity: Improving the remember/know procedure. Consciousness and Cognition, 21(3), 1435–1455. doi:10.1016/j.concog.2012.04.014
Montaldi, D., & Mayes, A. R. (2010). The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus, 20(11), 1291–1314. doi:10.1002/hipo.20853
Montaldi, D., Spencer, T. J., Roberts, N., & Mayes, A. R. (2006). The neural system that mediates familiarity memory. Hippocampus, 16(5), 504–520. doi:10.1002/hipo.20178
Müller, V. I., Cieslik, E. C., Laird, A. R., Fox, P. T., & Eickhoff, S. B. (2013). Dysregulated left inferior parietal activity in schizophrenia and depression: Functional connectivity and characterization. Frontiers in Human Neuroscience, 7, 268. doi:10.3389/fnhum.2013.00268
Nelson, S. M., McDermott, K. B., Wig, G. S., Schlaggar, B. L., & Petersen, S. E. (2013). The critical roles of localization and physiology for understanding parietal contributions to memory retrieval. The Neuroscientist, 19(6), 578–591. doi:10.1177/1073858413492389
Parks, C. M., Murray, L. J., Elfman, K., & Yonelinas, A. P. (2011). Variations in recollection: The effects of complexity on source recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition, 37(4), 861–873. doi:10.1037/a0022798
Phelps, E. A. (2004). Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology, 14(2), 198–202. doi:10.1016/j.conb.2004.03.015
Pourtois, G., Schettino, A., & Vuilleumier, P. (2013). Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biological Psychology, 92(3), 492–512. doi:10.1016/j.biopsycho.2012.02.007
Qin, P., & Northoff, G. (2011). How is our self related to midline regions and the default-mode network? NeuroImage, 57(3), 1221–1233. doi:10.1016/j.neuroimage.2011.05.028
Qin, P., Liu, Y., Shi, J., Wang, Y., Duncan, N., Gong, Q., . . . Northoff, G. (2012). Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: A combined fMRI-meta-analytic study. Human Brain Mapping, 33(1), 154-164. doi:10.1002/hbm.21201
Qin, X. A., Koutstaal, W., & Engel, S. A. (2014). The hard-won benefits of familiarity in visual search: Naturally familiar brand logos are found faster. Attention, Perception, & Psychophysics, 76(4), 914–930. doi:10.3758/s13414-014-0623-5
Ragland, J. D., Blumenfeld, R. S., Ramsay, I. S., Yonelinas, A., Yoon, J., Solomon, M., . . . Ranganath, C. (2012). Neural correlates of relational and item-specific encoding during working and long-term memory in schizophrenia. NeuroImage, 59(2), 1719-1726. doi:10.1016/j.neuroimage.2011.08.055
Ramon, M., Dricot, L., & Rossion, B. (2010). Personally familiar faces are perceived categorically in face-selective regions other than the fusiform face area. The European Journal of Neuroscience, 32(9), 1587–1598. doi:10.1111/j.1460-9568.2010.07405.x
Ramon, M., Caharel, S., & Rossion, B. (2011). The speed of recognition of personally familiar faces. Perception, 40(4), 437–449.
Ranganath, C., & Ritchey, M. (2012). Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience, 13(10), 713–726. doi:10.1038/nrn3338
Ranganath, C., Yonelinas, A. P., Cohen, M. X., Dy, C. J., Tom, S. M., & D’Esposito, M. (2004). Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia, 42(1), 2–13. doi:10.1016/j.neuropsychologia.2003.07.006
Richter-Levin, G., & Akirav, I. (2000). Amygdala-hippocampus dynamic interaction in relation to memory. Molecular Neurobiology, 22(1/3), 11–20. doi:10.1385/MN:22:1-3:011
Ridderinkhof, K. R., Van den Wildenberg, W. P. M., Segalowitz, S. J., & Carter, C. S. (2004). Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition, 56(2), 129–140. doi:10.1016/j.bandc.2004.09.016
Rossion, B., Schiltz, C., Robaye, L., Pirenne, D., & Crommelinck, M. (2001). How does the brain discriminate familiar and unfamiliar faces?: A PET study of face categorical perception. Journal of Cognitive Neuroscience, 13(7), 1019–1034. doi:10.1162/089892901753165917
Rugg, M. D., Fletcher, P. C., Frith, C. D., Frackowiak, R. S., & Dolan, R. J. (1996). Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain: A Journal of Neurology, 119(Pt. 6), 2073–2083.
Ryals, A. J., Cleary, A. M., & Seger, C. A. (2013). Recall versus familiarity when recall fails for words and scenes: The differential roles of the hippocampus, perirhinal cortex, and category-specific cortical regions. Brain Research, 1492, 72–91. doi:10.1016/j.brainres.2012.10.068
Sabatinelli, D., Fortune, E. E., Li, Q., Siddiqui, A., Krafft, C., Oliver, W. T., . . . Jeffries, J. (2011). Emotional perception: Meta-analyses of face and natural scene processing. NeuroImage, 54(3), 2524-2533. doi:10.1016/j.neuroimage.2010.10.011
Singer, T., Critchley, H. D., & Preuschoff, K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences, 13(8), 334–340. doi:10.1016/j.tics.2009.05.001
Skinner, E. I., & Fernandes, M. A. (2007). Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia, 45(10), 2163–2179. doi:10.1016/j.neuropsychologia.2007.03.007
Song, Z., Jeneson, A., & Squire, L. R. (2011). Medial temporal lobe function and recognition memory: A novel approach to separating the contribution of recollection and familiarity. The Journal of Neuroscience, 31(44), 16026–16032. doi:10.1523/JNEUROSCI.3012-11.2011
Spaniol, J., Davidson, P. S., Kim, A. S., Han, H., Moscovitch, M., & Grady, C. L. (2009). Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia, 47(8/9), 1765–1779. doi:10.1016/j.neuropsychologia
Squire, L. R., Wixted, J. T., & Clark, R. E. (2007). Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews: Neuroscience, 8(11), 872–883. doi:10.1038/nrn2154
Staresina, B. P., Fell, J., Lam, A. T. A. D., Axmacher, N., & Henson, R. N. (2012). Memory signals are temporally dissociated in and across human hippocampus and perirhinal cortex. Nature Neuroscience, 15(8), 1167–1173. doi:10.1038/nn.3154
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain—3-dimensional proportional system: An approach to cerebral imaging. Stuttgart: Thieme.
Tarr, M. J., & Gauthier, I. (2000). FFA: A flexible fusiform area for subordinate-level visual processing automatized by expertise. Nature Neuroscience, 3(8), 764–769. doi:10.1038/77666
Trinkler, I., King, J. A., Doeller, C. F., Rugg, M. D., & Burgess, N. (2009). Neural bases of autobiographical support for episodic recollection of faces. Hippocampus, 19(8), 718–730. doi:10.1002/hipo.20556
Turkeltaub, P. E., Eden, G. F., Jones, K. M., & Zeffiro, T. A. (2002). Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. NeuroImage, 16(3, Pt. 1), 765–780.
Turkeltaub, P. E., Eickhoff, S. B., Laird, A. R., Fox, M., Wiener, M., & Fox, P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping, 33(1), 1–13. doi:10.1002/hbm.21186
Vilberg, K. L., & Rugg, M. D. (2008). Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia, 46(7), 1787–1799. doi:10.1016/j.neuropsychologia.2008.01.004
Waidergoren, S., Segalowicz, J., & Gilboa, A. (2012). Semantic memory recognition is supported by intrinsic recollection-like processes: “The butcher on the bus” revisited. Neuropsychologia, 50(14), 3573–3587. doi:10.1016/j.neuropsychologia.2012.09.040
Wais, P. E. (2008). fMRI signals associated with memory strength in the medial temporal lobes: A meta-analysis. Neuropsychologia, 46(14), 3185–3196. doi:10.1016/j.neuropsychologia.2008.08.025
Walton, M. E., & Mars, R. B. (2007). Probing human and monkey anterior cingulate cortex in variable environments. Cognitive, Affective, & Behavioral Neuroscience, 7(4), 413.
Wheeler, M. E., & Buckner, R. L. (2003). Functional dissociation among components of remembering: Control, perceived oldness, and content. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(9), 3869–3880.
Wheeler, M. E., & Buckner, R. L. (2004). Functional-anatomic correlates of remembering and knowing. NeuroImage, 21(4), 1337–1349. doi:10.1016/j.neuroimage.2003.11.001
White, T., Cullen, K., Rohrer, L. M., Karatekin, C., Luciana, M., Schmidt, M., . . . Lim, K. O. (2008). Limbic structures and networks in children and adolescents with schizophrenia. Schizophrenia Bulletin, 34(1), 18-29. doi:10.1093/schbul/sbm110
Wixted, J. T., & Squire, L. R. (2011). The medial temporal lobe and the attributes of memory. Trends in Cognitive Sciences, 15(5), 210–217. doi:10.1016/j.tics.2011.03.005
Yonelinas, A. P. (1994). Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition, 20(6), 1341–1354.
Yonelinas, A. P. (2001). Components of episodic memory: The contribution of recollection and familiarity. Philosophical Transactions of the Royal Society, B: Biological Sciences, 356(1413), 1363–1374. doi:10.1098/rstb.2001.0939
Yonelinas, A. P. (2002). The nature of recollection and familiarity: A review of 30 years of research, 46, 441-517.
Yonelinas, A. P., Otten, L. J., Shaw, K. N., & Rugg, M. D. (2005). Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience, 25(11), 3002–3008. doi:10.1523/JNEUROSCI.5295-04.2005
Yonelinas, A. P., Aly, M., Wang, W.-C., & Koen, J. D. (2010). Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus, 20(11), 1178–1194. doi:10.1002/hipo.20864
Yovel, G., & Paller, K. A. (2004). The neural basis of the butcher-on-the-bus phenomenon: When a face seems familiar but is not remembered. NeuroImage, 21(2), 789–800. doi:10.1016/j.neuroimage.2003.09.034
Acknowledgments
Fabien D’Hondt was funded by an FSR incoming post-doc fellowship from the Université Catholique de Louvain, Belgium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary File 1
Significant activation likelihood estimates for the contrast specific familiar > unfamiliar [(SF) > (UF)] for face stimuli. (DOC 245 kb)
Supplementary File 2
Distribution of the foci (for the three contrasts) prior to the ALE analysis. Representative slices in the axial plane. (GIF 144 kb)
Rights and permissions
About this article
Cite this article
Horn, M., Jardri, R., D’Hondt, F. et al. The multiple neural networks of familiarity: A meta-analysis of functional imaging studies. Cogn Affect Behav Neurosci 16, 176–190 (2016). https://doi.org/10.3758/s13415-015-0392-1
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-015-0392-1