Published online May 14, 2022. doi: 10.3748/wjg.v28.i18.1996
Peer-review started: November 13, 2021
First decision: January 9, 2022
Revised: January 22, 2022
Accepted: March 26, 2022
Article in press: March 26, 2022
Published online: May 14, 2022
Incidental gallbladder cancer (IGBC) represents 50%-60% of gallbladder cancer cases. Data are conflicting on the role of IGBC diagnosis in oncological outcomes. Some studies suggest that IGBC diagnosis does not affect outcomes, while others that overall survival (OS) is longer in these cases compared to non-incidental diagnosis (NIGBC). Furthermore, some studies reported early tumour stages and histopathologic characteristics as possible confounders, while others not.
To investigate the role of IGBC diagnosis on patients’ overall survival, especially after surgical treatment with curative intent.
Retrospective analysis of all patient referrals with gallbladder cancer between 2008 and 2020 in a tertiary hepatobiliary centre. Statistical comparison of patient and tumour characteristics between IGBC and NIGBC subgroups was performed. Survival analysis for the whole cohort, surgical and non-surgical subgroups was done with the Kaplan-Meier method and the use of log rank test. Risk analysis was performed with univariable and multivariable Cox regression analysis.
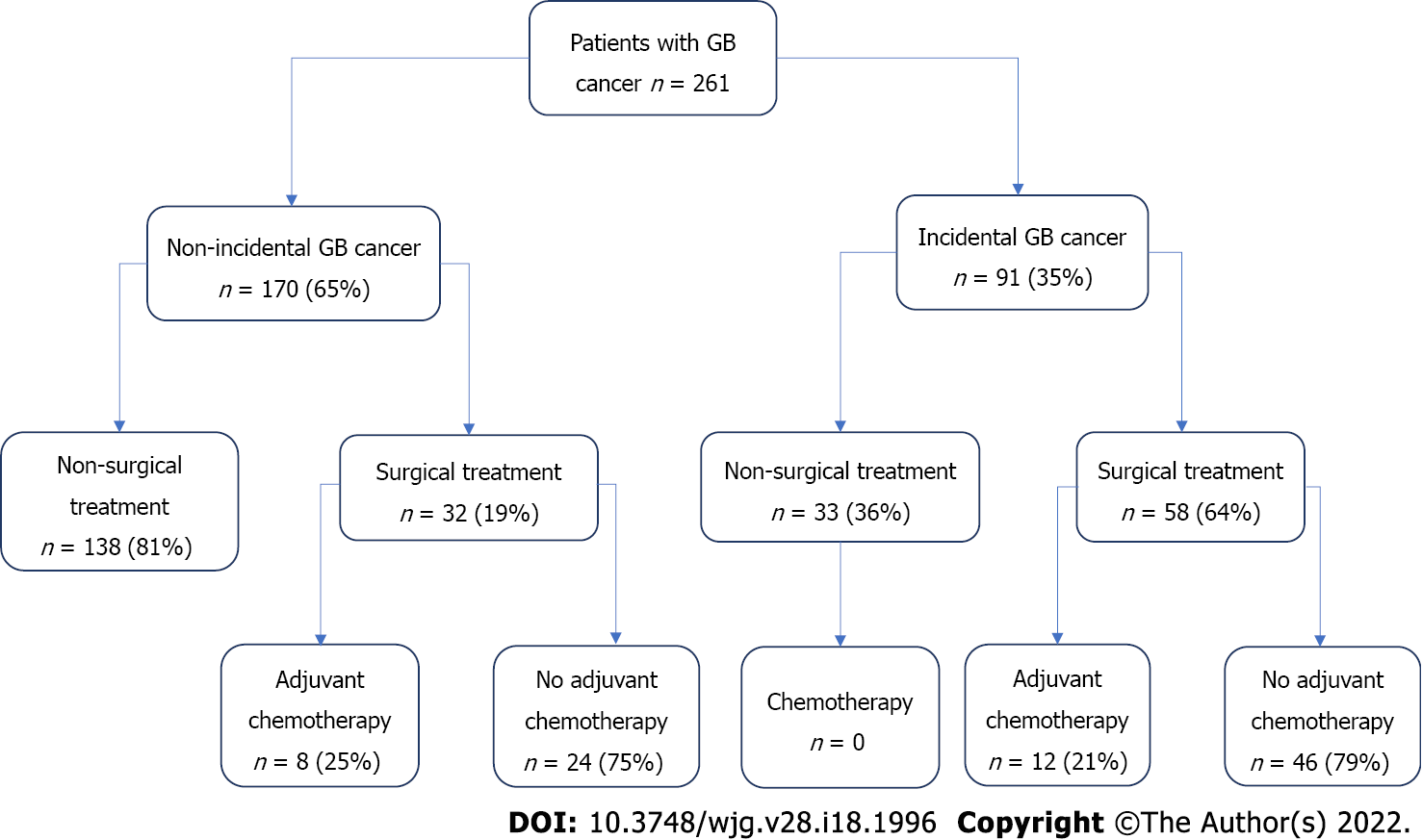
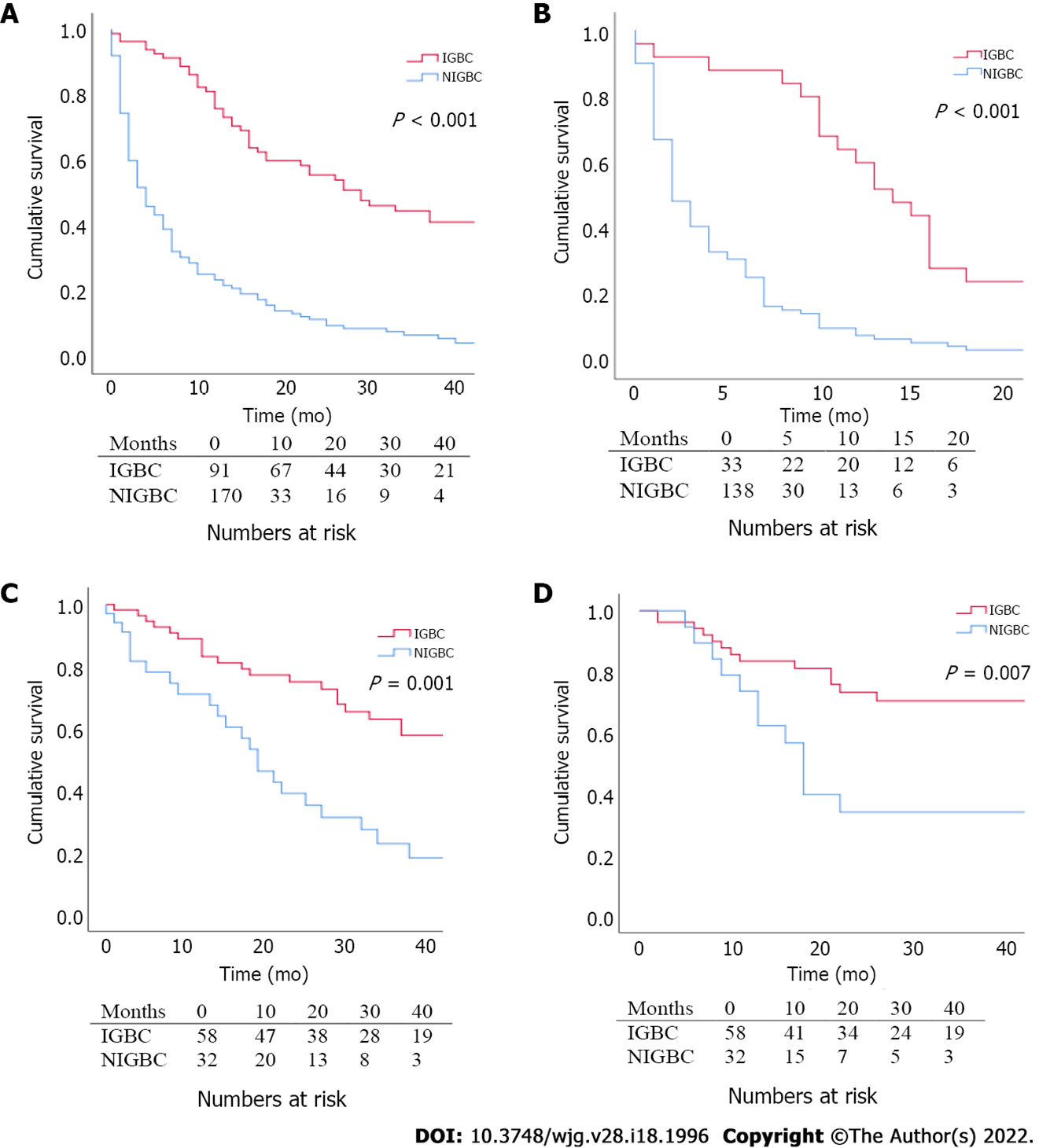
The cohort included 261 patients with gallbladder cancer. 65% of cases had NIGBC and 35% had IGBC. A total of 90 patients received surgical treatment (66% of IGBC cases and 19% of NIGBC cases). NIGBC patients had more advanced T stage and required more extensive resections than IGBC ones. OS was longer in patients with IGBC in the whole cohort (29 vs 4 mo, P < 0.001), as well as in the non-surgical (14 vs 2 mo, P < 0.001) and surgical subgroups (29 vs 16.5 mo, P = 0.001). Disease free survival (DFS) after surgery was longer in patients with IGBC (21.5 mo vs 8.5 mo, P = 0.007). N stage and resection margin status were identified as independent predictors of OS and DFS. NIGBC diagnosis was identified as an independent predictor of OS.
IGBC diagnosis may confer a survival advantage independently of the pathological stage and tumour characteristics. Prospective studies are required to further investigate this, including detailed pathological analysis and molecular gene expression.
Core Tip: Data are conflicting on the role of incidental gallbladder cancer (IGBC) diagnosis in oncological outcomes. Some studies suggest that IGBC diagnosis does not affect outcomes, while others that overall survival (OS) is longer in these cases compared to non-incidental diagnosis (NIGBC). In our study, IGBC diagnosis conferred better OS in all patients with gallbladder cancer, as well as within the surgical and non-surgical groups. Similarly, disease free survival was significantly longer in patients with IGBC. NIGBC diagnosis was identified as an independent predictor of OS along with N stage and resection margin status.
- Citation: Alarabiyat M, Raza SS, Isaac J, Mirza D, Marudanayagam R, Roberts K, Abradelo M, Bartlett DC, Dasari BV, Sutcliffe RP, Chatzizacharias NA. Incidental gallbladder cancer diagnosis confers survival advantage irrespective of tumour stage and characteristics. World J Gastroenterol 2022; 28(18): 1996-2007
- URL: https://www.wjgnet.com/1007-9327/full/v28/i18/1996.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i18.1996
Gallbladder cancer (GBC) is associated with poor prognosis even after treatment, with median overall survival (OS) ranging in the literature between 3 and 22 mo[1,2]. Incidental gallbladder cancer (IGBC) discovered on routine histological examination of gallbladder specimens after cholecystectomy is more common than non-incidental gallbladder cancer (NIGBC) and represents 50%-60% of all cases[3-5]. The prognostic implication of incidental or non-incidental diagnosis in oncological outcomes is still a matter of debate as is the effect of the timing of curative intent resection which is performed as a secondary operation in IGBC.
Published evidence are contradictory with some studies suggesting that incidental diagnosis does not affect survival[5-8], while others showed longer survival with IGBC[9-11]. Earlier tumour stages in the IGBC group have been suggested as a confounding factor for any potential survival benefit[5]. On the contrary, other studies identified a survival benefit in IGBC even after controlling for tumour stage and degree of differentiation[9,10].
The aim of this study was to investigate the role of IGBC diagnosis in patient OS and especially after surgical treatment with curative intent.
This is a retrospective single tertiary centre cohort study between January 2008 and December 2020. The sample included all patients with a histological diagnosis of GBC obtained by surgery or biopsy. The management of all patients was discussed and agreed in the hepatobiliary multidisciplinary (MDT) meeting. IGBC diagnosis was established after histopathological examination of specimens following cholecystectomy for benign aetiology. This was followed by complete staging with a computerized tomography scan of the thorax, abdomen and pelvis (CT-TAP) with subsequent curative intent resection if appropriate. NIGBC diagnosis was made based on imaging and/or biopsy after MDT discussion of referred patients. All patients had staging with CT-TAP, followed by surgery if clinically appropriate. In patients with locally advanced disease, neoadjuvant chemotherapy was administered and resection was contemplated after restaging. Liver magnetic resonance imaging and positron emission tomography scans were used selectively in both groups if liver metastases or extrahepatic disease was suspected on CT. Following surgical resection all patients were referred to oncology for assessment of adjuvant chemotherapy (AC).
Data were collected and recorded for patient's demographics, American society of anesthesiology (ASA) score, extent of surgical resection, histology, chemotherapy, recurrence and survival. The extent of surgery was defined as minor if radical cholecystectomy, gallbladder (GB) bed resection or liver segments IVb/V resection with or without bile duct resection was performed. It also included patients who only had bile duct resection. The resection was defined as major if a major hepatectomy (three or more liver segments) or multi-visceral resection was performed. Recurrence was defined as local/regional (GB bed, hilar lymph nodes), distant or both. The primary outcome of the study was difference in OS between IGBC and NIGBC and the secondary outcome was difference in disease-free survival (DFS).
T-test, Chi-Square, Fisher’s Exact and Mann-Whitney U tests were used as appropriate to compare variables and outcomes between the two groups, with statistical significance set at P < 0.05. Survival analysis was performed with the Kaplan-Meier method and log rank test was used to compare survival curves between the study groups. Univariable and multivariable time to event analyses were performed using the Cox proportional hazard model to determine risk factors for OS and DFS. Variables were subjected to a univariable analysis first and those with P < 0.2 were introduced into a multivariable model. Hazard ratios (HR) and associated 95% confidence intervals (CI) were calculated. A two-tailed P value < 0.05 was considered statistically significant. All statistical analyses were performed using the software package SPSS Statistics for Windows (version 25.0; SPSS Inc., Chicago, IL, United States).
The study cohort comprised of 261 patients, with 35% presenting as IGBC and 65% had non incidental presentation (NIGBC) at the time of diagnosis (Figure 1). Median age was 69 years [interquartile range (IQR) 61-77] and male to female ratio was 1:3. Eighty-one percent of NIGBC and 34% of IGBC patients did not undergo resection. For the majority of these (82%) locally advanced or metastatic disease was the main reason. Other causes included patient’s choice, poor medical status and pathological stage < 1b (where resection is not indicated) (Table 1). Reasons for not having AC after resection were patients’ choice or comorbidities and early tumour stages (CIS, T1/T2, N0) with negative resection margins.
| Reason | IGBC (n = 33, 19%) | NIGBC (n = 138, 81%) | Total number (n = 171)1 |
| Locally advanced inoperable disease | 0 | 41 (30) | 41 (24) |
| Metastatic disease | 21 (64) | 78 (56) | 99 (58) |
| Patient declined surgery | 5 (15) | 3 (2) | 8 (5) |
| Unfit for surgery | 4 (12) | 15 (11) | 19 (11) |
| Surgery not required | 3 (9) | 0 | 3 (2) |
A total of 90 patients had curative intent resection. The type of resection depended on IGBC vs NIGBC diagnosis, pre-operative staging, intra-operative findings, cystic duct margin status and the T stage of IGBC patients. Hepatoduodenal (portal) lymphadenectomy was performed in all patients. For IGBC cases, the median time from the time of the index cholecystectomy to the curative resection was 13.5 wk (IQR: 11-16 wk).
Patient and tumour characteristics are shown on Table 2. Patients with NIGBC had more advanced T stage and underwent more extensive resections compared to those with IGBC. Similarly, N stage approached but did not reach significance. The types of procedures performed are shown on Table 3.
| Variable | IGBC (n = 58) | NIGBC (n = 32) | P value |
| Median age (range) | 63 yr (55.8-71.3) | 66 yr (66.0-71.5) | 0.302 |
| Gender | 0.08 | ||
| Male | 9 (16) | 10 (32) | |
| Female | 49 (84) | 22 (68) | |
| Ethnicity | 0.507 | ||
| Caucasian | 39 (67) | 23 (72) | |
| Asian | 15 (26) | 5 (16) | |
| Black | 3 (5) | 2 (6) | |
| Missing | 1 (2) | 2 (6) | |
| Median BMI (range) | 28.2 (24.3-32.1) | 28.2 (24.2-30.7) | 0.563 |
| ASA score | 0.829 | ||
| 1 | 4 (7) | 2 (6) | |
| 2 | 43 (74) | 23 (72) | |
| 3 | 10 (17) | 6 (19) | |
| Missing | 1 (2) | 1 (3) | |
| Median CCI (range) | 4 (3-5) | 4 (4-6) | 0.635 |
| Extent of surgery | < 0.001 | ||
| Minor resection | 57 (98) | 24 (75) | |
| Major resection | 1 (2) | 8 (25) | |
| T stage | < 0.001 | ||
| CIS | 1 (2) | 1 (3) | |
| T1a | 3 (5) | 0 | |
| T1b | 4 (7) | 1 (3) | |
| T2 | 42 (72) | 12 (38) | |
| T3 | 7 (12) | 15 (47) | |
| T4 | 1 (2) | 3 (9) | |
| N status | 0.051 | ||
| N0 | 41 (71) | 16 (50) | |
| N1/2 | 17 (29) | 16 (50) | |
| Histologic type | 0.746 | ||
| CIS | 1 (2) | 1 (3) | |
| Adenocarcinoma | 55 (94) | 30 (94) | |
| Adeno-squamous | 1 (2) | 1 (3) | |
| Mixed | 1 (2) | 0 | |
| Degree of differentiation (CIS excluded) | 0.614 | ||
| Well | 7 (12) | 4 (13) | |
| Moderate | 30 (52) | 20 (63) | |
| Poor | 12 (21) | 5 (16) | |
| Undifferentiated/unclassified | 8 (14) | 2 (6) | |
| Resection margin | 0.169 | ||
| Negative | 53 (91) | 26 (81) | |
| Positive | 5 (9) | 6 (19) | |
| Cystic duct margin on index cholecystectomy | 6 (10) | N/A | N/A |
| Post-operative complications | 15 (26) | 11 (34) | 0.394 |
| Pattern of recurrence | 0.628 | ||
| Local/regional | 5 (9) | 5 (16) | |
| Distant | 6 (10) | 5 (16) | |
| Local and distant | 2 (3) | 1 (3) | |
| Adjuvant chemotherapy | 12 (21) | 8 (25) | 0.502 |
| Type of surgery | IGBC (n = 58) | NIGBC (n = 32) | Total number (n = 90) |
| Radical cholecystectomy | 0 | 18 (56) | 18 (20) |
| Radical cholecystectomy +extrahepatic bile duct excision | 0 | 6 (19) | 6 (7) |
| Gallbladder bed resection | 36 (62) | 0 | 36 (40) |
| Gallbladder bed resection + extrahepatic bile duct excision | 17 (30) | 0 | 17 (19) |
| Segment IVb + V liver resection | 2 (3) | 0 | 2 (2) |
| Extrahepatic bile duct excision | 2 (3) | 0 | 2 (2) |
| Extended resections: | 4 (4) | ||
| Central hepatectomy + caudate lobe resection + extrahepatic bile duct excision | 0 | 1 (3) | 1 (1) |
| Pancreaticoduodenectomy + Gallbladder bed resection + right hemicolectomy | 0 | 1 (3) | |
| Pancreaticoduodenectomy + right hepatectomy | 0 | 1 (3) | |
| Radical cholecystectomy + right hemicolectomy + partial gastrectomy | 0 | 1 (3) |
For the whole cohort, median OS was longer in patients with IGBC diagnosis, (29 vs 4 mo, P < 0.001). OS of IGBC patients was significantly longer compared to NIGBC patients in the non-surgical group (14 vs 2 mo, P < 0.001), as well as the surgical group (29 vs 16.5 mo, P = 0.001). Similarly, DFS was significantly longer in patients with IGBC (21.5 mo vs 8.5 mo, P = 0.007) (Figure 2).
Univariable Cox regression analysis (Table 4) identified that age, ASA, T stage, N stage, resection margin status and NIGBC diagnosis were significantly related to OS (P < 0.05). On multivariable analysis, N stage, resection margin status and NIGBC diagnosis were identified as independent predictors of survival, increasing the risk of mortality by 3, 5 and 2 times respectively (Table 4).
| Variable | P value | HR (95%CI)
| P value | HR (95%CI)
| P value | HR (95%CI) |
| Univariable analysis | Multivariable analysis | Multivariable analysis (excluding timing of diagnosis variable) | ||||
| Age | 0.011 | 1.04 (1.01-1.07) | 0.453 | 1.02 (0.97-1.08) | 0.153 | 1.04 (0.99-1.10) |
| Gender | 0.96 | 1.01 (0.71-1.44) | - | - | - | - |
| Race | 0.853 | 1.05 (0.61-1.81) | - | - | - | - |
| BMI | 0.601 | 0.983 (0.92-1.05) | - | - | - | - |
| ASA score | 0.024 | 1.96 (1.09-3.52) | 0.121 | 2.26 (0.81-6.31) | 0.061 | 2.53 (0.96-6.66) |
| CCI score | 0.064 | 1.19 (0.99-1.42) | 0.66 | 0.91 (0.58-1.41) | 0.269 | 0.78 (0.50-1.21) |
| T stage | < 0.001 | 2.73 (1.72-4.34) | 0.913 | 1.04 (0.56-1.90) | 0.088 | 1.62 (0.93-2.81) |
| N stage | < 0.001 | 5.65 (2.84-11.25) | < 0.001 | 3.07 (1.72-5.46) | 0.001 | 3.59 (1.72-7.49) |
| R status | < 0.001 | 7.35 (3.38-15.95) | < 0.001 | 5.26 (2.10-13.13) | 0.012 | 3.00 (1.27-7.07) |
| Post-operative complications | 0.885 | 1.05 (0.53-2.09) | - | - | - | - |
| Non-incidental diagnosis | 0.004 | 2.41 (1.32-4.38) | 0.026 | 2.25 (1.10-4.58) | Not included in analysis | Not included in analysis |
| Degree of differentiation | 0.443 | 1.40 (0.61-3.21) | - | - | - | - |
| Adjuvant chemotherapy | 0.87 | 1.07 (0.47-2.44) | - | - | - | - |
Similarly, T stage, N stage, resection margin status and NIGBC diagnosis were identified to be associated with worse DFS on univariable analysis, however only N stage and resection margin status were found to be independent predictors on multivariable analysis (Table 5).
| Variable | P value | HR (95%CI)
| P value | HR (95%CI)
| P value | HR (95%CI) |
| Univariable analysis | Multivariable analysis | Multivariable analysis (excluding timing of diagnosis variable) | ||||
| Age | 0.275 | 1.02 (0.98-1.06) | - | - | - | - |
| Gender | 0.732 | 0.85 (0.34-2.13) | - | - | - | - |
| Race | 0.997 | 1.13 (0.15-8.51) | - | - | - | - |
| BMI | 0.782 | 0.99 (0.91-1.07) | - | - | - | - |
| ASA score | 0.817 | 0.91 (0.42-1.98) | - | - | - | - |
| CCI score | 0.842 | 0.98 (0.76-1.26) | - | - | - | - |
| T stage | 0.021 | 2.06 (1.12-3.82) | 0.504 | 1.26 (0.64-2.47) | 0.21 | 1.53 (0.79-2.97) |
| N stage | 0.001 | 4.08 (1.83-9.08) | 0.006 | 3.18 (1.39-7.26) | 0.008 | 3.08 (1.35-7.06) |
| R status | < 0.001 | 11.05 (3.31-36.94) | 0.006 | 5.76 (1.67-19.88) | 0.002 | 6.95 (1.98-24.34) |
| Post-operative complications | 0.404 | 0.63 (0.22-1.85) | - | - | - | - |
| Non-incidental diagnosis | 0.011 | 2.78 (1.26-6.12) | 0.09 | 2.13 (0.89-5.09) | Not included in analysis | Not included in analysis |
| Degree of differentiation | 0.211 | 0.65 (0.33-1.28) | - | - | - | - |
| Adjuvant chemotherapy | 0.457 | 1.42 (0.56-3.58) | - | - | - | - |
In an effort to statistically investigate if NIGBC diagnosis acted as a confounding factor for the T stage of the disease despite the use of time-dependent regression analysis, models without this parameter were produced. Again, only N stage and margin status were identified as independent prognostic factors for OS and DFS, while T stage was not (Tables 4 and 5).
GBC is a rare malignancy with unfavorable prognosis despite the advances in oncological treatments[1,2,12]. The timing of GBC diagnosis, whether incidental after cholecystectomy for benign causes or pre-operative on imaging and/or biopsy, has been previously under investigation for the potential effect in outcomes, however evidence is scarce. Violation of the anatomical planes around the tumour and incomplete clearance during the index laparoscopic cholecystectomy with residual disease in 35%-46% of the patients have been proposed as factors responsible for adverse oncological outcomes in IGBC patients[13-15]. Furthermore, inadvertent GB perforation during cholecystectomy has been reported in up to 22%[16,17], and this could theoretically lead to tumour seeding and metastatic disease. Interestingly, the site of invasion of local disease has also been shown to play an important role in T2 disease[15]. Nonetheless, some published evidence suggested that IGBC diagnosis confers favourable survival, regardless of the stage or degree of differentiation of the disease[9,11]. On the other hand, in other studies, NIGBC diagnosis did not adversely affect survival[5-8]. In a meta-analysis of 51 studies by Pyo et al[18], IGBC patients had favorable survival in comparison to NIGBC. However, not all studies included in this meta-analysis were able to show the same difference between both groups.
Sixty five percent of referred patients in our cohort had a preoperative radiological diagnosis of GBC. Sixty six percent of all patients did not proceed to an oncological resection (81% of NIGBC and 36% of IGBC patients), 16% of these due to locally advanced disease (all NIGBC patients) and 38% due to metastatic disease on staging imaging (23% of IGBC and 46% of NIGBC patients). Only 3% of IGBC were stage T1a or below and therefore, no further resection was indicated. Of note only 7% of patients were deemed unfit for surgical treatment. It is clear that the majority of the patients that did not proceed to management with curative intent were due to the late presentation of the disease, a fact that is well described for GBC[19]. The percentage of patients who had AC after curative intent surgery was low (22%). This is comparable with the published literature of around 24%[20]. The reasons for this may include patients’ choice and comorbidities, however, may also be attributed to the change in recommended best practice over the years of the study. According to a previously published expert consensus statement, AC was considered only in patients with high risk pathologic features: T3-T4 stages, metastatic lymph nodes and positive resection margins[21]. However, after the BILCAP trial showing improved survival with capecitabine (36.4 mo to 51.1 mo; P = 0.028), it is currently recommended that all patients with resected biliary tract malignancy, including GB cancer, receive 6 mo of adjuvant capecitabine[22]. The results of the currently ongoing ACTICCA-1 trial are eagerly awaited and will provide further evidence if the combination chemotherapy of gemcitabine and cisplatin is superior to capecitabine monotherapy in the adjuvant setting.
Our data suggests that NIGBC diagnosis adversely affected oncological outcomes. NIGBC patients were more likely to have higher stages of the disease (T3/4), consequently undergoing more extensive resections. The range of oncological procedures for selected cases included multi-visceral resections and major hepatectomies to achieve histologically clear resection margins. Routine performance of such procedures is not associated with survival benefit and has high morbidity rates; however, it is still indicated when the vascular inflow or resection margins are/may be compromised[23-25]. Positive lymph node status was more common in patients with NIGBC with the difference approaching statistical significance. OS of all patients with NIGBC diagnosis was substantially worse than those with IGBC and this was also noted in both surgical and non-surgical subgroups. Furthermore, in the surgical subgroup, NIGBC diagnosis was identified as an independent predictor of OS; doubling the risk of mortality. Stronger predictors were pN stage and margin status, increasing the risk by 3 and 5 times respectively. These findings persisted when models computed that accounted for the possibility of NIGBC diagnosis acting as a confounding factor for T stage (by not including this parameter in the analysis), indicating that it is not true. This seemingly paradoxical observation may be explained by the presence of micro-metastases in early stages of GBC [26], which would affect and in the end dictate OS and DFS, rather than pT stage. Similar were the results for DFS, with N stage and margin status conferring higher relative risk of recurrence, while NIGBC diagnosis approached but did not reach significance.
Lymph node status is an important prognostic factor in GBC. Widmann et al[27] in a meta-analysis of 18 observational studies which included more than 27000 patients, has shown that lymph node involvement has significant effect on OS and DFS. Lymphadenectomy also was associated with better OS and DFS in patients with T1b, T2 and T3 disease. This was not clearly demonstrated with T4 disease due to the low number of cases undergoing curative resection in this stage. Lymph node micrometastases, defined as disease detected on immune-histochemical staining, have also been described to correlate with the pathologic N stage of the disease and disease prognosis[28]. Nonetheless, the significance of the extent of lymphadenectomy and lymph node yield is controversial in the published literature, with data from two studies suggesting that harvesting more than four lymph nodes during surgery is associated with improved survival[29,30], whilst a third study concluded that lymph node yield does not correlate with improved survival[31]. These differences may be explained by the differences in pathological reporting (higher lymph node yield may result in more accurate pN staging) and/or variations in the non-surgical part of the patients’ management, such differences in the administration of neoadjuvant and adjuvant chemotherapy, regimens, duration etc.
Overall, our data suggest that the timing of diagnosis of GBC may play a significant role in the oncological outcomes. Due to the retrospective nature of the study and the long study period, data on the site of invasion (hepatic vs peritoneal) were not available, hence this could not be investigated as a possible explanation[32,33]. Another possibility is a difference in the genetic profile of the tumours which could account for different behavior, such as early micrometastases, that cannot be captured by the common radiological investigations and standard pathological parameters of stage and differentiation [34]. However, this cannot be investigated by the current study and would require a prospective molecular study design.
The limitations of the study include its single centre retrospective methodology. The long study period also included differences and evolution in the surgical approach during the oncological resection, with more bile duct resections done during the early study period to achieve a negative margin and a greater lymph node yield. In the later years, bile duct resection was only performed in the presence of a positive cystic duct margin. Nonetheless, this is the first study to include all patients referred for GBC to a tertiary regional centre, rather than only the ones receiving surgical treatment, therefore providing outcome data in an intention to treat basis over the whole referral cohort including the patients that did not receive surgical treatment. It is also one of few studies to demonstrate the effect of non-incidental diagnosis on the oncological outcomes.
Conclusively, the presented data suggest that IGBC diagnosis may confer a survival advantage, including patients that received surgical treatment, independently of the pathological stage and tumour characteristics. Prospective studies are required to investigate the reasons behind this, including detailed pathological analysis and molecular gene expression.
Incidental gallbladder cancer (IGBC) represents 50%-60% of gallbladder cancer cases. Data are conflicting on the role of IGBC diagnosis in oncological outcomes. Some studies suggest that IGBC diagnosis does not affect outcomes, while others that overall survival (OS) is longer in these cases compared to non-incidental diagnosis (NIGBC). Furthermore, some studies reported early tumour stages and histopathologic characteristics as possible confounders, while others not.
GB cancer is an uncommon malignancy with poor survival. Data suggest whether the diagnosis is incidental or not may play a role in the oncological outcomes. Confirmation of this observation may lead in further research aiming to better identify the reasons and refining the treatment strategy based on the presenting diagnosis.
This study aimed to investigate the role of IGBC diagnosis on patients’ overall survival, especially after surgical treatment with curative intent.
This is a retrospective analysis of all patient referrals with gallbladder cancer between 2008 and 2020 in a tertiary hepatobiliary centre. All patients had complete staging and were discussed in the multidisciplinary meeting prior to deciding on the treatment plan. Demographic, surgical and tumour variables were collected and compared between patients with IGBC and NIGBC using the appropriate statistical tests. Survival curves for OS and DFS were created using Kaplan-Meier method and compared with the log rank test. Risk analysis for independent predictors of OS and DFS was performed with univariable time to event analysis using the Cox proportional hazard model. Factors with a P value of < 0.200 were entered into a multivariable model and independent predictors (those with P < 0.05) were indentified. All statistical analysis was done using the software SPSS for Windows (Version 25.0, SPSS Inc., Chicago, IL, United States).
261 patients with GB cancer were included. Almost one-third pf patients had IGBC (91/261 patients) and two-thirds had NIGC. A total of 90/261 (34%) patients proceeded to have oncological resection. Metastatic disease was the most common reason for not having oncological resection. Patients with NIGBC were more likely to have advanced T stages of the disease and required more extensive resections than patients with IGBC. Survival analysis shows that patients with IGBC had better OS than patients with NIGBC in the whole cohort (29 vs 4 mo, P < 0.001), as well as in the non-surgical (14 vs 2 mo, P < 0.001) and surgical subgroups (29 vs 16.5 mo, P = 0.001). DFS was similarly better in patients with IGBC who underwent oncological resection (21.5 mo vs 8.5 mo, P = 0.007). After univariable and multivariable risk analysis, N stage, resection margin status and non-incidental diagnosis were identified as independent predictors of OS. N stage and resection margin status were also independent predictors of DFS. Within the limitations of a retrospective single centre study, our data suggest that difference in the oncological outcomes between the two groups cannot be solely explained by differences in pathologic or tumour features.
Our study suggests that IGBC diagnosis may confer a survival advantage, including patients that received surgical treatment, independently of the pathological stage and tumour characteristics. Prospective studies are required to investigate the reasons behind this, including detailed pathological analysis and molecular gene expression.
Published evidence is still contradicting. The theory that IGBC and NIGBC are two distinct variants of the same disease remains to be proven by detailed pathologic assessment and research in cancer molecular genetics.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aktas S, Turkey; Garg PK, India; Kai K, Japan; Yasukawa K, Japan S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Jaruvongvanich V, Yang JD, Peeraphatdit T, Roberts LR. The incidence rates and survival of gallbladder cancer in the USA. Eur J Cancer Prev. 2019;28:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Chen C, Geng Z, Shen H, Song H, Zhao Y, Zhang G, Li W, Ma L, Wang L. Long-Term Outcomes and Prognostic Factors in Advanced Gallbladder Cancer: Focus on the Advanced T Stage. PLoS One. 2016;11:e0166361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Shih SP, Schulick RD, Cameron JL, Lillemoe KD, Pitt HA, Choti MA, Campbell KA, Yeo CJ, Talamini MA. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245:893-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH, O'Reilly EM. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008;98:485-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 283] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 5. | Löhe F, Meimarakis G, Schauer C, Angele M, Jauch KW, Schauer RJ. The time of diagnosis impacts surgical management but not the outcome of patients with gallbladder carcinoma. Eur J Med Res. 2009;14:345-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 300] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Yip VS, Gomez D, Brown S, Byrne C, White D, Fenwick SW, Poston GJ, Malik HZ. Management of incidental and suspicious gallbladder cancer: focus on early referral to a tertiary centre. HPB (Oxford). 2014;16:641-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Cavallaro A, Piccolo G, Di Vita M, Zanghì A, Cardì F, Di Mattia P, Barbera G, Borzì L, Panebianco V, Di Carlo I, Cavallaro M, Cappellani A. Managing the incidentally detected gallbladder cancer: algorithms and controversies. Int J Surg. 2014;12 Suppl 2:S108-S119. [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Mazer LM, Losada HF, Chaudhry RM, Velazquez-Ramirez GA, Donohue JH, Kooby DA, Nagorney DM, Adsay NV, Sarmiento JM. Tumour characteristics and survival analysis of incidental vs suspected gallbladder carcinoma. J Gastrointest Surg. 2012;16:1311-1317. [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Cha BH, Bae JM. Comparison of clinical outcomes of incidental and non- incidental gallbladder cancers: a single-centre cross- sectional study. Asian Pac J Cancer Prev. 2014;15:1281-1283. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Ethun CG, Le N, Lopez-Aguiar AG, Pawlik TM, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Krasnick BA, Weber SM, Salem A, Martin RCG, Scoggins CR, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Russell MC, Maithel SK. Pathologic and Prognostic Implications of Incidental versus Nonincidental Gallbladder Cancer: A 10-Institution Study from the United States Extrahepatic Biliary Malignancy Consortium. Am Surg. 2017;83:679-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Bacalbasa N, Balescu I, Dima S and Popescu I. Surgical Advances in the Treatment of Gallbladder Carcinoma at Different Stages, Bile Duct Cancer, Luis Rodrigo, IntechOpen, 2019. [DOI] [Cited in This Article: ] |
| 13. | Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, Adams RB, Staley CA, Trindade EN, Schulick RD, Choti MA, Capussotti L. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11:1478-86; discussion 1486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | de Savornin Lohman EAJ, van der Geest LG, de Bitter TJJ, Nagtegaal ID, van Laarhoven CJHM, van den Boezem P, van der Post CS, de Reuver PR. Re-resection in Incidental Gallbladder Cancer: Survival and the Incidence of Residual Disease. Ann Surg Oncol. 2020;27:1132-1142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Vega EA, Vinuela E, Okuno M, Joechle K, Sanhueza M, Diaz C, Jarufe N, Martinez J, Troncoso A, Diaz A, Chun YS, Tzeng CD, Lee JE, Vauthey JN, Conrad C. Incidental vs non-incidental gallbladder cancer: index cholecystectomy before oncologic re-resection negatively impacts survival in T2b tumors. HPB (Oxford). 2019;21:1046-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Goetze TO, Paolucci V. Use of retrieval bags in incidental gallbladder cancer cases. World J Surg. 2009;33:2161-2165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Lee HY, Kim YH, Jung GJ, Roh YH, Park SY, Kang NU, Yoon SH, Cho JH, Roh MH, Han SY, Lee SW, Baek YH, Jeong JS. Prognostic factors for gallbladder cancer in the laparoscopy era. J Korean Surg Soc. 2012;83:227-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Pyo JS, Son BK, Lee HY, Oh IW, Chung KH. Incidental Carcinoma after Cholecystectomy for Benign Disease of the Gallbladder: A Meta-Analysis. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Andrén-Sandberg A. Diagnosis and management of gallbladder cancer. N Am J Med Sci. 2012;4:293-299. [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Kemp Bohan PM, Kirby DT, Chick RC, Bader JO, Clifton GT, Vreeland TJ, Nelson DW. Adjuvant Chemotherapy in Resectable Gallbladder Cancer is Underutilized Despite Benefits in Node-Positive Patients. Ann Surg Oncol. 2021;28:1466-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V, Coimbra FJ, Jarnagin WR. Gallbladder cancer: expert consensus statement. HPB (Oxford). 2015;17:681-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 278] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 22. | Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, El-Khoueiry A, Feng M, Katz MHG, Primrose J, Soares HP, Valle J, Maithel SK. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol. 2019;37:1015-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 23. | D'Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16:806-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Araida T, Higuchi R, Hamano M, Kodera Y, Takeshita N, Ota T, Yoshikawa T, Yamamoto M, Takasaki K. Hepatic resection in 485 R0 pT2 and pT3 cases of advanced carcinoma of the gallbladder: results of a Japanese Society of Biliary Surgery survey--a multicenter study. J Hepatobiliary Pancreat Surg. 2009;16:204-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | D'Souza MA, Valdimarsson VT, Campagnaro T, Cauchy F, Chatzizacharias NA, D'Hondt M, Dasari B, Ferrero A, Franken LC, Fusai G, Guglielmi A, Hagendoorn J, Hidalgo Salinas C, Hoogwater FJH, Jorba R, Karanjia N, Knoefel WT, Kron P, Lahiri R, Langella S, Le Roy B, Lehwald-Tywuschik N, Lesurtel M, Li J, Lodge JPA, Martinou E, Molenaar IQ, Nikov A, Poves I, Rassam F, Russolillo N, Soubrane O, Stättner S, van Dam RM, van Gulik TM, Serrablo A, Gallagher TM, Sturesson C; E-AHPBA scientific and research committee. Hepatopancreatoduodenectomy -a controversial treatment for bile duct and gallbladder cancer from a European perspective. HPB (Oxford). 2020;22:1339-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Endo I, Shimada H, Takimoto A, Fujii Y, Miura Y, Sugita M, Morioka D, Masunari H, Tanaka K, Sekido H, Togo S. Microscopic liver metastasis: prognostic factor for patients with pT2 gallbladder carcinoma. World J Surg. 2004;28:692-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Widmann B, Warschkow R, Beutner U, Weitzendorfer M, Ukegjini K, Schmied BM, Tarantino I, Steffen T. Effect of lymphadenectomy in curative gallbladder cancer treatment: a systematic review and meta-analysis. Langenbecks Arch Surg. 2020;405:573-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Tanabe M, Endo I, Masunari H, Sugita M, Morioka D, Ishikawa T, Ichikawa Y, Shimada H. Is lymph-node micrometastasis in gallbladder cancer a significant prognostic factor? Hepatogastroenterology. 2012;59:31-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 29. | Ito H, Ito K, D'Angelica M, Gonen M, Klimstra D, Allen P, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Tran TB, Nissen NN. Surgery for gallbladder cancer in the US: a need for greater lymph node clearance. J Gastrointest Oncol. 2015;6:452-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 21] [Reference Citation Analysis (0)] |
| 31. | Ong SL, Garcea G, Thomasset SC, Neal CP, Lloyd DM, Berry DP, Dennison AR. Ten-year experience in the management of gallbladder cancer from a single hepatobiliary and pancreatic centre with review of the literature. HPB (Oxford). 2008;10:446-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Lee W, Jeong CY, Jang JY, Kim YH, Roh YH, Kim KW, Kang SH, Yoon MH, Seo HI, Yun SP, Park JI, Jung BH, Shin DH, Choi YI, Moon HH, Chu CW, Ryu JH, Yang K, Park YM, Hong SC. Do hepatic-sided tumours require more extensive resection than peritoneal-sided tumours in patients with T2 gallbladder cancer? Surgery. 2017;162:515-524. [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Lee H, Choi DW, Park JY, Youn S, Kwon W, Heo JS, Choi SH, Jang KT. Surgical Strategy for T2 Gallbladder Cancer According to Tumor Location. Ann Surg Oncol. 2015;22:2779-2786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Wardell CP, Fujita M, Yamada T, Simbolo M, Fassan M, Karlic R, Polak P, Kim J, Hatanaka Y, Maejima K, Lawlor RT, Nakanishi Y, Mitsuhashi T, Fujimoto A, Furuta M, Ruzzenente A, Conci S, Oosawa A, Sasaki-Oku A, Nakano K, Tanaka H, Yamamoto Y, Michiaki K, Kawakami Y, Aikata H, Ueno M, Hayami S, Gotoh K, Ariizumi SI, Yamamoto M, Yamaue H, Chayama K, Miyano S, Getz G, Scarpa A, Hirano S, Nakamura T, Nakagawa H. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol. 2018;68:959-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 226] [Article Influence: 37.7] [Reference Citation Analysis (1)] |