INTRODUCTION
Over 1 million people die from colorectal cancer (CRC) every year worldwide. According to the statistics by the United States National Cancer Institute, CRC is the third most common cancer in both men and women[1]. The risk of lymph node metastasis in patients with rectal cancer is 29%-52%[2-4]. Lymph node metastasis has predictive value for postoperative local recurrence and distant metastasis. High-resolution magnetic resonance imaging (MRI) is the preferred clinical imaging method to evaluate lymph node metastasis of rectal cancer[5]. Rectal MRI can accurately evaluate the tumor location, tumor stage, invasion depth, extramural vascular invasion (EMVI), and circumferential resection margin[6-8]. Accurate MRI assessment is of great significance to patients with rectal cancer, as it is related to the patients' prognosis[9]. It can be used to make a reasonable clinical treatment plan.
Nowadays, with the rapid development of advanced technology, artificial intelligence (AI) is being used in many fields. AI, represented by machine learning, is being increasingly used in the medical field too[10-12]. Machine learning-based AI uses big data collection and analysis to make effective decisions and predictions about new situations. In terms of algorithm application, artificial neural networks, support vector machines (SVM), and kernel methods are what are mainly being used[13-18] (Figure 1). With the increasing application of AI in the medical field, MRI based on big datasets helps in the provision of systematic and precise medical services to patients with rectal cancer in the treatment process.
Figure 1 Relationship between artificial neural networks and support vector machines in artificial intelligence.
ANN: Artificial neural networks; SVM: Support vector machines.
MRI-BASED AI MODEL IMPROVES THE ACCURACY OF STAGING RECTAL CANCER
Lymphatic spread is an important metastatic route of rectal cancer, and many studies have reported that lymph node metastasis increases the risk of local recurrence in patients. Only 10% of the patients with stage T1 rectal cancer had lymph node metastasis. More than 40% of rectal cancer patients with lymph node metastasis did not have distant metastasis when local recurrence occurred[19,20]. If a metastatic lymph node can be identified before surgery, it is necessary and reasonable to perform radical surgery involving regional lymph node dissection for these patients. According to the National Comprehensive Cancer Network guidelines and clinical practice, neoadjuvant chemoradiotherapy is recommended for patients with locally advanced rectal cancer. Therefore, accurate evaluation by using MRI to stage the disease is crucial for clinical decision-making.
High-resolution MRI has many advantages for clinically staging rectal cancer[21]. The images are reviewed by two radiologists who are experts in clinical work. It is time-consuming to make an accurate diagnosis. There is an inevitable subjective bias among different physicians[22]. Girshick et al [23] put forward faster region-based convolutional neural network (“Faster R-CNN”) in 2016, which was widely used in the simulation of medical image diagnosis. Ding et al[24] developed an AI model based on the “Faster R-CNN” algorithm to detect the lymph nodes of patients with rectal cancer. The accuracy of the model was verified by multicenter clinical practice. The MRI data of 414 CRC patients with pelvic lymph node metastasis were first identified using the R-CNN system, and the results were compared to those of radiologists and pathologists. The consistency between radiologists and the AI model was 0.912. The consistency of the AI model and pathology diagnosis was 0.448. The consistency between radiologists and pathologists was only 0.134. The conclusion was that the AI model based on “Faster R-CNN” was superior to the radiologists in predicting pelvic lymph node metastasis in rectal cancer patients, but its accuracy was inferior to that of the pathologists.
Lymph node detection is more complicated due to obvious individual differences in lymph node locations and sizes. He et al[25] proposed the “Mask R-CNN” that has excellent data processing ability. Multiparametric MRI (mpMRI) includes images such as T2WI and DWI. Zhao et al[26] attempted to combine mpMRI and “Mask R-CNN” into an automatic lymph node detection system. The sensitivity for lymph node detection in this model was 80%. The results are similar to those of Barbu et al (80%) and Feuersteins et al (82.1%), which were higher than those of Kitasaka et al (57%) and Feulner et al (65.4%)[27-29]. The positive predictive value of the AI model for lymph node detection was 73.5%, which was significantly higher than that of the other four studies. The average time required by the AI model to detect a patient was 1.43 s. The average time required for a radiologist with nine years of experience at the center was 134.4 s. The AI model reduces the time cost to some extent. The conclusion is that the AI detection system can improve the efficiency of lymph node detection, especially for lymph nodes ≥ 3 mm. Based on this study, we can infer that R-CNN combined with multi-parameter MRI can better improve the efficiency of lymph node detection.
A single-center retrospective study reported that high-resolution MRI based on an AI model can be used to evaluate the involvement of circumferential resection margins in rectal cancer[30]. A total of 240 CRC patients with positive circumferential resection margins were included, and image training was carried out based on the regional convolutional neural network AI model. In the training set, the ratio of positive to negative images was 1:2. The diagnosis results of the AI platform were compared with those of a senior radiologist. The accuracy, sensitivity, and specificity of the model were 0.932, 0.838, and 0.956, respectively. The area under the curve (AUC) was 0.953. This study suggested high-resolution MRI based on an AI model has a good prospect of application in evaluation for the involvement of circumferential resection margins in rectal cancer.
Concurrent peritoneal metastasis (PM) may occur in 5%–15% of CRC patients, and heterochronous peritoneal metastasis may occur in approximately 20% of CRC patients. The sensitivity of computed tomography (CT) to PM was positively correlated with lesion size, especially for lesions < 0.5 cm. The sensitivity was between 11% and 70%[31]. It is difficult for radiologists to detect simultaneous peritoneal metastasis in CRC. Yuan et al[32] constructed an AI model based on an SVM classifier using the RESnet-3D algorithm to evaluate simultaneous peritoneal metastatic carcinoma of CRC. In this model, CT images of 54 patients with peritoneal metastatic cancer and 76 patients with CRC without peritoneal metastatic cancer were included in the training set. The RESnet-3D features were combined with 12 specific signs of peritoneal metastatic cancer in order to construct an SVM classifier. The sensitivity, specificity, and accuracy of the AI model for PM detection were 93.75%, 94.44%, and 94.11%, respectively. It is therefore much better than conventional enhanced CT. It is believed that there will be a more developed image diagnosis system based on the AI model in the future that will make staging diagnosis in MR images more efficient, accurate, and stable, and reduce the judgment error caused by the differences in radiologists' diagnostic abilities to a certain extent.
APPLICATION OF AN MRI-BASED AI MODEL IN RADIOTHERAPY
It is a very important yet time-consuming process to manually draw tumor volume contours in rectal cancer radiotherapy. Wang et al[33] aimed to develop an automatic tumor segmentation algorithm based on deep learning to segment rectal tumors using T2-weighted MRI images. This study included 93 patients with locally advanced rectal cancer (cT3-4 and/or cN1-2) who underwent surgical treatment following neoadjuvant chemoradiotherapy (nCRT). The model was trained in two stages: Tumor recognition and tumor segmentation. The data sets were randomly divided into training sets (90%) and validation sets (10%). Four indices, namely, the Hausdorff distance (HD), average surface distance (ASD), Dice index (DSC), and Jaccard index (JSC), were calculated to evaluate the advantages and disadvantages of automatic and manual segmentation. The DSC, JSC, HD, and ASD results of the AI model and those of radiologists (manual segmentation) were compared as follows: DSC [(0.74 ± 0.14) vs (0.71 ± 0.13)] (P = 0.42), JSC [0.60 ± 0.16 vs 0.57 ± 0.15] (P = 0.35), HD [20.44 ± 13.35 vs 14.91 ± 7.62] (P = 0.35), and ASD [3.25 ± 1.69 mm vs 2.67 ± 1.46] (P = 0.16), which showed no statistically significant difference between the automatic segmentation group and the manual segmentation group. A total of 140 patients with locally advanced rectal cancer were included in another study[34]. The multi-parameter MRI images of patients were identified using an AI model based on a deep learning algorithm. Its deep learning algorithm was mainly based on a convolutional neural network. The model was used to segment the tumor after recognition of the MRI image. In this study, the results of the AI model were compared with those of two senior radiologists, and the AUC was 0.99. The AI model can be accurate in tumor location and tumor segmentation in CRC patients.
CNNs are an advanced algorithm applied at present. However, repeated pooling and undersampling can reduce the resolution of the features and may result in the loss of detailed information. Men et al[35] combined cascaded atrous convolution (CAC) and spatial pyramid pooling module (SPP), and developed a new-type CNN (CAC-SPP) that could further improve the accuracy of tumor segmentation. The detection efficiency of the CAC-SPP model was superior to that of the algorithm models U-NET and RESnet-101 (P < 0.05). The detection speed of the CAC-SPP model was similar to that of RESnet-101, which was faster than that of U-NET. In short, the CAC-SPP model with high-resolution MRI can improve the accuracy of rectal tumor segmentation by obtaining more tumor features.
APPLICATION OF AN MRI-BASED AI MODEL IN THE EVALUATION OF STAGE AFTER NEOADJUVANT THERAPY FOR RECTAL CANCER
Currently, total mesorectal excision has become the standard treatment for locally advanced rectal cancer[36]. In clinical practice, there are often patients with rectal cancer of the same stage for whom nCRT has different curative effects. Some patients achieved a pathological complete response (PCR) after nCRT, while others did not respond to treatment and some even had progressive disease. Over 30% of the patients could not benefit from nCRT[37]. For the patients who had a PCR after neoadjuvant therapy, the risk of local recurrence was relatively low. For these patients, minimally invasive and anal sphincter sparing surgery, or even the "watch and see" strategy is the mainstream opinion at present. However, due to the limitations of the current diagnostic methods, clinical complete response is not equivalent to PCR. Clinicians should focus on improving the accuracy of MRI by using high-resolution MRI and selecting a more effective MR contrast agent. The increase in the MR sequence increases the workload of the radiologists and leads to the consumption of imaging examination time. In addition, different hospitals have different clinical diagnostic abilities and different radiologists are inconsistent in making imaging diagnoses. Combined with radiomics and convolutional neural networks, increasing research has been conducted on construction of image recognition models based on AI in order to solve these problems. The use of MR images based on AI models to evaluate the response to neoadjuvant therapy in patients with locally advanced rectal cancer and identify patients with a complete pathological response in the early stages will be of great significance for the realization of individualized precision therapy for CRC in the future.
Ferrari et al[38] developed an AI model based on high-resolution MRI, hoping that this model can help identify patients with a complete pathological response after neoadjuvant therapy in locally advanced rectal cancer, and can identify the non-responders after neoadjuvant therapy in the early stages. This study included 55 patients with locally advanced rectal cancer who received surgical treatment after neoadjuvant therapy. The patients were randomly divided into two groups, one for the training cohort and the other for the validation cohort. In the training cohort, the MRI images of patients were combined with the histopathological results using the algorithm, and the model performance was verified in the validation cohort. The AUC for identifying complete reaction in the validation set was 0.86 (95%CI: 0.70. 0.94), and the AUC for identifying the non-responders was 0.83 (95%CI: 0.71, 0.92). In conclusion, the AI model based on high-resolution MR images has achieved good results, which is helpful in identifying patients with complete remission after neoadjuvant therapy and patients with no response to neoadjuvant therapy in the early stages.
Zhang et al[39] developed a deep learning algorithm model based on T2 weighting and diffusion kurtosis MR images. A prospective study was carried out based on this model, involving 383 patients with 308 as the training set and 104 as the verification set. The model aimed to evaluate PCR, tumor regression grade, and down-staging of locally advanced rectal cancer after neoadjuvant therapy. The study results showed that the AUC of the validation set in predicting PCR was 0.99. The AUC for T-cell receptor gamma and T staging assessments was 0.70 and 0.79, respectively. With the assistance of the deep learning algorithm model, the error rate of radiology physicians in reading films was significantly reduced. A deep learning model based on diffusion kurtosis MR can evaluate the PCR of patients after nCRT and assist radiologists in evaluating the chemotherapy responses after neoadjuvant therapy in locally advanced rectal cancer.
Shi et al[40] developed an AI model based on a deep learning algorithm using MRI images before and during neoadjuvant therapy. The study aimed to evaluate and predict the patient’s response to neoadjuvant therapy. It was found that the AI image recognition model based solely on MR images before nCRT or MRI images during neoadjuvant therapy was less effective than that combining two MR images in terms of PCR or good response after neoadjuvant therapy.
APPLICATION OF AN MRI-BASED AI MODEL IN PREDICTING CHEMORADIOTHERAPY RESPONSE IN PATIENTS WITH RECTAL CANCER
Fu et al[41] designed a study using experienced radiation oncologists and a deep learning algorithm model to map tumor volume contours on DWI images after neoadjuvant therapy in patients with locally advanced rectal cancer. In this study, a least absolute shrinkage and selection operator-logistic regression model was used to predict treatment response. The results of the two models were verified and compared with regard to the AUC. The AUC of the manual drawing group was 0.64, and that of the deep learning group was 0.73 (P < 0.05). On analysis of the findings from this study, the performance of the deep learning model was found to be better than that of manual mapping in predicting the response of rectal cancer to neoadjuvant therapy. A similar retrospective study was recently published by Yi et al[42]. A total of 134 patients were included, and T2-weighted MRI images were extracted using machine learning models to predict the patients' chemotherapeutic response and tumor downstaging. The results were verified using the subject work curve. The AUC of the PCR group was 0.91 (95%CI: 0.83-0.98), that of the good response group was 0.90 (95%CI: 0.83-0.97), and the tumor downstage AUC was 0.93 (95%CI: 0.87-0.98). The results suggest that radiomics based on machine learning has promising application prospects in predicting the response of patients to chemotherapy. A study compared a Bayesian network algorithm model with a model combining SVM, Bayesian networks, convolutional neural networks, and the K-nearest algorithm in predicting CRC patients' responses to neoadjuvant therapy[43]. The shape feature set, texture feature set, intensity feature set, and the other information of the MR images were extracted by the two models for comprehensive evaluation. The AUC and accuracy of the Bayesian network algorithm model in the validation queue were 74% and 79%, respectively, while the AUC and accuracy of the combined model were 95% and 90%, respectively. The deep learning model integrated with multiple algorithms showed better performance in predicting neoadjuvant therapy responsiveness in rectal cancer patients.
MRI-BASED AI MODEL MAY BE AN EFFECTIVE BIOMARKER FOR PREDICTING THE PROGNOSIS OF PATIENTS
Many scholars have realized the role of radiological characteristics in predicting the mutation status of the KRAS gene in patients. Recently, Cui et al[44] used an AI model based on MR images to predict individuals with KRAS gene mutations among CRC patients. In this study, 304 patients were included, and the model predicted performance with an AUC of 0.722 (95%CI, 0.654-0.790) in the training set and 0.714 (95%CI, 0.602-0.827) in the validation set.
Radiomics features based on T2-weighted images are effective in predicting the KRAS status in patients with rectal cancer to determine the expression of KRAS in patients with rectal cancer, which may be helpful in supplementing genomic analysis of patients. In the future, more MR images should be included, analyzed, and trained to improve the predictive efficiency of this model.
MrT staging, mrN staging, positive EMVI, and peritoneal reflection invasion were all associated with heterochronic distant metastasis after surgery. It has been confirmed that histological invasion of the vessels around the intestinal wall is an independent predictor of distant metastasis for rectal cancer[45]. Clinically, if the high-risk population of postoperative local recurrence and distant metastasis of rectal cancer can be identified and screened in the early stages, targeted systematic treatment can be administered to high-risk populations before surgery to adjust the treatment strategy and improve the prognosis of patients. It has been reported that the radiomics model based on MRI can be used to predict lymph node metastasis of bladder cancer and breast cancer[46,47]. The results suggest that the radiological information contained in MRI images may serve as an effective biomarker for predicting the prognosis of patients. In other words, the MR-based AI models use automated, high-throughput technologies to extract image information and features to capture intra-tumor heterogeneity in a non-invasive manner to guide personalized medicine and precision therapy.
CONCLUSION
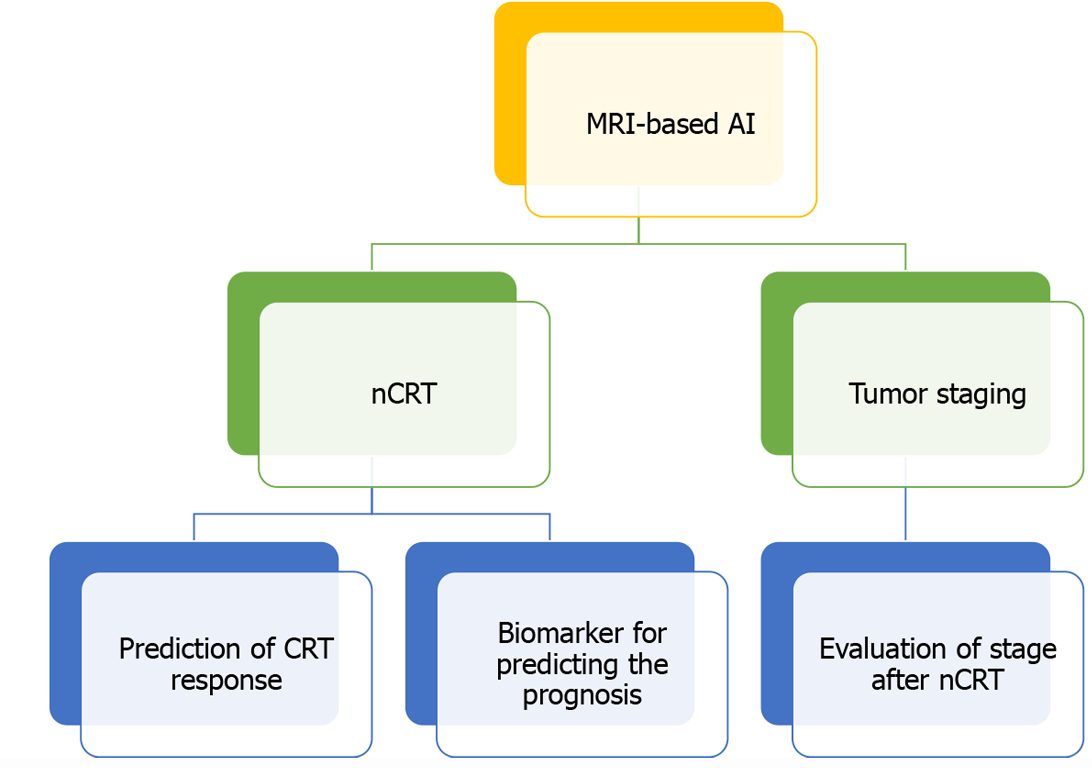
AI is a new technical science. AI methods represented by machine learning have been applied more and more widely in the field of medicine (Figure 2). Rectal MRI is considered the preferred method for the diagnosis of rectal cancer as recommended by national guidelines. Therefore, its importance is self-evident. Recently, there has been an increase in the use of AI models based on MR images in locally advanced rectal cancer. MRI combined with AI can not only improve the accuracy of staging and the efficiency of lymph node detection but also effectively evaluate the patients' response to neoadjuvant chemoradiotherapy. Through continuous optimization of the deep learning algorithm, MRI combined with a deep learning image recognition system can effectively predict the prognosis and treatment response of patients. It is believed that in the near future, AI models based on MRI will be vital in the provision of systematic and precise medical services for the treatment of CRC patients.
Figure 2 Application of artificial intelligence based on magnetic resonance images in rectal cancer.
MRI: Magnetic resonance imaging; AI: Artificial intelligence; nCRT: Neoadjuvant chemoradiotherapy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boscá L, Tanabe S S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Ma YJ