Abstract
Research has indicated that highly trait-anxious (HTA) individuals exhibit a specific deficit in filtering threat-related distractors from visual–spatial working memory (WM). Prior demonstrations of impaired inhibition control in HTA individuals have mainly focused on tasks that required the inhibition of prepotent response tendencies. Studies on the suppression of emotionally neutral distractors from WM in trait anxiety have also been minimal. In this article, we present a study on the manifestation of general inefficient filtering of neutral distractors during visual–spatial WM maintenance stages in HTA individuals. Female participants performed a visual–spatial WM task while event-related potentials were recorded. They were made to remember the orientations of red rectangles within half of the screen and to ignore all salient green rectangles. As predicted, no significant main effect of group and no interaction between group and condition were found in the N2pc component, suggesting that group differences did not manifest in the initial process of object individuation. During the subsequent WM maintenance phase, HTA individuals were highly inefficient at filtering the irrelevant items from WM, as reflected not only by parallel late contralateral delay activity (CDA; 450 to 900 ms) amplitudes for the distractor condition and the four red items, but also by a smaller filtering efficiency score in the HTA group than in the low-trait-anxiety group. Extending previous studies, our findings verify a general filtering impairment in HTA individuals for task-irrelevant salient distractors during a WM maintenance phase.
Similar content being viewed by others
Trait anxiety has long been associated with a preferential biasing of attentional resources toward threatening information sources (e.g., Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Bishop, 2007; Cisler & Koster, 2010). Such negative attentional bias in anxious individuals may result in the unnecessary entry of threat-related information into their working memory (WM), promoting their worry, intrusive thoughts, and other anxiety-related cognitions that interfere with ongoing behavior (Thiruchselvam, Hajcak, & Gross, 2012). However, the mechanism of how these individuals process task-irrelevant negative information after it has captured their attention and entered WM remains unclear. WM is a limited-capacity system in which information is temporarily retained and manipulated in the mind to guide subsequent behavior (Baddeley, 2012). Given that the ability to filter task-irrelevant negative information from WM or to shift attention from an arousing portion of a negative-image representation within WM is vital for emotion regulation (Levens & Gotlib, 2010; Thiruchselvam et al., 2012), less-efficient regulation of irrelevant negative content during WM representation may underlie the development and maintenance of anxiety and anxiety disorders.
Stout, Shackman, and Larson (2013) have shown that threat-related distractors (fearful faces) are difficult to filter from visual–spatial WM, a difficulty that is exacerbated among high-trait-anxious (HTA) individuals because they allocate excessive WM storage to threat, even if it is irrelevant to the task. This study provided evidence of a specific deficit to filtering threat-related distractors from WM in trait anxiety. However, the question is whether this filtering deficit in WM is more general to cognitively challenging situations—no matter what types of information have to be filtered.
Trait anxiety has long been associated with impaired inhibition of distractors when internal or external threatening stimuli are present (Cisler & Koster, 2010). However, researchers have recently reported that this impairment in trait anxiety can be observed even in the absence of threat (e.g., Ansari & Derakshan, 2011a, 2011b; Berggren & Derakshan, 2013; Bishop, 2009). Furthermore, such cognitive impairment in trait anxiety has mainly been seen in selective attention tasks, but not in WM tasks. A relatively recent and exciting observation by Moriya and Sugiura (2012) has provided behavioral evidence that impaired filtering of neutral distractors in WM is predicted by trait social anxiety. Since behavioral measures alone may prove insufficient to evaluate the influence of trait anxiety on filtering neutral distractors from WM, it would be useful to use electroencephalographic (EEG) measures to investigate the cognitive mechanism underlying this issue.
According to the attentional control theory (ACT; Berggren & Derakshan, 2013; Eysenck & Derakshan, 2011; Eysenck, Derakshan, Santos, & Calvo, 2007), trait anxiety interferes with the inhibition process of WM, causing less-efficient inhibition of task-irrelevant neutral information. Furthermore, this deficit in inhibition control mainly impairs processing efficiency (i.e., the manner in which more cognitive resources are utilized to achieve the desired performance outcome) and does not adversely influence performance effectiveness (i.e., the ability to perform the task). In many of the behavioral studies testing these specific premises of ACT, reaction time (RT) has been regarded as an index of processing efficiency and task accuracy as the measure of effectiveness (e.g., Ansari, Derakshan, & Richards, 2008). However, such indirect measures of performance efficiency/effectiveness have been criticized, as both RTs and accuracy measure the outcome of processing rather than processing per se (Basten, Stelzel, & Fiebach, 2012; Eysenck & Derakshan, 2011). Furthermore, several studies have shown no effects of trait anxiety on behavioral performance, but significant effects of trait anxiety on neuroimaging or EEG measures, suggesting that trait anxiety influences various neural processes (e.g., Ansari & Derakshan, 2011a; Basten et al., 2012; Eysenck & Derakshan, 2011; Osinsky, Alexander, Gebhardt, & Hennig, 2010). Thus, the online recording of brain activity during task processing is necessary to directly measure the processing efficiency of HTA individuals. Using EEG measures, in the present study we investigated the effects of trait anxiety on the neural filtering efficiency (FE) of cognitive processing in WM in the absence of threat-related stimuli.
Another factor prompted this study. Previously, observations of impaired inhibitory control in HTA individuals primarily focused on tasks that emphasized competition between low-potency correct responses and high-potency incorrect responses, such as the flanker task (Bishop, 2009; Pacheco-Unguetti, Acosta, Callejas, & Lupiáñez, 2010), Stroop task (Basten et al., 2011; Osinsky et al., 2010; Osinsky, Gebhardt, Alexander, & Hennig, 2012), and antisaccade task (Ansari & Derakshan, 2011a, 2011b). However, the inhibitory control of the central executive functions of WM involves at least two components: prepotent response inhibition and suppression of irrelevant information from WM (Friedman & Miyake, 2004). Inefficient suppression of task-unrelated fearful faces from working memory has been shown in anxious individuals, and this effect is not evident for task-irrelevant neutral faces (Stout et al., 2013). Research on the suppression of emotionally neutral distractors from WM in trait anxiety has been limited. The present study further tested whether inhibition control in trait anxiety is impaired during WM maintenance when salient neutral distractors compete with less-salient task-relevant stimuli. In this way, we could determine whether inhibition dysfunction in trait anxiety not only influences output-related responses, but also input-related stimulus processes in a WM task with neutral distractors.
Recent neuroimaging research has suggested that trait anxiety interferes with the recruitment of prefrontal mechanisms required for attentional control of task-irrelevant information, even in the absence of threat. Bishop (2009) has shown that trait anxiety is associated with weaker activation of the left dorsolateral prefrontal cortex (DLPFC) in a response-conflict task under low perceptual load. It was concluded that trait anxiety is associated with impoverished recruitment of prefrontal attentional control mechanisms to inhibit distractor processing, even when threat-related stimuli are absent. However, trait anxiety is also associated with increased activation of the right DLPFC for incongruent relative to congruent trials in a Stroop task (Basten, Stelzel, & Fiebach, 2011) and for the manipulation of neutral verbal information held in WM (Basten et al., 2012). All of this evidence together suggests that the DLPFC plays a vital role in attentional control deficits in trait anxiety, though the neural correlates of this impairment are still under debate.
On the basis of recently gained insights on individual differences in visual WM capacity, we used event-related potentials (ERPs) to directly test whether HTA individuals have difficulty filtering task-irrelevant unemotional distractors from WM. Vogel and colleagues (McCollough, Machizawa, & Vogel, 2007; Vogel & Machizawa, 2004) described an ERP component, the contralateral delay activity (CDA), which is a sustained negative voltage at posterior electrodes during the maintenance of WM. The amplitude of the CDA increases with the number of representations held in visual WM and reaches an asymptote at an individual’s WM capacity (Gao, Yin, Xu, Shui, & Shen, 2011; McCollough et al., 2007; Vogel & Machizawa, 2004). The intraparietal sulcus is a potential neural generator of the CDA, as it is strongly modulated by the number of items held in memory and is sensitive to individual differences in WM capacity (Todd & Marois, 2004, 2005; Xu & Chun, 2006). Furthermore, recent work has also suggested that the CDA amplitude is modulated by top-down attentional control (Kuo, Stokes, & Nobre, 2012; Machizawa, Goh, & Driver, 2012; Reinhart et al., 2012; Sander, Werkle-Bergner, & Lindenberger, 2011).
In Vogel, McCollough, and Machizawa (2005), participants were required to compare the orientations of red rectangles, sometimes in the presence of task-irrelevant distractors (blue rectangles). In people with high WM capacity, the CDA amplitudes for remembering two red items along with two blue distractors were equivalent to those for remembering two red items only, indicating that these people were perfectly efficient at representing only the red items and excluding the irrelevant items from memory. In people with low WM capacity, however, the CDA amplitudes for remembering two red items along with distractors were equivalent to those for remembering four red items alone, indicating that these people were inefficient at excluding the irrelevant items from memory. Thus, in this paradigm, the CDA can be used as a neurophysiological marker of the numbers of relevant and irrelevant items held in visual WM during the retention interval of the task.
The main objective of this study was to investigate whether trait anxiety is associated with the inefficient filtering of emotionally neutral distractors from WM during the encoding and subsequent maintenance stages. The ERPs were recorded during a lateralized change detection task, in which participants were cued to selectively remember the orientations of red items within half of the screen, while ignoring all salient green items (Vogel et al., 2005). We focused on the ERPs elicited by the memory array that indexed the attentional selection of the stimuli (the N2pc component; Eimer, 1996; Luck & Hillyard, 1994; Mazza, Turatto, & Caramazza, 2009) and the maintenance of WM contents (the CDA component; Vogel & Machizawa, 2004). The N2pc component reflects the focusing of covert attention on a potential target item and the filtering of the surrounding distractor items in a visual search task (Eimer, 1996; Luck & Hillyard, 1994; Mazza et al., 2009). In the enumeration task, the N2pc is often perceived to reflect a process of object individuation or, more precisely, the attentional mechanism that forms temporal representations of distinct objects from multistimulus arrays (Ester, Drew, Klee, Vogel, & Awh, 2012; Mazza & Caramazza, 2011; Pagano & Mazza, 2012).
Impaired inhibition control in HTA individuals is primarily reflected by processing efficiency rather than by performance effectiveness (Berggren & Derakshan, 2013; Eysenck & Derakshan, 2011; Eysenck et al., 2007). Thus, group differences in FE and WM capacity may not become evident in the behavioral data, but may emerge primarily in the ERP data. First, given that trait anxiety mainly influences attentional control (Bishop, 2009; Osinsky et al., 2010), we expected that nonsignificant group differences during the initial process of object individuation would be obtained, because the early individuation process is immune from attentional control (Pagano & Mazza, 2013), as evidenced by the lack of group differences in N2pc amplitudes. Second, on the basis of the assumption of the ACT that trait anxiety interferes with the inhibition process of WM (Berggren & Derakshan, 2013; Eysenck & Derakshan, 2011; Eysenck et al., 2007), we expected that HTA individuals would exhibit inefficient filtering of irrelevant information, as compared to low-trait-anxious (LTA) individuals. Specifically, in HTA individuals, the amplitude of the CDA for remembering two red items along with two green distractors would be similar to that for remembering four red items. In contrast, in LTA individuals, the amplitude of the CDA for remembering two red items along with two green distractors would be similar to that for remembering two red items. Third, we predicted that relative to LTA individuals, HTA individuals would be associated with reduced visual WM capacity for relevant information in the target-only conditions, as mainly reflected by their smaller CDA amplitudes.
Method
Participants
Initially, 1,634 undergraduate students from Southwest University participated in a mass screening using the Chinese version of trait anxiety portion of the Spielberger State–Trait Anxiety Inventory (TAI; Shek, 1993; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). Subsequently, participants who scored high in trait anxiety (HTA group; upper 27th percentile of the distribution) and had low levels of trait anxiety (LTA group; lower 27th percentile of the distribution) were selected for further consideration. From these groups, we invited 20 healthy female HTA participants (mean age of 20.3 years) and 19 female LTA participants (mean age of 20.6 years). We chose to invite female participants only in order to control for possible gender effects. Because most classes in Southwest University are dominated by female students, not enough male participants in the mass screening could have been recruited for a balanced gender distribution in our sample. Two participants (one HTA participant) were excluded due to excessive rates of ocular artifacts. All of the participants were tested within two weeks of their first assessment. During the test, each participant provided demographic information (Table 1) and was assessed with the TAI and the Chinese version of the Beck Depression Inventory (BDI-II; Beck, Steer, Ball, & Ranieri, 1996). An independent-samples t test revealed that the two groups differed significantly in trait anxiety scores in both a first, t(35) = 17.92, p < .001, and a second, t(35) = 8.99, p < .001, administration of the test. A significant group difference in BDI scores was measured, t(35) = 3.91, p < .001, showing that the HTA group (M = 15.84) had larger BDI scores than did the LTA group (M = 6.89). All of the participants denied regular use of medication or other nonmedical substances that could potentially affect the central nervous system. According to their telephone interviews, all of the participants were right-handed with normal or corrected-to-normal vision, as well as no history of psychiatric or neurological diseases. The participants were each paid 40 yuan (about $6.37).
Stimuli and procedure
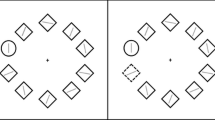
In an electromagnetically shielded room, participants were seated comfortably about 80 cm from a 19-in. screen. They performed a lateralized change detection task, adapted from Vogel et al. (2005), in which they were cued to remember the information on one side of the display and ignore the information on the other side. To isolate the CDA from other task-general brain activity, the CDA is measured as the difference in mean amplitudes between activity in the hemispheres contralateral and ipsilateral to the to-be-remembered information. Thus, bilateral displays are crucial for isolating the CDA. In each trial (Fig. 1a), the participants were presented with a brief bilateral array of colored rectangles (each 0.41º × 1.42º) of varying orientations (vertical, horizontal, left 45º, and right 45º). The two stimulus arrays were presented within 4º × 7.6º rectangular regions that were presented 2.8º to the left and right of a central fixation cross on a gray background. The stimulus positions were randomized, with the constraint that the distance between rectangles within a hemifield was at least 2º. The numbers of targets and distractors were always the same in both hemifields; only the location and color of the stimuli could differ between hemifields. The task was to remember the orientations only of the red items (RGB: 200, 0, 0) and to ignore the green ones (RGB: 25, 255, 52) in either the left or the right hemifield. The RGB values of the colors were converted to LCh values (red, LCh = 42/87/40; green, LCh = 88/113/138). Green distractors were more physically salient relative to the red items, as the luminance of green was higher than that of red.
Stimulus sequence and experimental conditions. a Example of the distractor condition in a change trial in which the orientations of the red rectangles (white) in the right hemifield are to be remembered (the distractors are black). b Three task conditions in which amplitudes of the contralateral delay activity (CDA) event-related potential were compared, so as to investigate filtering performance
Each trial began with a 200-ms arrow presented above a fixation cross. The arrow cued participants to remember the orientations of only the red items in either the left or the right side of the memory array. Following a variable interval of 200 to 400 ms, a memory array was presented for 100 ms. The memory array was removed from the display for 900 ms (delay/retention period). The test array was then displayed for a maximum of 5,000 ms. Participants responded by pressing one of two vertically aligned keys to indicate whether or not a change was present. On one half of the trials, the memory and test arrays were identical, whereas on the other half, the orientation of a single red rectangle within the to-be-remembered side of the memory array was different from its orientation in the test array. Key allocations were counterbalanced between the participants. The instructions emphasized accuracy rather than speed. Moreover, participants were also instructed to keep their eyes fixated throughout the task. The intertrial interval was 2,000 ms.
The three conditions differed in their numbers of targets (red rectangles) and distractors (green rectangles) (Fig. 1b). In the two-red-items condition, only two red items were shown on each side of the display. In the four-red-items condition, only four red items were shown on each side of the display. In the two red items plus two green items condition (hereafter called the distractor condition), two red items along with two green distractors were shown on each side of the display. These three conditions were presented in random order in each block. Ten blocks were presented, and each block included 60 trials. Overall, participants experienced 200 trials for each type of memory array (i.e., two red items, four red items, and two red items with two distractors). At the beginning of the session, the participants completed a practice block of 18 trials (six per condition). They were given a short break after each block.
EEG recording and processing
Brain electrical activity was recorded at 64 scalp sites using tin electrodes mounted on an elastic cap (Brain Product, München, Germany), with references on the left and right mastoids, and a ground electrode on the medial frontal aspect. Vertical electrooculograms (EOGs) were recorded supra- and infraorbitally at the left eye. The horizontal EOG was recorded from the left versus right orbital rim. The EEG and EOG were amplified using a 0.05- to 100-Hz bandpass and were continuously digitized at 500 Hz/channel. All interelectrode impedances were maintained below 5 kΩ. Offline, the data were referenced to the average of the left and right mastoids (average mastoid reference), and these signals were bandpass-filtered offline using a phase shift-free Butterworth filters with half-power cutoffs at 0.1 and 30 Hz and a roll-off of 12 dB/octave. Trials containing saccades (horizontal EOG exceeding ±25 μV), blinks (Fpz exceeding ±60 μV, vertical EOG exceeding ±75 μV), or muscle artifacts (all other electrodes exceeding ±75 μV) were removed from further analyses (as in Kiss, Grubert, Petersen, & Eimer, 2012). In addition, we computed the averaged horizontal-EOG signals on left-cue trials versus right-cue trials in order to assess the degree of residual eye movement activity. Two participants were excluded because their residual horizontal EOGs exceeded ±3 μV in at least one trial condition (Woodman & Luck, 2003). Finally, to exclude the possibility that residual horizontal-EOG activity that was not removed by the correction procedure had influenced the N2pc or CDA condition effects, we performed an extra check (Spronk, Vogel, & Jonkman, 2012): We computed correlations between the condition effects at horizontal-EOG and parietal–occipital electrodes (P3/4, P5/6, P7/8, PO3/4, and PO7/8) in the time window of 220 to 900 ms. These correlations were all nonsignificant [two red items, r(37) = −.26, p = .22; distractor condition, r(37) = −.22, p = .28; four red items, r(37) = −.25, p = .23], confirming that our N2pc or CDA results cannot be explained by ocular artifacts. The percentages of trials excluded from the averaging were 14.5 % for the LTA group and 16.2 % for the HTA group because of artifact rejection. Nonsignificant group differences emerged in the percentages of trials excluded from averaging, t(35) = 0.62, p = .54. Only trials with correct responses were analyzed.
Measures and analyses
Our primary behavioral measure was K, an estimate of the number of objects held in WM (Cowan, 2001; Pashler, 1988). Pashler’s formula was used because the task in this study used whole-display probes (Rouder, Morey, Morey, & Cowan, 2011). Specifically, K = N × (HR – FA)/(1 – FA), where K is WM capacity; N is the number of to-be-remembered items; HR is the hit rate, or the proportion of correct responses when a change is present; and FA is the false alarm rate, or the proportion of incorrect responses on no-change trials. Pashler’s K was used because it was developed for WM tasks using whole-display probes, whereas the more commonly used Cowan’s K was developed for single-probe displays (Rouder et al., 2011). We also computed K in the distractor condition by filling in 2 for N, because of the two target items (Lee et al., 2010). K scores were entered into a 2 × 3 mixed analysis of variance (ANOVA), with Group (HTA and LTA) as the between-subjects factor and Condition (two-red-items, four-red-items, and distractor conditions) as the within-subjects factor. Also an “unnecessary storage” measure was calculated by subtracting Pashler’s K for the distractor condition from the K for two red items, which could be used as behavioral filtering cost scores to assess behavioral performance (Lee et al., 2010; Spronk et al., 2012). In addition, the behavioral measures derived from the task in the three conditions were (1) Cowan’s K scores, K = N × (HR – FA); (2) reaction times (RTs) for correct detections; and (3) response accuracy. A mixed ANOVA was also conducted for these measures. Cowan’s K scores were reported only to allow comparisons to other studies that had used them.
The analysis of the underlying neural mechanisms of encoding and maintenance focused on lateralized ERP components elicited by the memory array. The averaged epoch for the ERPs was 1,400 ms, including 200 ms pre-memory-array and 1,200 ms post-memory-array onset. Separate averages were computed for each participant in each of the three different conditions and for contralaterality (electrode contralateral vs. ipsilateral to the location of memory arrays). Contralateral waveforms were calculated as the average of the left-sided electrodes to the right-sided items and of the right-sided electrodes to the left-sided items. Ipsilateral waveforms were calculated as the average of the left-sided electrodes to the left-sided objects and of the right-sided electrodes to the right-sided objects. Figure 2 depicts the contralateral and ipsilateral waveforms collapsed across posterior electrode sites for each group in each condition. The lateralized ERP components were then computed as the difference in mean amplitudes between the activity in hemispheres contralateral and ipsilateral to the memory array during the retention period. On the basis of previous work (Stormer, Li, Heekeren, & Lindenberger, 2013; Vogel et al., 2005), mean activity from five pairs of lateral posterior electrode sites (P3/4, P5/6, P7/8, PO3/4, and PO7/8) were used to calculate the lateralized ERP components. Figure 3 depicts the contralateral-minus-ipsilateral ERP waveforms for the three conditions and the two groups separately at posterior electrode pairs. With regard to the ERPs, we observed an early rising lateralized ERP component in the time windows of 300 to 450 ms, whose fluctuation was different from the subsequent CDA. Because of the temporal–spatial overlap between this lateralized ERP component and the subsequent CDA, we referred to it as the early CDA. Thus, we measured both an early CDA (300 to 450 ms) and a late CDA (450 to 900 ms). Note that the time window of early CDA was identified on the basis of the data and we presented it as an exploratory analysis. On the basis of previous studies (Lee et al., 2010; Leonard et al., 2013; Stormer et al., 2013) and our data, three measurement windows were selected: 220 to 300 ms (N2pc component), 300 to 450 ms (early-CDA component), and 450 to 900 ms (late-CDA component) after the onset of the memory array. The resulting mean amplitudes were entered into a 2 × 3 mixed ANOVA, with group (HTA and LTA) as the between-subjects factor and Condition (two red items, four red items, and the distractor condition) as the within-subjects factor. Appropriate Greenhouse–Geisser adjustments to the degrees of freedom were performed, and Bonferroni corrections were used for each comparison.
Although the CDA is a measure of working memory storage per se, it can be used as a neurophysiological marker to measure filtering efficiency. The FE analysis of each group used the formula of Vogel et al. (2005)—that is, FE = (F – D)/(F – T), where F is the late-CDA amplitude for four red items, D is the late-CDA amplitude in the distractor condition, and T is the late-CDA amplitude for two red items. The formula provides a quantitative measure of each participant’s ability to efficiently filter irrelevant information, and the scores ranged from 1 (efficient: identical to two items) to 0 (inefficient: identical to four items). An independent-samples t test (two-tailed) was used to test whether the HTA group has a lower FE than the LTA group.
Results
Behavioral results
Figure 4a shows Pashler’s K scores as a function of condition. A repeated measures ANOVA showed no significant main effect of group or interaction for K scores, F(1, 35) = 0.35, p = .558, η p 2 = .01, and F(2, 70) = 1.49, p = .23, η p 2 = .041, indicating that in each condition, both groups successfully retrieved a parallel number of objects from visual WM (see Table 2). However, a significant main effect of condition was observed, F(2, 70) = 244.1, p < .001, η p 2 = .875, indicating an increase in K scores as more objects were available to be remembered. For the four red items, the average capacity of HTA individuals was estimated at 2.8 items, whereas that of LTA individuals was estimated at 2.7 items. For Cowan’s K scores and response accuracy, similar effects were found. Details can be found in supplemental materials. In addition, an independent-samples t test revealed that the two groups did not differ significantly in behavioral filtering cost scores, t(35) = 0.42, p = .68, indicating that, at the behavioral level, HTA individuals have the same ability to filter distractors as LTA individuals do.
Behavioral data from the visual working memory (WM) change detection task: Bar graphs of a Pashler’s K, b Cowan’s K, c average reaction times (RTs, in milliseconds), and d accuracy for each group in the two-red-items, distractor, and four-red-items conditions. Error bars represent standard errors of the means
A repeated measures ANOVA also showed no significant main effect of the group or interaction for RTs (Fig. 4c), F(1, 35) = 0.67, p = .42, η p 2 = .019, and F(2, 70) = 0.22, p = .72, η p 2 = .006. However, a significant main effect of condition was observed, F(2, 70) = 56.37, p < .001, η p 2 = .721, indicating an increase in RTs as participants needed to make accurate memory decisions for more objects.
Lateralized ERP results
N2pc (220 to 300 ms)
In both groups, the N2pc amplitude was highest for the distractor condition, followed by four red items and then two red items (see Fig. 3). A repeated measures ANOVA yielded no significant main effect of group, F(1, 35) = 0.036, p = .85, η p 2 = .001, nor an interaction, F(2, 70) = 0.827, p = .44, η p 2 = .025, but a significant main effect of condition, F(2, 70) = 13.49, p < .001, η p 2 = .279, did emerge. Subsequent pairwise comparisons showed that N2pc amplitudes significantly increased from two red items (−0.86 ± 1.06) to four red items (p = .009) and to the distractor condition (p < .001). The N2pc was also larger for the distractor condition (−1.38 ± 1.32) than for four red items (−1.16 ± 1.21) (p = .032).
Late CDA (450 to 900 ms)
Figure 5b depicts the mean amplitude of late CDA as a function of condition for the HTA and LTA groups. For all groups, it appears that within the 450- to 900-ms time window, late-CDA amplitudes were highest for four red items, followed by the distractor condition, and then by two red items (see also Table 3). A repeated measures ANOVA yielded a significant main effect of condition, F(2, 70) = 57.41, p < .001, η p 2 = .621. The main effect of group was not significant, F(1, 35) = 3.14 , p = .085, η p 2 = .082. Importantly, the interaction of condition and group was significant, F(2, 70) = 3.36, p = .04, η p 2 = .088. One-way ANOVAs conducted within each group indicated that the CDA amplitudes for both the HTA group, F(2, 36) = 27.01, p < .001, η p 2 = .6, and the LTA group, F(2, 34) = 32.93, p < .001, η p 2 = .659, differed significantly across conditions. Subsequent pairwise comparisons showed that in the LTA group, the late-CDA amplitude of the distractor condition was significantly smaller than that for four red items (p < .001) and significantly larger than that for two red items (p = .01). This result indicated that LTA individuals did not completely exclude the distractors and stored at least some irrelevant information in visual WM. By contrast, the HTA group had an amplitude in the distractor condition that was significantly larger than that for two red items (p < .001), but not significantly different from that for four red items (p = .603). This result indicated that HTA individuals were highly inefficient at keeping the irrelevant items stored in memory. Post-hoc tests also showed a smaller late CDA for HTA individuals than for LTA individuals for four red items (p = .035), but not for two red items (p = .064) or the distractor condition (p = .386). These results indicated that HTA individuals exhibited reduced WM capacity when remembering four items was required.
Since depression has also been linked to modulation of the late-CDA amplitude (Owens, Koster, & Derakshan, 2012), we measured the possible effect of depression on late-CDA amplitudes. BDI scores were entered into an analysis of covariance (ANCOVA), similar to the ANOVA described above, as a covariate. The results showed no significant effect of BDI on late-CDA amplitudes, F(1, 34) = 0.024, p = .88, η p 2 = .001, and BDI did not significantly interact with condition, F(2, 68) = 0.98, p = .38, η p 2 = .028. Importantly, both the main effect of condition, F(2, 68) = 19.55, p < .001, η p 2 = .365, and the interaction of condition and group, F(2, 68) = 3.46, p = .038, η p 2 = .092, were significant. Therefore, individual differences in depression did not significantly influence the interaction between group and condition for late-CDA amplitudes.
Early CDA (300 to 450 ms)
Figure 5a depicts the mean amplitudes of the early CDA as a function of condition for the HTA and LTA groups. In both groups, the early CDA for four red items was larger than that for two red items. However, relative to the LTA group, the HTA group showed a larger early CDA for the distractor condition than for the four target items. A repeated measures ANOVA yielded a significant main effect of condition, F(2, 70) = 28.26, p < .001, η p 2 = .447, but the main effect of group was not significant, F(1, 35) = 0.254, p = .617, η p 2 = .007. Importantly, the interaction of condition and group was significant, F(2, 70) = 3.65, p = .032, η p 2 = .095. One-way ANOVAs conducted within each group indicated that early-CDA amplitudes for both the HTA group, F(2, 36) = 21.08, p < .001, η p 2 = .539, and the LTA group, F(2, 34) = 11.02, p < .001, η p 2 = .393, differed significantly across conditions. Subsequent pairwise comparisons showed that in the HTA group, the early-CDA amplitude increased significantly from two red items to four red items (p = .004) and to the distractor condition (p < .001). Furthermore, the early CDA for the distractor condition was larger than that for four red items (p = .008). In the LTA group, the early-CDA amplitude increased significantly from two red items to four red items (p = .001), and to the distractor condition (p = .001). However, the early CDA for the distractor condition did not differ significantly from that for four red items (p = .552). In sum, the early-CDA amplitude for the distractor condition was larger than that for four target items in the HTA group, whereas this difference was attenuated or disappeared in the LTA group.
In order to examine whether individual differences in depression moderated the effect of condition and its interaction with group on early-CDA amplitudes, we conducted an ANCOVA with Condition and Group as factors, by adding BDI score as a covariate. The results showed no significant effect of BDI on early-CDA amplitudes, F(1, 34) = 0.001, p = .97, η p 2 = .001, and BDI did not significantly interact with condition, F(2, 68) = 0.89, p = .42, η p 2 = .025. Importantly, both the main effect of condition, F(2, 68) = 12.38, p < .001, η p 2 = .267, and the interaction of condition and group, F(2, 68) = 3.31, p = .043, η p 2 = .089, were significant. Therefore, individual differences in depression did not significantly influence the interaction between group and condition on early-CDA amplitudes.
Filtering efficiency
FE was calculated using the formula FE = (F – D)/(F – T). The formula provides a quantitative measure of whether late-CDA amplitudes in the distractor condition are more similar to those for four red items or two red items. This quantitative measure can be used to assess whether a significant difference in FE exists between HTA and LTA groups. In the study, the FE scores ranged from .06 to .81. The LTA group had a mean FE score of .54 (SD = .23), and the HTA group had a mean FE score of .37 (SD = .23). An independent-samples t test (two-tailed) revealed that the two groups differed significantly in FE, t(35) = 2.11, p = .042, indicating that the HTA group was significantly inefficient at filtering irrelevant information from storage in visual WM, as compared to the LTA group. In addition, in order to measure the possible effect of depression on FE, we calculated the Pearson’s correlation between BDI scores and FE scores. The results revealed that the correlation between them did not reach significance (r = −.025, p = .883), indicating a unique influence of trait anxiety on FE.
Correlations between FE and behavior
Previous research has shown that WM capacity and FE scores are strongly correlated (Owens et al., 2012; Vogel et al., 2005). Figure 6 shows the correlation between each participant’s FE and WM capacity in each group. WM capacity was estimated by the K score for four red items. A significant correlation was found for the LTA group, Pearson’s r(18) = .67, p = .02, but not for the HTA group, r(19) = .21, p = .69. Furthermore, the correlations between FE and WM capacity were marginally significant between the LTA and HTA groups (z = 1.87, p = .06, two-tailed). Thus, the HTA group did not show the significant correlation pattern between K and FE shown in the LTA group—that low-capacity individuals show low FE scores, and that high-capacity individuals have much higher FE scores.
Discussion
In the present study, we used a well-validated neural measure of visual–spatial WM storage to investigate the extent to which anxiety is associated with impaired ability to prevent emotionally neutral distractors from entering WM. In the LTA group, the late-CDA amplitude of the distractor condition was smaller than that for four red items and was larger than that for two red items, suggesting that LTA individuals did not completely exclude the distractors and stored at least some irrelevant information in visual WM. More importantly, as expected, the HTA group had a late-CDA amplitude in the distractor condition that was significantly larger than that for two red items, but not significantly different from that for four red items. These results demonstrate that HTA individuals show inefficiency in filtering emotionally neutral distractors from WM. Therefore, the findings of the present study extend the recent literature investigating inefficient filtering of threat from WM in trait anxiety, and they underscore anxious individuals’ susceptibility to salient neutral distractors after they enter visual WM.
Attentional selection was quantified as the magnitude of the N2pc component, a well-established ERP component that reflects the focusing of attention onto multistimulus arrays. In our study, both HTA and LTA individuals showed clear N2pc components for the three conditions, and the amplitude of the N2pc for four red items alone was larger than that for two red items alone. Thus, our results were consistent with the proposition that the N2pc increased as a function of target numerosity, reflecting a process of object individuation (Ester et al., 2012; Mazza & Caramazza, 2011; Pagano & Mazza, 2012). Mazza and Caramazza also argued that the object individuation process reflected by the N2pc binds indexes to object properties and locations as needed for further cognitive operations. Participants needed to bind indexes to four locations and two colors in the distractor condition, while they were required to bind indexes to four locations and one color with four red items, so the distractor condition necessitated enhanced attentional focus relative to four red items. Accordingly, we observed a larger N2pc for the distractor condition than for the four red items. No significant main effect of group or interaction effect between group and condition was observed in the N2pc, suggesting that group differences do not manifest in the initial process of object individuation, but in the subsequent processes. These subsequent processes may involve the encoding of individuated objects in greater detail, operating on the WM contents, thus leading to the successful maintenance of task-relevant objects in visual WM.
Our results show an interaction between group and condition in the early CDA during the time interval of 300 to 450 ms. Specifically, the early-CDA amplitude for the distractor condition was larger than that for the four red items in HTA individuals, whereas in LTA individuals, its amplitude for the distractor condition was parallel to that for the four red items. In HTA individuals, the fact that the early-CDA amplitude was larger for the distractor condition (two red items plus two green items) than for the four red items is likely inconsistent with the fact that the traditional CDA amplitude is sensitive to the number of memory items. The early-CDA amplitude may be a measure of the amount of processing resources required to perform object identification after object individuation, in order to categorize objects in greater detail. In this sense, HTA individuals showed more use of processing resources for object identification for the distractor condition than for the four red items, as reflected by their larger early CDA for the distractor condition than for the four red items. By contrast, LTA individuals used equal amounts of processing resources for object identification in the distractor and four-red-items conditions, as reflected by their parallel early CDAs for both conditions. Given that efficiency is often measured by the amount of neural activity devoted to the task (Basten et al., 2012), our results demonstrate low processing efficiency in HTA individuals in the process of object identification when salient green distractors compete with less salient task-relevant red items. Further research would be necessary to determine whether this early-CDA effect was real, as the time window of the early CDA was identified on the basis of the data, and examination of the early CDA was exploratory in this study.
During the subsequent WM maintenance phase, HTA individuals were highly inefficient at filtering the irrelevant items from WM. HTA individuals showed parallel late-CDA amplitudes for remembering the two red items and two distractors and for remembering four red items alone, indicating that their increased WM storage for distractors was likely due to a deficit in filtering task-irrelevant neutral stimuli from WM. Thus, the late CDA can be used as a neural marker to measure FE, although CDA is a measure of working memory storage per se. Filtering impairment in HTA individuals was also quantified by the smaller FE score in the HTA group than in the LTA group. Our results imply that HTA individuals show impaired filtering of salient distractors and tend to allocate attentional resources to irrelevant information during the WM maintenance phase.
In addition, the HTA group did not show the significant correlation pattern between K and FE shown in the LTA group, that low-capacity individuals would show low FE scores and high-capacity individuals produce much higher efficiency scores. Given that K scores reflect the cumulative results of multiple processes, such as stimulus encoding, maintenance, and retrieval, the finding of the significant correlation between K and FE in the HTA group suggests that the WM capacity of the HTA group can derive from processing limitations during stimulus maintenance, as reflected by late-CDA amplitudes. In contrast, the finding of a nonsignificant correlation pattern in the LTA group suggests that WM capacity of the LTA group may not be definitively determined by processing limitations during stimulus maintenance, but likely derives instead from processing limitations during stimulus encoding or retrieval, as any constraints at early encoding stages would necessarily affect later maintenance or retrieval processes. These correlation results were not in line with the finding of Owens et al. (2012) of a significant positive correlation between K and FE for both a nondepression group and a depression group. This discrepancy may reflect a dissociating effect of subclinical anxiety and depression on the correlation between K and FE. Further research would be necessary to verify this effect by comparing an anxious group and a depression group in the same experimental design.
Relative to LTA individuals, HTA individuals were characterized by reduced filtering of irrelevant information and reduced WM capacity at the neural level. However, these findings did not emerge at the behavioral level. This dissociation appears to be consistent with the proposition of ACT that impaired inhibition control in anxiety is primarily reflected by impaired neural filtering efficiency (i.e., the manner in which cognitive resources are employed to achieve the desired performance outcome), rather than by performance effectiveness (i.e., the ability to perform tasks). In visual WM studies, the K score may be an index of one’s performance effectiveness because it reflects an individual’s ability to retrieve a set of objects from visual WM. This finding was also consistent with previous studies reporting dissociations between the CDA and K score (Leonard et al., 2013; Sander et al., 2011).
Although the intraparietal sulcus is a potential neural generator of the CDA in humans (Robitaille et al., 2009; Robitaille et al., 2010; Todd & Marois, 2004, 2005; Xu & Chun, 2006), studies focusing on patients with unilateral prefrontal cortex (PFC) lesions have shown that CDA load modulations are only observed in the ipsilesional, but not the contralesional, visual field; thus, the areas of PFC associated with top-down attentional control during visual WM maintenance have a significant function in the CDA-generating circuit (Voytek & Knight, 2010). Furthermore, Reinhart et al. (2012) demonstrated that the local-field potentials in the PFC and cognitive-control regions contribute to the generation of the CDA, indexing WM maintenance. Studies in healthy humans have also shown that the PFC exerts attentional control over access to working memory storage in the parietal cortex, and partly determines interindividual differences in WM capacity (Edin et al., 2009; McNab & Klingberg, 2008). According to these studies, the neuronal activity reflected in the CDA component is modulated by feedback processes from prefrontal regions. Given the poor spatial resolution of ERPs, no concrete conclusions on the subcortical source(s) of CDA may be inferred. However, the empirical evidence has demonstrated that the PFC contributes to the generation of the CDA component (Reinhart et al., 2012; Voytek & Knight, 2010) and to the filtering of irrelevant information in WM (Edin et al., 2009; McNab & Klingberg, 2008), and that the CDA amplitude is modulated by top-down attentional control (Kuo et al., 2012; Machizawa et al., 2012; Reinhart et al., 2012; Sander et al., 2011). Therefore, the reduced FE reflected by the later CDA in HTA individuals is likely due to the lesser activation of PFC involved in the top-down control of maintenance representations in visual WM. Further studies should examine this possibility directly in HTA individuals using fMRI.
On the one hand, our results provide electrophysiological evidence of a general impaired filtering of neutral distractors in HTA individuals. The previous study by Stout et al. (2013) showed that trait anxiety is linked to impairment in excluding threat-related distractors rather than emotionally neutral distractors from WM. Our findings of such a general filtering impairment were exhibited under high-attentional-demand conditions when physically salient green distractors competed with less salient, but task-relevant, red items. Attentional control processes might have been minimally challenged in the neutral distractor condition of Stout et al. because the competition between two neutral faces of equal salience was relatively weak. This explanation is consistent with the idea that the highly trait-anxious will exhibit impaired inhibition control depending on the levels of attentional demands placed on tasks (Berggren & Derakshan, 2013).
On the other hand, our findings demonstrate the generality of inhibition control failure in HTA individuals, not only in prepotent-response inhibition, but also in the suppression of salient distractors from WM. Our results extend prior observations of inhibition control failures at the stage of response selection (Ansari & Derakshan, 2011a, 2011b; Bishop, 2009; Pacheco-Unguetti et al., 2010) and add to previous indications that deficits in inhibitory control of input selection are present in HTA individuals. In other words, HTA individuals have difficulty inhibiting not only prepotent response tendencies, but also salient but task-irrelevant perceptual inputs in WM. The present results are analogous to the findings of Moser, Becker, and Moran (2012) that trait anxiety is related to increased attentional distraction by a salient, task-irrelevant color singleton in a visual search task. Our findings, together with those from a previous investigation (Moser et al., 2012), point to the imbalance between a stimulus-driven attentional system and a goal-driven attentional system in trait anxiety. Future studies should directly investigate the underlying neural mechanisms for the imbalance between these two different types of attentional selection in trait anxiety.
Several limitations of the present study should be noted. First, one limitation is that we confined our sample to female participants only. Even though this methodological approach controls for gender effects, it constrains the generalizability of the findings to males. Future studies should replicate the present findings in a male sample or in a sample with a balanced gender distribution. Second, although the present study did not reveal interactive effects of anxiety and depression in relation to FE, future research should enlarge the sample to examine this issue, because of the comorbidity between anxiety and depression. Third, our study did not consider the influence of other factors (personality traits, socioeconomic status, etc.) that can influence WM function; it will be important to control these factors in future research.
To summarize, this study is the first to directly measure the neural correlates of inefficient filtering of emotionally neutral distractors in HTA individuals during a visual–spatial WM maintenance phase. The results indicate that HTA individuals are associated with generally inefficient filtering of task-irrelevant information, as illustrated by the processes of object identification and maintenance in visual–spatial WM. Furthermore, the underlying neural mechanisms for the inefficient filtering may point to the lesser activation of areas of the PFC involved in the top-down control of maintenance representations in visual WM.
References
Ansari, T. L., & Derakshan, N. (2011a). The neural correlates of cognitive effort in anxiety: Effects on processing efficiency. Biological Psychology, 86, 337–348. doi:10.1016/j.biopsycho.2010.12.013
Ansari, T. L., & Derakshan, N. (2011b). The neural correlates of impaired inhibitory control in anxiety. Neuropsychologia, 49, 1146–1153. doi:10.1016/j.neuropsychologia.2011.01.019
Ansari, T. L., Derakshan, N., & Richards, A. (2008). Effects of anxiety on task switching: Evidence from the mixed antisaccade task. Cognitive, Affective, & Behavioral Neuroscience, 8, 229–238. doi:10.3758/CABN.8.3.229
Baddeley, A. (2012). Working memory: Theories, models, and controversies. Annual Review of Psychology, 63, 1–29. doi:10.1146/annurev-psych-120710-100422
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & van IJzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133, 1–24. doi:10.1037/0033-2909.133.1.1
Basten, U., Stelzel, C., & Fiebach, C. J. (2011). Trait anxiety modulates the neural efficiency of inhibitory control. Journal of Cognitive Neuroscience, 23, 3132–3145. doi:10.1162/jocn_a_00003
Basten, U., Stelzel, C., & Fiebach, C. J. (2012). Trait anxiety and the neural efficiency of manipulation in working memory. Cognitive, Affective, & Behavioral Neuroscience, 12, 571–588. doi:10.3758/s13415-012-0100-3
Beck, A., Steer, R., Ball, R., & Ranieri, W. (1996). Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment, 67, 588–597.
Berggren, N., & Derakshan, N. (2013). Attentional control deficits in trait anxiety: Why you see them and why you don’t. Biological Psychology, 92, 440–446.
Bishop, S. J. (2007). Neurocognitive mechanisms of anxiety: An integrative account. Trends in Cognitive Sciences, 11, 307–316. doi:10.1016/j.tics.2007.05.008
Bishop, S. J. (2009). Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12, 92–98.
Cisler, J. M., & Koster, E. H. (2010). Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review, 30, 203–216. doi:10.1016/j.cpr.2009.11.003
Cowan, N. (2001). The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences, 24, 87–114. doi:10.1017/S0140525X01003922. discussion 114–185.
Edin, F., Klingberg, T., Johansson, P., McNab, F., Tegner, J., & Compte, A. (2009). Mechanism for top-down control of working memory capacity. Proceedings of the National Academy of Sciences, 106, 6802–6807.
Eimer, M. (1996). The N2pc component as an indicator of attentional selectivity. Electroencephalography and Clinical Neurophysiology, 99, 225–234. doi:10.1016/0013-4694(96)95711-9
Ester, E. F., Drew, T., Klee, D., Vogel, E. K., & Awh, E. (2012). Neural measures reveal a fixed item limit in subitizing. Journal of Neuroscience, 32, 7169–7177. doi:10.1523/JNEUROSCI.1218-12.2012
Eysenck, M. W., & Derakshan, N. (2011). New perspectives in attentional control theory. Personality and Individual Differences, 50, 955–960.
Eysenck, M. W., Derakshan, N., Santos, R., & Calvo, M. G. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7, 336–353. doi:10.1037/1528-3542.7.2.336
Friedman, N. P., & Miyake, A. (2004). The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology. General, 133, 101–135. doi:10.1037/0096-3445.133.1.101
Gao, Z., Yin, J., Xu, H., Shui, R., & Shen, M. (2011). Tracking object number or information load in visual working memory: Revisiting the cognitive implication of contralateral delay activity. Biological Psychology, 87, 296–302.
Kiss, M., Grubert, A., Petersen, A., & Eimer, M. (2012). Attentional capture by salient distractors during visual search is determined by temporal task demands. Journal of Cognitive Neuroscience, 24, 749–759. doi:10.3758/s13414-012-0391-z
Kuo, B.-C., Stokes, M. G., & Nobre, A. C. (2012). Attention modulates maintenance of representations in visual short-term memory. Journal of Cognitive Neuroscience, 24, 51–60. doi:10.1162/jocn_a_00087
Lee, E. Y., Cowan, N., Vogel, E. K., Rolan, T., Valle-Inclan, F., & Hackley, S. A. (2010). Visual working memory deficits in patients with Parkinson’s disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain, 133, 2677–2689.
Leonard, C. J., Kaiser, S. T., Robinson, B. M., Kappenman, E. S., Hahn, B., Gold, J. M., & Luck, S. J. (2013). Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cerebral Cortex, 23, 1582–1592. doi:10.1093/cercor/bhs148
Levens, S. M., & Gotlib, I. H. (2010). Updating positive and negative stimuli in working memory in depression. Journal of Experimental Psychology. General, 139, 654–664. doi:10.1037/a0020283
Luck, S. J., & Hillyard, S. A. (1994). Spatial filtering during visual search: Evidence from human electrophysiology. Journal of Experimental Psychology. Human Perception and Performance, 20, 1000–1014. doi:10.1037/0096-1523.20.5.1000
Machizawa, M. G., Goh, C. C., & Driver, J. (2012). Human visual short-term memory precision can be varied at will when the number of retained items is low. Psychological Science, 23, 554–559. doi:10.1177/0956797611431988
Mazza, V., & Caramazza, A. (2011). Temporal brain dynamics of multiple object processing: The flexibility of individuation. PLoS ONE, 6, e17453. doi:10.1371/journal.pone.0017453
Mazza, V., Turatto, M., & Caramazza, A. (2009). Attention selection, distractor suppression and N2pc. Cortex, 45, 879–890. doi:10.1016/j.cortex.2008.10.009
McCollough, A. W., Machizawa, M. G., & Vogel, E. K. (2007). Electrophysiological measures of maintaining representations in visual working memory. Cortex, 43, 77–94.
McNab, F., & Klingberg, T. (2008). Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience, 11, 103–107. doi:10.1038/nn2024
Moriya, J., & Sugiura, Y. (2012). High visual working memory capacity in trait social anxiety. PLoS ONE, 7, e34244. doi:10.1371/journal.pone.0034244
Moser, J. S., Becker, M. W., & Moran, T. P. (2012). Enhanced attentional capture in trait anxiety. Emotion, 12, 213–216.
Osinsky, R., Alexander, N., Gebhardt, H., & Hennig, J. (2010). Trait anxiety and dynamic adjustments in conflict processing. Cognitive, Affective, & Behavioral Neuroscience, 10, 372–381. doi:10.3758/CABN.10.3.372
Osinsky, R., Gebhardt, H., Alexander, N., & Hennig, J. (2012). Trait anxiety and the dynamics of attentional control. Biological Psychology, 89, 252–259.
Owens, M., Koster, E. H., & Derakshan, N. (2012). Impaired filtering of irrelevant information in dysphoria: An ERP study. Social Cognitive and Affective Neuroscience, 7, 752–763. doi:10.1093/scan/nsr050
Pacheco-Unguetti, A. P., Acosta, A., Callejas, A., & Lupiáñez, J. (2010). Attention and anxiety: Different attentional functioning under state and trait anxiety. Psychological Science, 21, 298–304. doi:10.1177/0956797609359624
Pagano, S., & Mazza, V. (2012). Individuation of multiple targets during visual enumeration: New insights from electrophysiology. Neuropsychologia, 50, 754–761.
Pagano, S., & Mazza, V. (2013). Multiple object individuation during numerical Stroop. Psychophysiology, 50, 292–296.
Pashler, H. (1988). Familiarity and visual change detection. Perception & Psychophysics, 44, 369–378. doi:10.3758/BF03210419
Reinhart, R. M., Heitz, R. P., Purcell, B. A., Weigand, P. K., Schall, J. D., & Woodman, G. F. (2012). Homologous mechanisms of visuospatial working memory maintenance in macaque and human: Properties and sources. Journal of Neuroscience, 32, 7711–7722.
Robitaille, N., Grimault, S., & Jolicœur, P. (2009). Bilateral parietal and contralateral responses during maintenance of unilaterally encoded objects in visual short-term memory: Evidence from magnetoencephalography. Psychophysiology, 46, 1090–1099. doi:10.1111/j.1469-8986.2009.00837.x
Robitaille, N., Marois, R., Todd, J., Grimault, S., Cheyne, D., & Jolicœur, P. (2010). Distinguishing between lateralized and nonlateralized brain activity associated with visual short-term memory: fMRI, MEG, and EEG evidence from the same observers. NeuroImage, 53, 1334–1345.
Rouder, J. N., Morey, R. D., Morey, C. C., & Cowan, N. (2011). How to measure working memory capacity in the change detection paradigm. Psychonomic Bulletin & Review, 18, 324–330. doi:10.3758/s13423-011-0055-3
Sander, M. C., Werkle-Bergner, M., & Lindenberger, U. (2011). Contralateral delay activity reveals life-span age differences in top-down modulation of working memory contents. Cerebral Cortex, 21, 2809–2819.
Shek, D. T. (1993). The Chinese version of the State–Trait Anxiety Inventory: Its relationship to different measures of psychological well-being. Journal of Clinical Psychology, 49, 349–358.
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R., & Jacobs, G. A. (1983). Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press.
Spronk, M., Vogel, E. K., & Jonkman, L. M. (2012). Electrophysiological evidence for immature processing capacity and filtering in visuospatial working memory in adolescents. PLoS ONE, 7, e42262. doi:10.1371/journal.pone.0042262
Stormer, V. S., Li, S. C., Heekeren, H. R., & Lindenberger, U. (2013). Normative shifts of cortical mechanisms of encoding contribute to adult age differences in visual-spatial working memory. NeuroImage, 73C, 167–175.
Stout, D. M., Shackman, A. J., & Larson, C. L. (2013). Failure to filter: Anxious individuals show inefficient gating of threat from working memory. Frontiers in Human Neuroscience, 7, 58.
Thiruchselvam, R., Hajcak, G., & Gross, J. J. (2012). Looking inwards: Shifting attention within working memory representations alters emotional responses. Psychological Science, 23, 1461–1466.
Todd, J. J., & Marois, R. (2004). Capacity limit of visual short-term memory in human posterior parietal cortex. Nature, 428, 751–754. doi:10.1038/nature02466
Todd, J. J., & Marois, R. (2005). Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cognitive, Affective, & Behavioral Neuroscience, 5, 144–155. doi:10.3758/CABN.5.2.144
Vogel, E. K., & Machizawa, M. G. (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428, 748–751. doi:10.1038/nature02447
Vogel, E. K., McCollough, A. W., & Machizawa, M. G. (2005). Neural measures reveal individual differences in controlling access to working memory. Nature, 438, 500–503. doi:10.1038/nature04171
Voytek, B., & Knight, R. T. (2010). Prefrontal cortex and basal ganglia contributions to visual working memory. Proceedings of the National Academy of Sciences, 107, 18167–18172.
Woodman, G. F., & Luck, S. J. (2003). Serial deployment of attention during visual search. Journal of Experimental Psychology. Human Perception and Performance, 29, 121–138. doi:10.1037/0096-1523.29.1.121
Xu, Y., & Chun, M. M. (2006). Dissociable neural mechanisms supporting visual short-term memory for objects. Nature, 440, 91–95. doi:10.1038/nature04262
Author note
We thank three anonymous reviewers and Steve Luck for their very helpful comments. This research was supported by grants from the National Natural Science Foundation of China (Grant No. NSFC81171289), the Special Scientific Research Fund of Public Welfare Profession of the Ministry of Health (Grant No. 201002003), the Fundamental Research Funds for the Central Universities (Grant No. SWU1209475), and the Postgraduate Scientific and Technical Innovation Foundation at Southwest University (Grant No. ky2011001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qi, S., Ding, C. & Li, H. Neural correlates of inefficient filtering of emotionally neutral distractors from working memory in trait anxiety. Cogn Affect Behav Neurosci 14, 253–265 (2014). https://doi.org/10.3758/s13415-013-0203-5
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-013-0203-5