Abstract
Research has indicated that regions of left and right dorsolateral prefrontal cortex (DLPFC) are involved in integrating the motivational and executive function processes related to, respectively, approach and avoidance goals. Given that sensitivity to pleasant and unpleasant stimuli is an important feature of conceptualizations of approach and avoidance motivation, it is possible that these regions of DLPFC are preferentially activated by valenced stimuli. The present study tested this hypothesis by using a task in which goal pursuit was threatened by distraction from valenced stimuli while functional magnetic resonance imaging data were collected. The analyses examined whether the impact of trait approach and avoidance motivation on the neural processes associated with executive function differed depending on the valence or arousal level of the distractor stimuli. The present findings support the hypothesis that the regions of DLPFC under investigation are involved in integrating motivational and executive function processes, and they also indicate the involvement of a number of other brain areas in maintaining goal pursuit. However, DLPFC did not display differential sensitivity to valence.
Similar content being viewed by others
Motivation is fundamental to the pursuit of goals, as it is involved in selecting goals on the basis of their predicted value (e.g., reward or punishment), initiating behavior to achieve goals, and maintaining goal-directed action (e.g., Campbell & Pritchard, 1976; Jones, 1955; Lindsley, 1957). A number of theorists have proposed the existence of two fundamental motivational systems, one oriented toward potentially desirable outcomes, termed the approach motivational system, and one oriented toward potentially aversive outcomes, termed the avoidance motivational system (for reviews, see Elliot & Covington, 2001; Lang, Bradley, & Cuthbert, 1998). These motivational systems are hypothesized to form the “basic building blocks that underlie the complexity of human behavior” (Carver, Sutton, & Scheier, 2000, p. 741), because they are central to attaining the goals necessary for survival.
Approach and avoidance motivation are thought to be instantiated in neurobiological systems that are sensitive to the positive/desirable or negative/undesirable properties of stimuli, respectively (Elliot & Thrash, 2002). These systems are theorized to influence attention to and emotional processing of the rewarding and punishing features of stimuli, as well as behavioral responses to motivationally relevant stimuli (Elliot & Thrash, 2002). Individual differences in the activity and/or reactivity of these motivational systems are conceptualized as temperament types, on the basis of research indicating that they are heritable, present early in life, and stable over the lifespan (Clark, Watson, & Mineka, 1994; Elliot & Thrash, 2002).
Another set of processes hypothesized to be necessary for the pursuit of goals are those related to executive function, which are conceptualized as processes involved in the execution of goal-directed action (Banich, 2009). Although both motivation and executive function are thought to be essential to the pursuit of goals, the manner in which they interact is still a matter of debate (Pessoa, 2009).
Integration of motivation and executive function in dorsolateral prefrontal cortex
Consistent with conceptualizations of prefrontal cortex (PFC) as being necessary “to orchestrate thought and action in accordance with internal goals” (E. K. Miller & Cohen, 2001), recent research has implicated dorsolateral prefrontal cortex (DLPFC) and surrounding areas as being involved in the integration of the motivation and executive function processes (e.g., Lee & Wang, 2009; Spielberg, Miller, et al., 2011). For example, research has shown that DLPFC activation increases as both working memory demands and reward levels increase (Pochon et al., 2002), and other research has suggested that the interaction of state motivational processes and working memory load is instantiated in bilateral DLPFC (Taylor et al., 2004).
Inconsistencies have emerged in this research regarding the role of hemispheric lateralization in the relationship of DLPFC and motivational processes, with at least one study reporting activation selectively in left DLPFC in response to a reward manipulation (Pochon et al., 2002), and other studies reporting bilateral DLPFC activation in response to a manipulation that included both reward and punishment (e.g., Taylor et al., 2004). A long line of research has suggested that PFC is lateralized with respect to motivational/emotional valence, with right PFC associated with avoidance motivation and unpleasant emotion, and left PFC associated with approach motivation and pleasant emotion (for reviews, see Davidson & Irwin, 1999; Heller, 1993). Thus, differences in the motivational manipulation(s) used across studies could account for the discrepancies regarding lateralization in the literature (e.g., left DLPFC being active when only a reward manipulation is used, whereas bilateral DLPFC is active when the manipulation includes both reward and punishment). Another relevant factor not examined in these studies has been the influence of individual differences in motivational temperament on DLPFC activation during executive function tasks. To address these questions, Spielberg, Miller, et al. (2011) investigated moderation of the neural activation associated with incongruent versus congruent words on the color-word Stroop (1935) task by approach and avoidance temperament. Consistent with previous research, approach temperament moderated activation in areas of left DLPFC, whereas avoidance moderated activation in right DLPFC, and all of these effects were lateralized. Avoidance also unexpectedly moderated activation in left DLPFC, overlapping an area associated with approach, suggesting that laterality research, which often examines only hemispheric difference scores, had previously overlooked important bilateral contributions to motivation.
The regions of DLPFC identified in Spielberg, Miller, et al. (2011) have been associated with a number of other functions, including behavioral inhibition, planning upcoming action, attending to cues predictive of a motivationally salient event, and responding when such events occur (e.g., Abler, Walter, Erk, Kammerer, & Spitzer, 2006; Bickel, Pitcock, Yi, & Angtuaco, 2009; Kaladjian et al., 2009; Volle et al., 2005). Incorporating this research with their findings, Spielberg, Miller, et al. hypothesized that these regions of DLPFC are involved in implementing a motivational set that biases lower-order processing to be congruent with goals. Thus, DLPFC appears to play a central role in the pursuit of goals.
Brain areas involved in other aspects of goal pursuit
Anterior cingulate cortex
In addition to DLPFC, a number of other brain areas have been implicated in the instantiation of processes important for the pursuit of goals. Anterior cingulate cortex (ACC) has been implicated in encoding the predicted values associated with actions (for a review, see Rushworth & Behrens, 2008). Information represented in ACC is needed to efficiently create action plans to pursue goals, suggesting that ACC provides this information to DLPFC. A recent study identified a region of ACC (similar to the dorsal ACC region identified by Bush, Luu, & Posner, 2000) that was heavily connected to DLPFC and surrounding cortex and was reliably activated by reward manipulations (M. Beckmann, Johansen-Berg, & Rushworth, 2009), suggesting that this region of ACC provides motivational information regarding actions to DLPFC.
Amygdala
Although this area is often discussed solely in the context of unpleasantly valenced emotions, particularly fear (Baxter & Murray, 2002), research has supported a role for the amygdala in pleasantly valenced emotions and reward learning as well (Baxter & Murray, 2002; Holland & Gallagher, 2004; Sabatinelli, Bradley, Fitzsimmons, & Lang, 2005). In particular, the amygdala has been implicated as important for the identification of the motivational relevance of stimuli and the enhancement of feature processing in salient stimuli (Pessoa & Adolphs, 2010). Thus, previous studies have suggested that amygdala is differentially engaged by the salience of stimuli, independent of valence. In summary, the role of the amygdala in goal pursuit appears to be in biasing processing of perceptual information such that salient information is given more weight.
Basal ganglia
The basal ganglia, a set of subcortical nuclei that includes striatum, globus pallidus, substantia nigra, and subthalamic nucleus, has been heavily implicated in a number of reward processes (Haber, 2009) and, to a lesser extent, in punishment processes (e.g., Delgado, Nystrom, Fissell, Noll, & Fiez, 2000; Redgrave, Coizet, & Reynolds, 2010). For example, research has found nucleus accumbens to be activated during anticipation of appetitive stimuli (Knutson, Taylor, Kaufman, Peterson, & Glover, 2005). Additionally, striatum receives projections from diverse areas involved in goal pursuit, including DLPFC, ACC, orbitofrontal cortex, amygdala, and midbrain dopaminergic nuclei, and is thought to integrate information from processes in these areas (Haber, 2010). This integrated information can feed back through basal ganglia output nodes to influence processing in the cortical areas listed above (Gerfen & Bolam, 2010).
Orbitofrontal cortex
Orbitofrontal cortex (OFC) has been linked to the maintenance of the current and the expected motivational value of stimuli (O’Doherty & Dolan, 2006) and likely provides information about stimulus value to superior areas such as DLPFC (Szatkowska, Bogorodzki, Wolak, Marchewka, & Szeszkowski, 2008). O’Doherty (2007) proposed a medial-versus-lateral distinction in OFC, with the medial and lateral areas representing the values of rewards and punishments, respectively. However, there is disagreement in the literature regarding the role of lateral OFC in motivation. Elliott, Dolan, and Frith (2000) suggested that lateral OFC is activated when previously rewarded behavior must be inhibited, and thus that it does not represent the values of punishments per se. Kringelbach and Rolls (2004) incorporated both views by suggesting that lateral OFC represents the value of punishments and signals that behavior should change.
The present study
One important aspect of motivational processing not tested by Spielberg, Miller, et al. (2011) was the differential sensitivity to the valence of stimuli that has been hypothesized to be fundamental to the construct of motivational temperament—that is, that approach temperament is associated with sensitivity to pleasant valence, and avoidance temperament with sensitivity to unpleasant valence. Sensitivity to pleasant valence, for example, could lead to increased distraction from goals if the pleasantly valenced stimuli are salient and task-irrelevant. Therefore it is possible that, rather than being related solely to enhanced goal pursuit, greater levels of temperamental motivation also lead to disrupted goal pursuit in the presence of motivationally salient but task-irrelevant distractors. Consequently, greater recruitment of brain areas associated with maintaining goals would be needed to compensate. Thus, one aim of the present study was to test the hypothesis that approach and avoidance temperaments are associated with increased sensitivity to pleasant and unpleasant stimuli (valence manipulation), respectively, accompanied by greater compensatory recruitment of brain regions to maintain goal pursuit.
Emotionally arousing stimuli are often salient for goals independent of their valence, and therefore should attract attention. In a context in which the arousing aspect of the stimuli is irrelevant, this would lead to distraction from the goal and subsequent engagement of brain regions involved in maintaining task performance. A second aim of the present study was thus to test the hypothesis that brain areas observed to be differentially moderated by motivation in the context of tasks without an explicit emotional manipulation would be similarly engaged to ignore emotionally arousing information (arousal manipulation), which would suggest that these areas are engaged in the integration of motivational and executive function processes, regardless of the nature of the distraction that threatens to interrupt the goal.
To examine these hypotheses, the present study used fMRI to examine moderation of neural activation by trait approach and avoidance motivation in an emotion-word Stroop task (Williams, Mathews, & MacLeod, 1996). Unlike the color-word Stroop, the word meaning is distracting in the emotion-word Stroop because it is emotionally valenced and arousing. The emotion-word Stroop task used in the present study included both a valence manipulation (pleasant vs. unpleasant words) and an arousal manipulation (low vs. high arousal words).
Hypotheses
Approach temperament was hypothesized to be associated with greater distraction by pleasant words, and avoidance was hypothesized to be associated with greater distraction by unpleasant words. Both temperament types were hypothesized to be associated with distraction by arousing words. The hypothesized effects of this distraction on brain activation are specified below.
DLPFC
Approach and avoidance were hypothesized to be associated with increased engagement of the DLPFC areas identified by Spielberg, Miller, et al. (2011), as a compensatory strategy to maintain goal pursuit and ignore emotionally arousing words. Additionally, approach was hypothesized to differentially moderate left DLPFC with respect to the valence of the distractors, such that greater approach was associated with greater activation to pleasant (relative to unpleasant) distractors. Similarly, avoidance was hypothesized to be associated with greater activation to unpleasant (relative to pleasant) distractors in bilateral DLPFC.
ACC
Approach and avoidance were hypothesized to be associated with greater activation to pleasant and unpleasant distractors, respectively, in the ACC region that research has suggested provides action-related value information to DLFPC.
Amygdala
As discussed above, research has suggested that amygdala is engaged by the salience of stimuli, independent of valence. However, because approach is thought to increase the salience of pleasant stimuli, it was hypothesized that amygdala activation to pleasant stimuli would increase as a function of approach. Furthermore, it was hypothesized that amygdala activation to unpleasant stimuli would increase as a function of avoidance.
Basal ganglia
Given the proposed role of basal ganglia in integrating information from DLPFC, ACC, amygdala, and OFC, we hypothesized that this area would show a pattern of activation similar to that of the areas that project to it. Specifically, basal ganglia was hypothesized to show greater activation to pleasant distractors as a function of approach, greater activation to unpleasant distractors as a function of avoidance, and greater activation to arousing distractors as a function of both temperament types.
OFC
Approach was hypothesized to be associated with greater activation to pleasant distractors in medial OFC, whereas avoidance was hypothesized to be associated with greater activation to unpleasant distractors in lateral OFC, given research that has implicated these areas in the maintenance of appetitive and aversive value, respectively.
Method
Participants
The participants were recruited from a large pool of undergraduates who completed a series of questionnaires as partial fulfillment of enrollment in an introductory psychology course. The questionnaires included the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990; Molina & Borkovec, 1994), as a measure of anxious apprehension, and portions of the Mood and Anxiety Symptom Questionnaire (MASQ; Watson, Clark, et al., 1995; Watson, Weber, et al., 1995)—specifically, the Anxious Arousal scale (MASQ-AA) and the Loss of Interest subscale of the Anhedonic Depression scale (MASQ-AD-LI; Nitschke, Heller, Imig, McDonald, & Miller, 2001). Participants were contacted (1) if they scored at or above the 80th percentile ( ≥ 63 on the PSWQ, ≥ 33 on the MASQ-AA, or ≥ 22 on the MASQ-AD-LI) on one of the three psychopathology dimensions (anxious apprehension, anxious arousal, or anhedonic depression) and at or below the 50th percentile ( ≤ 49 on the PSWQ, ≤ 25 on the MASQ-AA, and ≤ 17 on the MASQ-AD-LI) on the other two dimensions (creating three “pure” groups); (2) if they scored at or above the 80th percentile on all three psychopathology dimensions (creating a “comorbid” group); or (3) at or below the 50th percentile on all three psychopathology dimensions (creating a “control” group). Group membership was ignored in the data analyses for the present study, except when testing whether group membership was a confounding effect. The participants were then screened for claustrophobia, left-handedness, history of serious brain injury, abnormal hearing or vision, metal in their body, pregnancy, and nonnative English-speaking.
A total of 107 participants completed the laboratory protocol. Participants were not used (a) if they moved more than 3.3 mm relative to the volume used for registration (the middle volume of the time series) or more than 2 mm between adjacent volumes (one participant exceeded this criterion only during the last block of words, and this block was not used); (b) if they committed errors on 15% or more of the trials; (c) if they exhibited reaction times greater than three standard deviations from the mean of the entire sample; (d) if their scans exhibited apparent signal loss due to magnetic susceptibility in areas of interest; or (e) if their scans exhibited activation patterns that appeared to be due to residual motion-related variance. These exclusions left 80 participants (47 female, 33 male; mean age = 19 years). In the present sample, 76 of the participants overlapped with the sample used in Spielberg, Miller, et al. (2011), who used data from a different task. One participant’s scans exhibited a scanner artifact throughout the time series. Independent-components analysis, as implemented in MELODIC (C. Beckmann & Smith, 2004), was used to isolate and remove this artifact. After removal, no artifact was apparent.
Questionnaires
To measure approach and avoidance temperament, three questionnaires were administered that have been associated with these constructs (Elliot & Thrash, 2002): the Behavioral Inhibition and Behavioral Activation Scales (Carver & White, 1994), the Neuroticism and Extraversion subscales of the NEO–Five Factor Inventory (Costa & McRae, 1992), and the Negative and Positive Temperament subscales of the General Temperament Survey (Watson & Clark, 1993). These scales were used as indicators in confirmatory factor analysis using AMOS. On the basis of previous research (Elliot & Thrash, 2002; Spielberg, Miller, et al., 2011), two latent factors were modeled, with behavioral activation, extraversion, and positive temperament used as indicators for Approach Temperament, and behavioral inhibition, neuroticism, and negative temperament used as indicators for Avoidance Temperament. Maximum-likelihood estimation was used, and the two latent factors were allowed to covary freely. The factor scores were extracted with the regression method to use as measures of approach and avoidance temperament.
Stimuli and experimental design
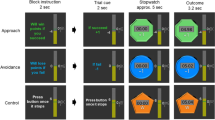
Participants completed two tasks, an emotion-word Stroop and a color-word Stroop (findings from the latter task are presented in Spielberg, Miller, et al., 2011). The order of presentation of the two tasks was counterbalanced across participants. In the emotion-word Stroop task, 256 trials were presented in 16 blocks (four pleasant, four unpleasant, and eight neutral) of 16 trials each, with a variable intertrial interval (2,000 ± 225 ms) between trial onsets. A trial began with presentation of a word for 1,500 ms, followed by a fixation cross for an average of 500 ms. Each trial consisted of one word presented in one of four ink colors (red, yellow, green, and blue), each color occurring equally often with each word type. Blocks of pleasant or unpleasant words alternated with blocks of neutral words. The order of presentation of the blocks in the present investigation was counterbalanced for each participant. In addition to the word blocks, we included four fixation blocks (one at the beginning, one at the end, and two in the middle of the session) and five rest blocks (one at the beginning, one at the end, and one between each word block). In the fixation condition, a fixation cross intensified in place of word presentation, and in the rest condition, the participants were instructed to rest and keep their eyes open.
The 256 word stimuli were selected from the Affective Norms for English Words set (Bradley & Lang, 1999). Of these words, 64 were pleasant (e.g., birthday, ecstasy, and laughter), 64 were unpleasant (e.g., suicide, war, and victim), and two sets of 64 were neutral (e.g., hydrant, moment, and carpet). The words were carefully selected on the basis of established norms for valence, arousal, frequency of usage in the English language, and number of letters (see Table 1). The words ranged from three to eight letters long (visual angle: 5–18 deg). Each word was centered on a black background and projected. The participants responded with their index and middle fingers using a four-button response box (James Long Co.) under each hand.
fMRI data collection
The fMRI data were 370 three-dimensional (3-D) images acquired using a Siemens gradient-echo echo-planar imaging sequence (TR 2,000 ms, TE 25 ms, flip angle 80º, FOV = 220 mm) on a Siemens Allegra 3 T scanner. Each image consisted of 38 oblique axial slices (slice thickness 3 mm, 0.3-mm gap, in-plane resolution 3.4375 × 3.4375 mm) acquired parallel to the anterior and posterior commissures. After the fMRI acquisition, a 160-slice MPRAGE structural sequence was acquired (spatial resolution 1 × 1 × 1 mm) and used to warp the participant’s functional data into standard space.
fMRI data reduction and preprocessing
Image processing and statistical analysis were implemented primarily using the FMRI Expert Analysis Tool, version 5.98 (FEAT, www.fmrib.ox.ac.uk/analysis/research/feat/), part of the FSL analysis package (www.fmrib.ox.ac.uk/fsl). The first three time points (fMRI volumes) of the data set for each participant were discarded to allow the magnetic resonance signal to reach a steady state. Functional data for each participant were motion corrected using rigid-body registration, implemented in FSL’s linear registration tool, MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002). The data were intensity normalized, such that the mean intensity (across time and across voxels in the brain) was constrained to be equal across participants. Next, the data were temporally filtered with a nonlinear high-pass filter that attenuated frequencies below 1/212 Hz and spatially smoothed using a 3-D Gaussian kernel (full width at half maximum = 5 mm). Temporal low-pass filtering was carried out using AFNI’s 3dDespike tool (http://afni.nimh.nih.gov/) to remove intensity spikes.
fMRI data processing
Regression analyses were performed on the processed functional time series of each participant using FMRIB’s Improved Linear Model (FILM) with autocorrelation correction (Woolrich, Ripley, Brady, & Smith, 2001). Four predictors, one for each word type block (pleasant, neutral, and unpleasant) and one modeling the rest condition, were included in the regression model (fixation was left unmodeled). For each predictor, the vector of assigned weights corresponding to word type was convolved with a gamma function to better approximate the temporal course of the blood-oxygen-dependent (BOLD) hemodynamic response function. Each predictor yielded a per-voxel effect-size parameter estimate (β) map representing the magnitude of activation associated with that predictor.
In order to create comparisons of interest, β values for the relevant parameters were contrasted. Two comparisons of interest were created. A valence comparison (VAL) was created by contrasting the pleasant condition with the unpleasant condition. An arousal comparison (ARO) was created by averaging the pleasant and unpleasant conditions and contrasting this average against the neutral condition. For each participant, these functional activation maps were nonlinearly warped into a common stereotaxic space (the 2009 Montreal Neurological Institute [MNI] 152 symmetrical 1 × 1 × 1 mm template; Fonov, Evans, McKinstry, Almli, & Collins, 2009) using FMRIB’s Non-Linear Image Registration Tool (FNIRT; Andersson, Jenkinson, & Smith, 2007).
Group inferential statistical analyses were carried out using FMRIB’s Local Analysis of Mixed Effects (FLAME). To examine the task main effects, a t test of the means across all participants was conducted for VAL and ARO. To examine moderation by motivational temperaments, VAL and ARO were entered as dependent variables into second-level multiple regression analyses with approach and avoidance scores as the predictor variables.Footnote 1 Three covariates of no interest were included that modeled the different counterbalancing orders of task blocks, and these covariates were not correlated with approach or avoidance (see G. A. Miller & Chapman, 2001, on this use of covariates). Each moderation-related regression analysis produced two β maps, one corresponding to the unique variance associated with approach temperament (with the shared variance associated with avoidance removed) and one corresponding to the unique variance associated with avoidance temperament (with the shared variance associated with approach removed). Two-tailed t tests were conducted on the βs for approach and avoidance and then converted to z scores to determine the significance of the βs. On the basis of a priori hypotheses, several masks were used to constrain the number of voxels under consideration. These masks were of (1) bilateral superior-lateral-prefrontal gray matter, (2) bilateral amygdala, (3) bilateral basal ganglia, (4) bilateral ventral-prefrontal gray matter, and (5) cingulate and paracingulate gray matter. Additionally, in order to examine the task main effects, a whole-brain gray-matter mask was used, because no a priori hypotheses were made regarding these effects.
In order to correct for multiple voxel comparisons for the temperament analyses, Monte Carlo simulations via AFNI’s AlphaSim program (Ward, 2000) were used to estimate the appropriate cluster size for each mask, which provided a two-tailed familywise error rate of .01. An error rate of .01 for each mask was employed (as opposed to the conventional .05) in order to correct for the fact that five masks were examined (.05/5 = .01), thus giving an overall error rate of .05 across all five masks. An individual voxel-level threshold of p = .04 was used in combination with minimum cluster sizes of 1,092 mm3 (superior lateral prefrontal), 780 mm3 (amygdala), 780 mm3 (basal ganglia), 1,170 mm3 (ventral prefrontal), and 1,092 mm3 (cingulate/paracingulate). Multiple voxel comparisons were corrected similarly for the analyses examining task main effects, except that the error rate per mask was held at .05 because only one mask was examined, a whole-brain gray-matter mask (cluster threshold = 2,340 mm3).
Lateralization analyses
Lateralization in DLPFC was tested using a locally written MATLAB program. This program conducted a repeated measures homogeneity-of-slopes analysis of covariance, with hemisphere as the repeated measure, approach and avoidance scores as continuous between-subjects predictors (along with the covariates of no interest mentioned above), and VAL or ARO fMRI activation as the dependent variable. The interaction of each temperament score with hemisphere was examined, which tested whether the relationship between temperament score and brain activation differed significantly across hemispheres. Given that the Hemisphere factor had only two levels, nonsphericity was not a concern (because the sphericity assumption requires that the variance of the difference between a pair of levels be the same for all pairs, and there was only one pair of levels). These analyses were conducted on a per-voxel basis, and correction for multiple comparisons was conducted on the resultant β maps using cluster-thresholding via AlphaSim, in the same manner as in the main analyses. For the lateralization analyses, the superior lateral prefrontal cortex mask was edited to contain only the right hemisphere, and an individual voxel-level threshold of p = .04 was used in combination with a minimum cluster size of 702 mm3.
Analyses to examine bias by sampling strategy
The interaction of temperament and sampling group was examined in order to determine whether the sampling strategy (based on psychopathology scores not of interest in the present study) biased the present findings. Psychopathology Group was entered as a between-subjects factor in the main analyses, and the interaction between this factor and each temperament score was examined in the regions in which clusters were observed in the main analyses. These analyses were conducted in a voxel-wise manner and were thresholded in the manner described above (using the same masks), except that an overall corrected p value of .10 per mask was used. This resulted in cluster thresholds of 702 mm3 (superior lateral prefrontal), 468 mm3 (amygdala), 468 mm3 (basal ganglia), 780 mm3 (ventral prefrontal), and 702 mm3 (cingulate/paracingulate). A more liberal p value was used in order to increase the chance of locating biasing effects of the sampling strategy.
Behavioral analyses
The mean reaction time (RT) and error frequency were calculated for each condition and each participant. To calculate the effect of task on RTs, two orthogonal paired-sample t tests were conducted, one in which pleasant was compared to unpleasant and one in which the average of pleasant and unpleasant was compared to neutral. Similar comparisons were used with related-samples Wilcoxon signed rank tests to calculate the effect of task on error rates. A VAL RT interference score was calculated by subtracting the unpleasant RT from the pleasant RT. A VAL error rate difference score was calculated similarly. An ARO RT interference score was calculated by subtracting the neutral RT from the average of the pleasant and unpleasant RTs. An ARO error difference rate score was calculated by subtracting the neutral error frequency from the sum of the pleasant and unpleasant error frequencies (it was not necessary to calculate the mean of pleasant and unpleasant because the number of neutral trials was equal to the sum of the pleasant and unpleasant trials). The RT interference and error rate difference scores were entered as dependent variables in regression analyses (Poisson regression was used with error difference scores), with approach and avoidance temperament entered simultaneously as predictors.
Results
Confirmatory factor analysis
The two-factor model of scales contributing to the approach and avoidance scores was successfully estimated and was associated with a nonsignificant χ 2 value of 10.3 (p = .25, 8 dfs). The comparative-fit index value (Bentler, 1990) was 0.993, and the root-mean square error of approximation value (Brown & Cudeck, 1993) was 0.060, indicating that the model provided excellent fit to the data. Consistent with prior research (e.g., Spielberg, Heller, Silton, Stewart, & Miller, 2011), the latent factors were negatively correlated (r = – .58, p < .001). All measurement weights were significant at p < .001, and the standardized estimates are provided in Table 2.
Behavioral analysis
Paired-sample t tests were conducted to detect differences in RTs between emotion conditions. Pleasant RTs (mean = 684 ms) did not differ from unpleasant RTs (mean = 685 ms; mean difference = –1 ms, p = .817, Cohen’s d = –0.03). Also, high arousal (i.e., average of pleasant and unpleasant; mean = 684 ms) did not differ from the neutral condition (mean = 681 ms; mean difference = 3 ms, p = .530, Cohen’s d = 0.07).
Related-samples Wilcoxon signed rank tests were conducted to detect differences in error rates between the emotion conditions. Error rates did not differ for the pleasant (mean = 3.2) versus the unpleasant (mean = 3.6; mean difference = –.4, p = .122, r = –.17) conditions. However, high arousal (sum of pleasant and unpleasant; mean = 6.8) did lead to a greater error rate than did the neutral condition (mean = 5.6; mean difference = 1.2, p < .001, r = .42).
Linear and Poisson regression analyses were conducted to determine the effect of temperament on RT interference and differences in error rates, respectively. Neither approach (β = .20, p = .172, ΔR 2 = .024) nor avoidance (β = .06, p = .656, ΔR 2 = .003) predicted valence-related RT interference, nor did they predict arousal-related RT interference (approach, β = .04, p = .813, ΔR 2 = .001; avoidance, β = .02, p = .883, ΔR 2 = .000). Similarly, neither approach (β = .02, Wald χ 2 = .1, p = .763) nor avoidance (β = –.05, Wald χ 2 = .9, p = .355) predicted valence-related differences in error rates, nor did they predict arousal-related differences in error rates (approach, β = .06, Wald χ 2 = 1.5, p = .223); avoidance, β = .05, Wald χ 2 = 1.4, p = .234).
Main effects of task
Table 3 lists the brain regions where main effects of VAL and ARO were observed.
Valence-related activation moderated by temperament
Table 4 lists the brain regions where activation related to VAL was moderated by trait approach or avoidance temperament. As is illustrated in Fig. 1, two clusters emerged in which VAL activation was moderated by avoidance temperament. In line with the present hypotheses, higher avoidance temperament was associated with a larger response to unpleasant than to pleasant distractors in left putamen and right amygdala. No areas emerged in which avoidance was associated with more activation to pleasant distractors. Unexpectedly, approach temperament was associated with less activation to pleasant than to unpleasant distractors in right caudate. No areas emerged in which approach was associated with more activation to pleasant distractors.
Moderation of activation to emotionally valenced distractors by motivational temperaments: (a) Approach temperament correlates with activation in right caudate. (b) Avoidance temperament correlates with activation in right amygdala. (c) Avoidance temperament correlates with activation in left putamen. Blue/green cluster colors indicate negative correlations with pleasant – unpleasant activation. R = right, y = coordinates in MNI 2009a space
Arousal-related activation moderated by temperament
Table 5 lists the brain regions where activation related to ARO was moderated by trait approach or avoidance temperament. In line with the present hypotheses, activation to arousing distractors in left DLPFC and right putamen increased as approach temperament increased, as illustrated in Fig. 2. The area of left DLPFC identified in the present study overlapped both areas found to be associated with approach temperament in Spielberg, Miller, et al. (2011). Unexpectedly, three additional clusters emerged in which activation to arousing distractors increased as a function of approach temperament. These included a cluster in genual ACC/paracingulate and two clusters in posterior cingulate (PCC).
Moderation of activation to emotionally arousing distractors by approach temperament: (a) Left dorsolateral prefrontal cortex. (b) Genual anterior cingulate cortex and posterior cingulate cortex. (c)Right putamen. Yellow/red cluster colors indicate positive correlations with high arousal – low arousal activation. R = right, x and y = coordinates in MNI 2009a space
As is illustrated in Fig. 3, the results for avoidance motivation were also consistent with our hypotheses. Specifically, activation to arousing distractors in regions of right and left DLPFC, overlapping the areas identified in Spielberg, Miller, et al. (2011), increased as avoidance temperament increased. The left DLPFC cluster partially overlapped with the left DLPFC cluster associated with approach temperament by 561 mm3 (39% of the approach cluster, 21% of the avoidance cluster). Also in line with the present hypotheses, activation to arousing distractors in right putamen and caudate increased as a function of avoidance temperament. Unexpectedly, activation to arousing distractors also increased in a large cluster that included genual ACC, dorsal ACC, PCC, and paracingulate as avoidance temperament increased. No clusters emerged in OFC. However, this may have been due to a lack of power, because avoidance was associated with a cluster in right agranular OFC (cluster size = 1,110 mm3, mean z = 2.45; center of mass: x = 38, y = 23, z = –11) when the overall p value for that mask was relaxed to .05.
Moderation of activation to emotionally arousing distractors by avoidance temperament: (a) Right dorsolateral prefrontal cortex. (b)Left dorsolateral prefrontal cortex. (c)Genual and dorsal anterior cingulate cortex/posterior cingulate cortex/paracingulate. (d)Right caudate. (e)Right putamen. Yellow/red cluster colors indicate positive correlations with high arousal – low arousal activation. R = right, x and y = coordinates in MNI 2009a space
Lateralization analyses
Given work indicating that motivation influences the laterality of activation in DLPFC, analyses were carried out to locate regions in which the relationship between temperament type (approach, avoidance) and brain activation was asymmetric across the hemispheres. As is indicated in Table 6, no clusters exhibited significant lateralized relationships between temperament and valence-related activation. In line with the present hypotheses, a cluster was observed in left DLPFC in which approach temperament exhibited a larger correlation with arousal-related activation in the left hemisphere. Importantly, this cluster almost completely overlapped the left DLPFC cluster associated with approach temperament observed in the above analyses. Also in line with present hypotheses, a cluster was observed in right DLPFC in which avoidance temperament exhibited a larger correlation with arousal-related activation in the right hemisphere. Importantly, this cluster largely overlapped the right DLPFC cluster associated with avoidance temperament observed in the above analyses.
Analyses to examine bias by sampling strategy
No clusters were observed in which psychopathology group interacted with temperament to predict VAL activation. One cluster was observed in which psychopathology group interacted with approach temperament to predict ARO activation. Specifically, a cluster was observed in paracingulate/dorsal ACC (center of mass: x = –6, y = 31, z = 29). However, this cluster did not overlap with the cingulate clusters observed to be related to approach temperament in the analyses above. No clusters were observed in which psychopathology group interacted with avoidance temperament to predict ARO activation.
Discussion
The findings of the present study partially supported the hypothesis that approach and avoidance are differentially sensitive to valence. Avoidance motivation was positively associated with activation to unpleasant distractors in two of the hypothesized brain areas, although this sensitivity was not manifested in behavioral performance. No areas exhibited greater activation to pleasant distractors as a function of approach temperament. Unexpectedly, approach temperament was negatively associated with activation to pleasant distractors in right caudate. The hypothesis that approach and avoidance temperament would both be positively associated with recruitment of brain areas involved in maintaining goal pursuit when emotionally arousing distractors were present was supported. This increased recruitment was observed for both approach and avoidance in a number of areas, including DLPFC. Taken together, these findings support the involvement of DLPFC in the integration of motivation and executive function, but they fail to support the hypothesis that this integration in DLPFC would be differentially sensitive to the valence of the distracting stimuli.
Dorsolateral prefrontal cortex
The present findings confirm the importance of DLPFC in integrating motivational and executive function processes. As hypothesized, approach temperament was positively associated with activation to arousing distractors in a region of left DLPFC that substantially overlapped the two clusters found to be associated with approach temperament in Spielberg, Miller, et al. (2011), and this effect was lateralized as predicted. Also in line with our hypotheses, avoidance temperament was positively associated with activation to arousing distractors in regions of right and left DLPFC that substantially overlapped the two clusters found by Spielberg, Miller, et al. to be associated with avoidance temperament. Again similar to the findings of Spielberg, Miller, et al., the right DLPFC effect was lateralized, whereas the left DLFPC effect was not. Consistent with Spielberg, Miller, et al., these findings indicate that motivational temperaments are positively associated with engagement of DLPFC to maintain goal pursuit.
One interpretation of these findings is that these DLPFC areas are recruited to exert top-down control in order to compensate for the distraction induced by the arousing nature of the distractors. The present findings then suggest that motivational temperaments are associated with greater engagement in compensatory processing to maintain goals. An alternative interpretation is that activation in these areas of DLPFC reflects sustained attention to the arousing properties of the stimuli, rather than being compensatory in nature. In support of this hypothesis, Yarkoni, Barch, Gray, Conturo, and Braver (2009) found that the amount of time spent on task was proportional to activation in several prefrontal regions. Given this second interpretation, the present findings suggest that motivational temperaments are associated with greater levels of task-related sustained attention.
The present hypotheses regarding moderation of valence-related activation in DLPFC by motivational temperaments were not supported. Specifically, approach temperament did not moderate activation to pleasant distractors in left DLPFC, and avoidance temperament did not moderate activation to unpleasant distractors in bilateral DLPFC. Additionally, approach and avoidance temperament did not moderate valence-related behavioral performance. One potential explanation for the failure to support these hypotheses is that the effects under examination are relatively small, and the present study lacks the power to detect them. Although the sample was very substantial for an fMRI study, it was smaller than those in many relevant nonneuroscience studies in psychology.
An alternative explanation for the failure to find moderation of valence-related activation in DLPFC is the fact that the valenced stimuli were task-irrelevant, and differential sensitivity to valence in DLPFC may be evident only when the valenced stimuli are the focus of goal pursuit (e.g., approach temperament associated with increased sensitivity to appetitive goals). Future research could examine whether activation in the regions of DLPFC observed in the present study varies differentially as a function of approach and avoidance temperament when the goal itself is valenced. For example, activation in left DLPFC may increase as a function of approach temperament when the goal is appetitive (e.g., monetary reward), but not when the goal is aversive. Additionally, distractor word meaning was not directly relevant to the goal being pursued (attending to ink color) in the present study. It is possible that differential sensitivity to valence associated with motivational temperaments would be observed in DLPFC if the distractors were directly relevant to the task at hand. For example, if the goal were to identify whether a face was expressing disgust, approach temperament might show increased reaction times to pleasant versus unpleasant faces.
Cingulate cortex
Similar to the findings for DLPFC, several clusters emerged in cingulate in which approach or avoidance temperament was associated with more arousal-related activation, rather than valence-related activation as hypothesized. Approach was positively associated with activation in genual ACC, in the anterior end of the cingulate area hypothesized to be related to maintaining the value of actions, as well as in PCC. Avoidance was positively associated with activation in a larger swath of cortex, including both areas moderated by approach and extending farther, both dorsally and rostrally, from genual ACC.
Although we had not hypothesized moderation of activation in PCC, this finding is consistent with research implicating this region as having an important role in goal pursuit. Specifically, one theory has suggested that PCC, along with other areas such as genual ACC, is involved in using “past experiences adaptively to imagine perspectives and events beyond those that emerge from the immediate environment” (Buckner & Carroll, 2007, p. 49). Thus, PCC appears to be important for the anticipation of potential future outcomes. PCC involvement has also been found in studies directly examining approach and avoidance motivation. For example, PCC activation has been found to increase when participants self-reflected on approach- and avoidance-related goals (Johnson et al., 2006).
Amygdala
As hypothesized, amygdala activation to unpleasant distractors was positively associated with avoidance temperament. Given research indicating that amygdala is involved in identifying the motivational relevance of stimuli (Pessoa & Adolphs, 2010), this finding suggests that avoidance temperament is associated with the assessment of unpleasant stimuli as being more salient than pleasant stimuli. The present hypothesis that amygdala activation to pleasant distractors would increase as a function of approach temperament was not supported. Although this may be due to a lack of power, the present findings are consistent with research indicating that, when valenced stimuli are task-irrelevant, amygdala responses are greater to unpleasantly than to pleasantly valenced stimuli (Straube, Pohlack, Mentzel, & Miltner, 2008).
Basal ganglia
The hypothesis that arousal-related basal ganglia activation would be positively associated with approach and avoidance temperament was supported in right putamen for both approach and avoidance, and in the head of the caudate for avoidance temperament. The region of putamen observed to be active for both approach and avoidance receives projections from premotor cortex (Haber, 2010) and is thought to be involved in action preparation (Tremblay, Worbe, & Hollerman, 2009). Therefore, moderation of activation in this area of putamen may reflect the influence of motivation on action preparation. Research has suggested that the area of caudate found in the present study receives projections from DLPFC, ACC, and OFC and is involved in integrating information from these areas (Haber, 2010). This caudate region projects back to cortex through connections with globus pallidus and substantia nigra (Gerfen & Bolam, 2010), providing a route by which integrated information from DLPFC, ACC, and OFC can influence ongoing processing in these cortical areas.
The present hypotheses regarding the basal ganglia and valence-related activation were partially supported. Specifically, activation to unpleasant distractors in left putamen was positively associated with avoidance temperament. This cluster partially overlapped the left putamen cluster in which arousal-related activation was moderated by avoidance and is within the region of putamen that receives projections from premotor cortex (Haber, 2010) and is implicated in action preparation (Tremblay et al., 2009). This suggests that avoidance motivational information differentially influences the preparation of actions related to unpleasant stimuli.
The hypothesis that activation in basal ganglia to pleasant distractors would increase as a function of approach temperament was not supported. Instead, approach temperament was negatively associated with activation to pleasant, relative to unpleasant, distractors in right caudate. Given research indicating that this area of caudate receives projections from DLPFC (Haber, 2010), one potential explanation for this finding is that DLPFC is exerting top-down control on caudate to suppress processing of the pleasant distractors. However, the fact that approach temperament did not significantly moderate valence-related activation in DLPFC undermines this explanation. An alternative explanation is that approach temperament is not associated with sensitivity to pleasant stimuli. Rather, unpleasant stimuli may generally be more salient, and thus both motivational temperaments are associated with increased processing of such stimuli. If true, this would call into question important aspects of current conceptualizations of approach and avoidance motivation.
Orbitofrontal cortex
Contrary to our hypotheses, no clusters were observed in which motivational temperaments moderated activation in OFC. As discussed above in relation to other regions, this may be due to a lack of power to detect these effects. In support of this explanation is the fact that, when the overall p value for each mask was relaxed to .05, a cluster emerged in right agranular OFC in which avoidance was positively associated with activation to arousing stimuli.
Strengths and limitations
The present study benefited from direct tests of laterality, the use of a relatively large sample size (for the fMRI literature), and careful measurement of approach and avoidance temperament by estimation of latent factors from multiple indices. It extends the literature on the neural integration of approach and avoidance motivation and executive function processes by examining this integration in the context of emotionally valenced and arousing distraction. As with any study, however, several limitations must be considered when interpreting the results. First, the present study used only self-report measures of approach and avoidance temperament. Future research would benefit from additional behavioral performance measures, such as differential detection of cues indicating monetary reward and punishment (see, e.g., Henriques, Glowacki, & Davidson, 1994). Second, not all potentially relevant aspects of executive function were recruited by the task used in the present study. Future research could extend the present findings by examining how processes related to other aspects of executive function, such as shifting and updating (Miyake et al., 2000), are integrated with motivational processes. Third, it is unclear whether the high- and low-arousal words led to actual changes in physiological arousal in the present study. Given that the purpose of the arousal manipulation in the present study was to employ stimuli that differed in salience, rather than physiological arousal, this appears to be a relatively minor limitation. However, future research that uses a measure of sympathetic nervous system activity could provide useful information regarding the relationships between approach and avoidance motivation and goal maintenance.
A final limitation is that the sample used in the present study was selected on the basis of measures of anxiety and depression (creating groups with high and low levels of psychopathology), which may affect the generalizability of the present findings. One mitigating factor is that the data used in the present study were collected 1–6 months after the screening, during which time regression to the mean on the selection questionnaires occurred. Another factor is that the sampling strategy covered all but about 1 SD of the distribution of each scale, thus representing most of the population. Additionally, the fact that sampling group did not interact with motivational temperaments in any of the observed clusters suggests that the findings were not biased. However, the present study may lack appropriate power to detect these interactions. Given research suggesting that anxiety/depression and motivational temperaments share important variance (Spielberg, Heller, et al., 2011), one potential effect of the sampling strategy used in the present study was the creation of something similar to extreme groups on motivational temperament. Specifically, because participants in the present study were selected to be high or low in anxiety/depression, they would also tend to be high or low on motivational temperaments. Thus, the actual effects may be smaller than those observed here. If the sampling strategy used in the present study did create something akin to extreme groups, this would be a benefit as well as a drawback, because the power to detect effects of interest would be increased. However, it does limit the interpretation of the present findings, and future research should test these hypotheses in an unselected sample in order to determine whether the effects observed generalize to such populations.
In spite of these limitations, the present study adds to the literature by supporting the proposed role for DLPFC in the integration of motivational and executive function processes and by implicating a network of other brain areas as being involved in maintaining goal pursuit, including amygdala, ACC, PCC, and basal ganglia. Additionally, approach temperament was not associated with sensitivity to pleasant stimuli as hypothesized. Rather, approach was associated with decreased activation to pleasant stimuli in right caudate, calling into question aspects of current conceptualizations of approach motivation. The results from the present study indicate that avoidance temperament is associated with differential sensitivity to unpleasant stimuli, given the finding that valence-related activation in amygdala and left putamen varied as a function of avoidance temperament.
Notes
The fMRI analyses conducted using FEAT were rerun using FSL’s outlier deweighting (Woolrich, 2008) procedure to test whether the findings were driven by outliers. The sets of findings were virtually identical, indicating that the original findings were not due to outliers. Two additional analyses were conducted in order to rule out the potential confounding effects of structural differences that might correlate with approach or avoidance temperament. First, voxel-based morphometry analysis was performed with approach and avoidance temperament predicting gray-matter density. Approach and avoidance did not predict gray-matter density in any of the areas in which approach and avoidance predicted fMRI activation. Second, the approach and avoidance FEAT analyses were rerun with gray-matter density as a voxel-dependent covariate, thus removing any shared variance between temperament and gray-matter density. The new findings were extremely similar, indicating that the original findings were not due to temperament-related structural differences.
References
Abler, B., Walter, H., Erk, S., Kammerer, H., & Spitzer, M. (2006). Prediction error as a linear function of reward probability is coded in human nucleus accumbens. NeuroImage, 31, 790–795. doi:10.1016/j.neuroimage.2006.01.001
Andersson, J. L. R., Jenkinson, M., & Smith, S. (2007). Non-linear optimization (Technical Report No. TR07JA1). Oxford, U.K.: University of Oxford, FMRIB Centre.
Banich, M. T. (2009). Executive function: The search for an integrated account. Current Directions in Psychological Science, 18, 89–94. doi:10.1111/j.1467-8721.2009.01615.x
Baxter, M. G., & Murray, E. A. (2002). The amygdala and reward. Nature Reviews Neuroscience, 3, 563–573. doi:10.1038/nrn875
Beckmann, C., & Smith, S. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging, 23, 137–152. doi:10.1109/TMI.2003.822821
Beckmann, M., Johansen-Berg, H., & Rushworth, M. (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience, 29, 1175–1190. doi:10.1523/JNEUROSCI.3328-08.2009
Bentler, P. M. (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107, 238–246. doi:10.1037/0033-2909.107.2.238
Bickel, W. K., Pitcock, J. A., Yi, R., & Angtuaco, E. J. (2009). Congruence of BOLD response across intertemporal choice conditions: Fictive and real money gains and losses. Journal of Neuroscience, 29, 8839–8846. doi:10.1523/JNEUROSCI.5319-08.2009
Bradley, M. M., & Lang, P. J. (1999). Affective norms for English words (ANEW): Stimuli, instruction manual and affective ratings (Technical Report No. C-1). Gainesville, FL: University of Florida, NIMH Center for Research in Psychophysiology.
Brown, M. W., & Cudeck, R. (1993). Alternative ways of assessing model fit. In K. A. Bollen & J. S. Long (Eds.), Testing structural equation models (pp. 136–162). Newbury Park, CA: Sage.
Buckner, R. L., & Carroll, D. C. (2007). Self-projection and the brain. Trends in Cognitive Sciences, 11, 49–57. doi:10.1016/j.tics.2006.11.004
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4, 215–222. doi:10.1016/S1364-6613(00)01483-2
Campbell, J. P., & Pritchard, R. D. (1976). Motivation theory in industrial and organizational psychology. In M. D. Dunnette (Ed.), Handbook of industrial and organizational psychology. Chicago: Rand McNally.
Carver, C. S., & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319–333. doi:10.1037/0022-3514.67.2.319
Carver, C. S., Sutton, S. K., & Scheier, M. F. (2000). Action, emotion, and personality: Emerging conceptual integration. Personality and Social Psychology Bulletin, 26, 741–751. doi:10.1177/0146167200268008
Clark, L. A., Watson, D., & Mineka, S. (1994). Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology, 103, 103–116. doi:10.1037/0021-843X.103.1.103
Costa, P. T., & McCrae, R. R. (1992). Revised NEO personality inventory: NEOPI-R and Five Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources.
Davidson, R. J., & Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3, 11–21. doi:10.1016/S0896-6273(04)00264-8
Delgado, M. R., Nystrom, L. E., Fissell, C., Noll, D. C., & Fiez, J. A. (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84, 3072–3077.
Elliot, A. J., & Covington, M. V. (2001). Approach and avoidance motivation. Educational Psychology Review, 13, 73–92. doi:10.1023/A:1009009018235
Elliot, A. J., & Thrash, T. M. (2002). Approach-avoidance motivation in personality: Approach and avoidance temperaments and goals. Journal of Personality and Social Psychology, 82, 804–818. doi:10.1037/0022-3514.82.5.804
Elliott, R., Dolan, R. J., & Frith, C. D. (2000). Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cerebral Cortex, 10, 308–317. doi:10.1093/cercor/10.3.308
Fonov, V. S., Evans, A. C., McKinstry, R. C., Almli, C. R., & Collins, D. L. (2009). Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage, 47, S102. doi:10.1016/S1053-8119(09)70884-5
Gerfen, C. R., & Bolam, J. P. (2010). The neuroanatomical organization of the basal ganglia. In H. Steiner & K. Tseng (Eds.), Handbook of basal ganglia structure and function (pp. 3–28). Amsterdam: Academic Press.
Haber, S. N. (2009). Anatomy and connectivity of the reward circuit. In J. C. Dreher & L. Tremblay (Eds.), Handbook of reward and decision making (pp. 3–27). Amsterdam: Academic Press.
Haber, S. N. (2010). Integrative networks across basal ganglia circuits. In H. Steiner & K. Tseng (Eds.), Handbook of basal ganglia structure and function (pp. 409–427). Amsterdam: Academic Press.
Heller, W. (1993). Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology, 7, 476–489. doi:10.1037/0894-4105.7.4.476
Henriques, J. B., Glowacki, J. M., & Davidson, R. J. (1994). Reward fails to alter response bias in depression. Journal of Abnormal Psychology, 103, 460–466. doi:10.1037/0021-843X.103.3.460
Holland, P. C., & Gallagher, M. (2004). Amygdala–frontal interactions and reward expectancy. Current Opinion in Neurobiology, 14, 148–155. doi:10.1016/j.conb.2004.03.007
Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. doi:10.1016/S1053-8119(02)91132-8
Johnson, M. K., Raye, C. L., Mitchell, K. J., Touryan, S. R., Greene, E. J., & Nolen-Hoeksema, S. (2006). Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience, 1, 56–64. doi:10.1093/scan/nsl004
Jones, M. R. (1955). Nebraska symposium on motivation (Vol. 3). Lincoln, NE: University of Nebraska Press.
Kaladjian, A., Jeanningros, R., Azorin, J.-M., Nazarian, B., Roth, M., Anton, J.-L., & Mazzola-Pomietto, P. (2009). Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar Disorders, 11, 530–538. doi:10.1111/j.1399-5618.2009.00722.x
Knutson, B., Taylor, J., Kaufman, M., Peterson, R., & Glover, G. (2005). Distributed neural representation of expected value. Journal of Neuroscience, 25, 4806–4812. doi:10.1523/JNEUROSCI.0642-05.2005
Kringelbach, M. L., & Rolls, E. T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72, 341–372. doi:10.1016/j.pneurobio.2004.03.006
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1998). Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry, 44, 1248–1263. doi:10.1037/0735-7044.112.5.1069
Lee, D., & Wang, X. J. (2009). Mechanisms for stochastic decision making in the primate frontal cortex: Single-neuron recording and circuit modeling. In P. W. Glimcher, C. F. Camerer, E. Fehr, & R. A. Poldrack (Eds.), Neuroeconomics, decision making, and the brain (pp. 481–501). Oxford, U.K.: Elsevier.
Lindsley, D. B. (1957). Psychophysiology and motivation. In M. R. Jones (Ed.), Nebraska symposium on motivation (Vol. 5). Lincoln, NE: University of Nebraska Press.
Meyer, T. J., Miller, M. L., Metzger, R. L., & Borkovec, T. D. (1990). Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy, 28, 487–495. doi:10.1016/0005-7967(90)90135-6
Miller, G. A., & Chapman, J. P. (2001). Invited paper: Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110, 40–48. doi:10.1037/0021-843X.110.1.40
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. doi:10.1146/annurev.neuro.24.1.167
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. doi:10.1006/cogp. 1999.0734
Molina, S., & Borkovec, T. D. (1994). The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In G. C. L. Davey & F. Tallis (Eds.), Worrying: Perspectives on theory, assessment, and treatment (pp. 265–283). Chichester, U.K.: Wiley.
Nitschke, J. B., Heller, W., Imig, J. C., McDonald, R. P., & Miller, G. A. (2001). Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research, 25, 1–22. doi:10.1023/A:1026485530405
O’Doherty, J. P. (2007). Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Annals or the New York Academy of Sciences, 1121, 254–272. doi:10.1196/annals.1401.036
O’Doherty, J. P., & Dolan, R. J. (2006). The role of human orbitofrontal cortex in reward prediction and behavioral choice: Insights from neuroimaging. In D. H. Zald & S. L. Rauch (Eds.), The orbitofrontal cortex (pp. 265–284). Oxford, U.K.: Oxford University Press.
Pessoa, L. (2009). How do emotion and motivation direct executive control? Trends in Cognitive Sciences, 13, 160–166. doi:10.1016/j.tics.2009.01.006
Pessoa, L., & Adolphs, R. (2010). Emotion processing and the amygdala: From a “low road” to “many roads” of evaluating biological significance. Nature Reviews Neuroscience, 11, 773–782. doi:10.1038/nrn2920
Pochon, J. B., Levy, R., Fossati, P., Lehéricy, S., Poline, J. B., Pillon, B., & Dubois, B. (2002). The neural system that bridges reward and cognition in humans: An fMRI study. Proceedings of the National Academy of Sciences, 99, 5669–5674. doi:10.1073/pnas.082111099
Redgrave, P., Coizet, V., & Reynolds, J. (2010). Phasic dopamine signaling and basal ganglia function. In H. Steiner & K. Tseng (Eds.), Handbook of basal ganglia structure and function (pp. 549–559). Amsterdam: Academic Press.
Rushworth, M., & Behrens, T. (2008). Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience, 11, 389–397. doi:10.1038/nn2066
Sabatinelli, D., Bradley, M. M., Fitzsimmons, J. R., & Lang, P. J. (2005). Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. NeuroImage, 4, 1265–1270. doi:10.1016/j.neuroimage.2004.12.015
Spielberg, J. M., Heller, W., Silton, R. L., Stewart, J. L., & Miller, G. A. (2011a). Approach and avoidance profiles distinguish dimensions of anxiety and depression. Cognitive Therapy and Research, 35, 359–371. doi:10.1007/s10608-011-9364-0
Spielberg, J. M., Miller, G. A., Engels, A. S., Herrington, J. D., Sutton, B. P., Banich, M. T., & Heller, W. (2011b). Trait approach and avoidance motivation: Lateralized neural activity associated with executive function. NeuroImage, 54, 661–670. doi:10.1016/j.neuroimage.2010.08.037
Straube, T., Pohlack, S., Mentzel, H. J., & Miltner, W. H. R. (2008). Differential amygdala activation to negative and positive emotional pictures during an indirect task. Behavioral Brain Research, 191, 285–288. doi:10.1016/j.bbr.2008.03.040
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology: General, 18, 643–662. doi:10.1037/h0054651
Szatkowska, I., Bogorodzki, P., Wolak, T., Marchewka, A., & Szeszkowski, W. (2008). The effect of motivation on working memory: An fMRI and SEM study. Neurobiology of Learning and Memory, 90, 475–478. doi:10.1016/j.nlm.2008.06.001
Taylor, S. F., Welsh, R. C., Wager, T. D., Phan, K. L., Fitzgerald, K. D., & Gehring, W. J. (2004). A functional neuroimaging study of motivation and executive function. NeuroImage, 21, 1045–1054. doi:10.1016/j.neuroimage.2003.10.032
Toglia, M. P., & Battig, W. F. (1978). Handbook of semantic word norms. Hillsdale, NJ: Erlbaum.
Tremblay, L., Worbe, Y., & Hollerman, J. R. (2009). The ventral striatum: A heterogenous structure involved in reward processing, motivation, and decision-making. In J. C. Dreher & L. Tremblay (Eds.), Handbook of reward and decision making (pp. 51–77). Amsterdam: Academic Press.
Volle, E., Pochon, J. B., Lehéricy, S., Pillon, B., Dubois, B., & Levy, R. (2005). Specific cerebral networks for maintenance and response organization within working memory as evidenced by the “double delay/double response” paradigm. Cerebral Cortex, 15, 1064–1074. doi:10.1093/cercor/bhh207
Ward, D. B. (2000). Simultaneous inference for FMRI data. Retrieved July 27, 2006, from http://afni.nimh.nih.gov./pub/dist/doc/manual/AlphaSim.pdf
Watson, D., & Clark, L. A. (1993). Behavioral disinhibition versus constraint: A dispositional perspective. In D. M. Wegner & J. W. Pennebaker (Eds.), Handbook of mental control (pp. 506–527). New York, NY: Prentice Hall.
Watson, D., Clark, L. A., Weber, K., Assenheimer, J. S., Strauss, M. E., & McCormick, R. A. (1995a). Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology, 104, 15–25. doi:10.1037/0021-843X.104.1.15
Watson, D., Weber, K., Assenheimer, J. S., Clark, L. A., Strauss, M. E., & McCormick, R. A. (1995b). Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology, 104, 3–14. doi:10.1037/0021-843X.104.1.3
Williams, J. M. G., Mathews, A., & MacLeod, C. (1996). The emotional Stroop task and psychopathology. Psychological Bulletin, 120, 3–24. doi:10.1037/0033-2909.120.1.3
Woolrich, M. (2008). Robust group analysis using outlier inference. NeuroImage, 41, 286–301. doi:10.1016/j.neuroimage.2008.02.042
Woolrich, M. W., Ripley, B. D., Brady, M., & Smith, S. M. (2001). Temporal autocorrelation in univariate linear modeling fMRI data. NeuroImage, 14, 1370–1386. doi:10.1006/nimg.2001.0931
Yarkoni, T., Barch, D., Gray, J. R., Conturo, T. E., & Braver, T. S. (2009). BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PLOS One, 4, e4257. doi:10.1371/journal.pone.0004257
Author note
This work was supported by the National Institute of Mental Health (Grants R01 MH61358, T32 MH19554, and P50 MH079485). The authors thank Katie Mimnaugh, Kyle Gerst, and Naomi Sadeh for their assistance in the completion of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spielberg, J.M., Miller, G.A., Warren, S.L. et al. Trait motivation moderates neural activation associated with goal pursuit. Cogn Affect Behav Neurosci 12, 308–322 (2012). https://doi.org/10.3758/s13415-012-0088-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-012-0088-8