Published online Mar 7, 2020. doi: 10.3748/wjg.v26.i9.960
Peer-review started: November 28, 2019
First decision: December 23, 2019
Revised: January 12, 2020
Accepted: January 19, 2020
Article in press: January 19, 2020
Published online: March 7, 2020
In clinical practice, the diagnosis is sometimes difficult with contrast-enhanced ultrasound (CEUS) when the case has an atypical perfusion pattern. Color parametric imaging (CPI) is an analysis software for CEUS with better detection of temporal differences in CEUS imaging using arbitrary colors. It measures the differences in arrival time of the contrast agent in lesions so that the perfusion features of atypical hemangioma and colorectal cancer (CRC) liver metastasis can be distinguished.
To evaluate the role of a novel type of CPI of CEUS in the differential diagnosis of atypical hemangioma from liver metastases in patients with a history of CRC.
From January 2016 to July 2018, 42 patients including 20 cases of atypical hemangioma and 22 cases of liver metastases from CRC were enrolled. These patients had a mean age of 60.5 ± 9.3 years (range: 39-75 years). All patients received ultrasound, CEUS and CPI examinations. Resident and staff radiologists independently and retrospectively reviewed CEUS and CPI images. Two sets of criteria were assigned: (1) Routine CEUS alone; and (2) CEUS and CPI. The diagnostic sensitivity, specificity, accuracy and receiver operating characteristic (ROC) curve of resident and staff radiologists were analyzed.
The following CPI features were significantly different between liver hemangioma and liver metastases analyzed by staff and resident radiologists: Peripheral nodular enhancement (65%-70.0% vs 4.5%-13.6%, P < 0.001, P = 0.001), mosaic/chaotic enhancement (5%-10% vs 68.2%-63.6%, P < 0.001, P < 0.001) and feeding artery (20% vs 59.1%-54.5%, P = 0.010, P = 0.021). CPI imaging offered significant improvements in detection rates compared with routine CEUS in both resident and staff groups. By resident radiologists, the specificity and accuracy of CEUS+CPI were significantly increased compared with that of CEUS (77.3% vs 45.5%, P = 0.030; 78.6% vs 50.0%, P = 0.006). In addition, the area under the curve (AUC) of CEUS+CPI was significantly higher than that of CEUS (0.803 vs 0.757, P = 0.036). By staff radiologists, accuracy was improved in CEUS+CPI (81.0% vs 54.8%, P = 0.010), whereas no significant differences in specificity and sensitivity were found (P = 0.144, P = 0.112). The AUC of CEUS+CPI was significantly higher than that of CEUS (0.890 vs 0.825, P = 0.013) by staff radiologists.
Compared with routine CEUS, CPI could provide specific information on the hemodynamic features of liver lesions and help to differentiate atypical hemangioma from liver metastases in patients with CRC, even for senior radiologists.
Core tip: Features of atypical hemangioma and liver metastases on routine contrast-enhanced ultrasound are complicated. Color parametric imaging is a new approach that provides specific information on hemodynamic features. The following color parametric imaging features were significantly different between atypical liver hemangioma and liver metastases analyzed by staff and resident radiologists: Peripheral nodular enhancement, mosaic/chaotic enhancement and feeding artery. These findings could help radiologists and even senior radiologists with better identification of the diseases.
- Citation: Wu XF, Bai XM, Yang W, Sun Y, Wang H, Wu W, Chen MH, Yan K. Differentiation of atypical hepatic hemangioma from liver metastases: Diagnostic performance of a novel type of color contrast enhanced ultrasound. World J Gastroenterol 2020; 26(9): 960-972
- URL: https://www.wjgnet.com/1007-9327/full/v26/i9/960.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i9.960
Sonography is the most common imaging modality to detect focal hepatic lesions, but its diagnostic ability to differentiate between benign and malignant lesions is comparatively low. Many studies have shown that contrast-enhanced sonography (CEUS) with low–mechanical index techniques could provide important information about tissue perfusion and vascularity architecture and improve the differential diagnosis in focal hepatic lesions[1-3]. However, the diagnosis is difficult with CEUS when the case has an atypical perfusion pattern.
On CEUS, the typical feature of liver hemangioma is peripheral nodular and centripetal enhancement during the arterial phase followed by hyper- or isoenhancement during the portal venous and late phases[4-7]. In contrast, the feature of liver metastases is complete or rim-like hyperenhancement during the arterial phase followed by hypoenhancement in the portal venous and late phase. There were different perfusion patterns between the two diseases. However, hemangioma also has an atypical pattern on CEUS, such as rapid homogeneous hyperenhancement in arterial phase like malignant tumors or lack of enhancement in the center, which may be misinterpreted as wash out[8-10]. The atypical pattern makes the differentiated diagnosis quite difficult from liver metastases, especially in patients with a previous history of malignant tumor. In our center, we misdiagnosed several lesions of atypical hemangioma as liver metastases on CEUS in patients with a history of colorectal cancer (CRC). Consequently, these patients received unnecessary surgical resection for these lesions. These misdiagnosed cases encouraged us to explore a better way to differentiate between them.
Color parametric imaging (CPI) is an image analysis software for CEUS with better detection of temporal differences in CEUS imaging using arbitrary colors. It measures the differences in arrival time of the contrast agent between the target region and reference points determined arbitrarily at a structure in the liver such as the hepatic artery and portal vein. The arrival time was defined as zero and represented the time differences in different colors. A few studies demonstrated that CPI was useful in the diagnosis of hepatic parenchymal diseases[11,12] and in identifying spoke-wheel patterns of FNH[13]. To our knowledge, this is the first study to analyze whether CPI could provide useful information to differentially diagnose atypical hemangioma and liver metastases.
The aim of this study was to evaluate the role of CPI in the differential diagnosis of atypical hemangioma and liver metastases and the diagnostic performance of staff and resident radiologists.
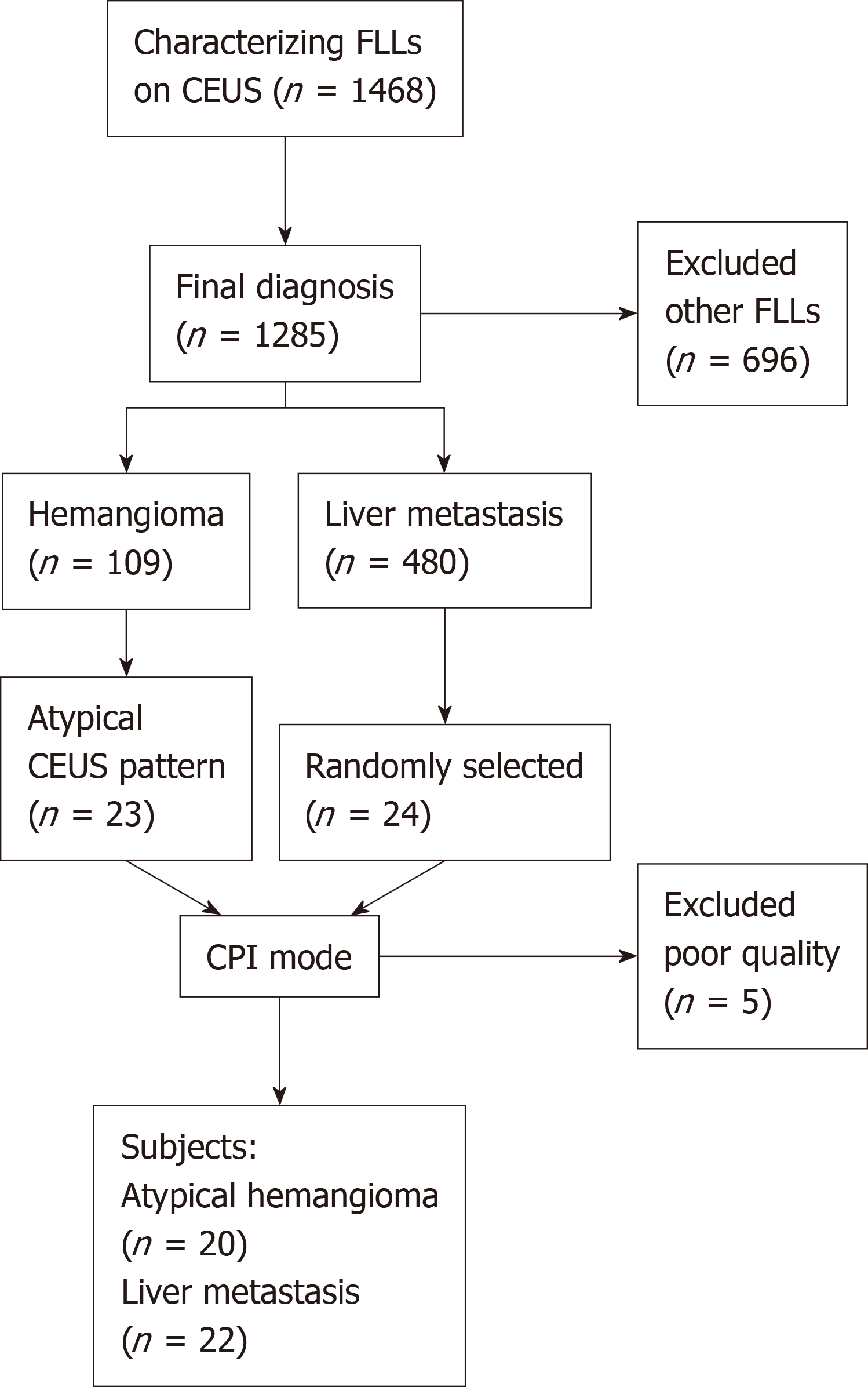
This retrospective study was approved by the Institutional Review Board of the Peking University School of Oncology, and written informed consent was waived. From January 2016 to July 2018, a total of 1468 consecutive patients with focal liver lesions were referred to our department for CEUS examinations. Of the 109 hemangioma cases, 23 cases had atypical CEUS patterns and previous CRC histories, which were difficult to exclude from liver metastasis. During the same period, 480 patients with suspected liver metastasis from CRCs were diagnosed based on CEUS. Because there were much more liver metastasis cases (n = 480) than hemangioma cases (n = 20), we randomly selected 24 cases from the liver metastasis pool according to a 1:20 proportion. If patients had more than one lesion, the largest and most clearly presented lesion was chosen for CPI evaluation. Five patients who had deep breath during the arterial phase and poor imaging quality were excluded. Finally, 20 hemangiomas and 22 liver metastases were entered into this retrospective study (Figure 1).
According to the liver CEUS guidelines[14] and other studies[10], the atypical pattern of CEUS for hemangioma included rapid homogeneous hyperenhancement at the arterial phase and hypoenhancement at the portal/late phase and peripheral nodular enhancement at the arterial phase and lack of enhancement in the center at the late phase. The CEUS pattern for liver metastasis included hyperenhancement at the arterial phase and washout at the late phase or rim-like enhancement at the arterial phase and a nonenhancement area at the late phase. Among the 22 patients with liver metastasis, the final diagnosis was confirmed by pathologic analysis of specimens obtained via US-guided percutaneous biopsy (n = 16) or surgical resection (n = 6). Among the 20 hemangiomas, the final diagnosis was based on either pathological results (n = 9) or contrast-enhanced computed tomography or contrast-enhanced magnetic resonance imaging findings with at least one year follow-up (n = 11). Among the 42 patients, 19 were male and 23 were female. The average age was 60.5 ± 9.3 years (range: 39-75 years). The mean size of liver lesions was 3.2 ± 1.8 cm (range: 1.3-11 cm).
A Logiq E9 ultrasonic machine (GE Healthcare, Milwaukee, WI, United States) was used with a C1-5 convex probe to obtain routine US and CEUS images. The ultrasound contrast agent was SonoVue (Bracco, Milan, Italy). Lyophilized SonoVue powder was dissolved in 5 mL saline. Bolus injection (2 mL the suspension) was performed at the antecubital vein via a 20G cannula within 2-3 s, followed by a 5-mL saline flush.
Before the examination, the patients were required to lie in the left lateral position or supine position and breathe steadily. The liver lesions were scanned and located using routine US. The echogenicity, diameter, border, morphology, necrosis, halo sign and vessels were observed. It was defined as necrosis in ultrasound imaging if there was an anechoic area within the lesions, clear boundaries, and color Doppler flow imaging showed no blood flow within the anechoic area. The halo sign was defined as a hypoechoic rim found around the solid mass with a distinct difference between the lesion and the surrounding liver. At least two vessels inside the lesion on color Doppler flow imaging indicated rich flow.
Then, the contrast mode was entered. The imaging settings, such as gain, depth, and focus, were optimal. The mechanical index was set at 0.11-0.13. After injecting contrast agent, the liver was scanned using contrast-enhanced harmonic grayscale sonography, and timer was initiated simultaneously. The dynamic blood perfusion of lesions was observed from baseline to the late phase. Consecutive cine clips (90 s each) were recorded and stored on the hard disk for further analysis. The enhancement phases were divided into the arterial phase (10-30 s), portal vein phase (31-120 s), and late phase (120-360 s) after injection of contrast agent. The same ultrasound machine with the procedure software of parametric imaging was used to obtain CPI.
Two resident radiologists (W. X. F. and W. H.) each with at least one year of experience in the evaluation of liver CEUS images as well as two staff radiologists (Y. W. and W. W.) each with at least 10 years of experience in the liver CEUS images retrospectively read all the CEUS and CPI images independently. Two radiologists at the same level retrospectively interpreted the CEUS images without knowledge of the patients’ final diagnosis. In all cases, consensus agreement between the two radiologists was used to determine the CEUS feature and possible diagnosis. According to CEUS guidelines[14], the enhancement patterns at the arterial phase to portal phase of liver lesions included four patterns: Peripheral nodular enhancement (peripheral focal enhancement in the arterial phase, progressing in a centripetal direction to partial fill-in.), rim-like enhancement (peripheral enhancement in the arterial phase, without gradual fill-in.), homogenous hyperenhancement, and heterogeneous enhancement.
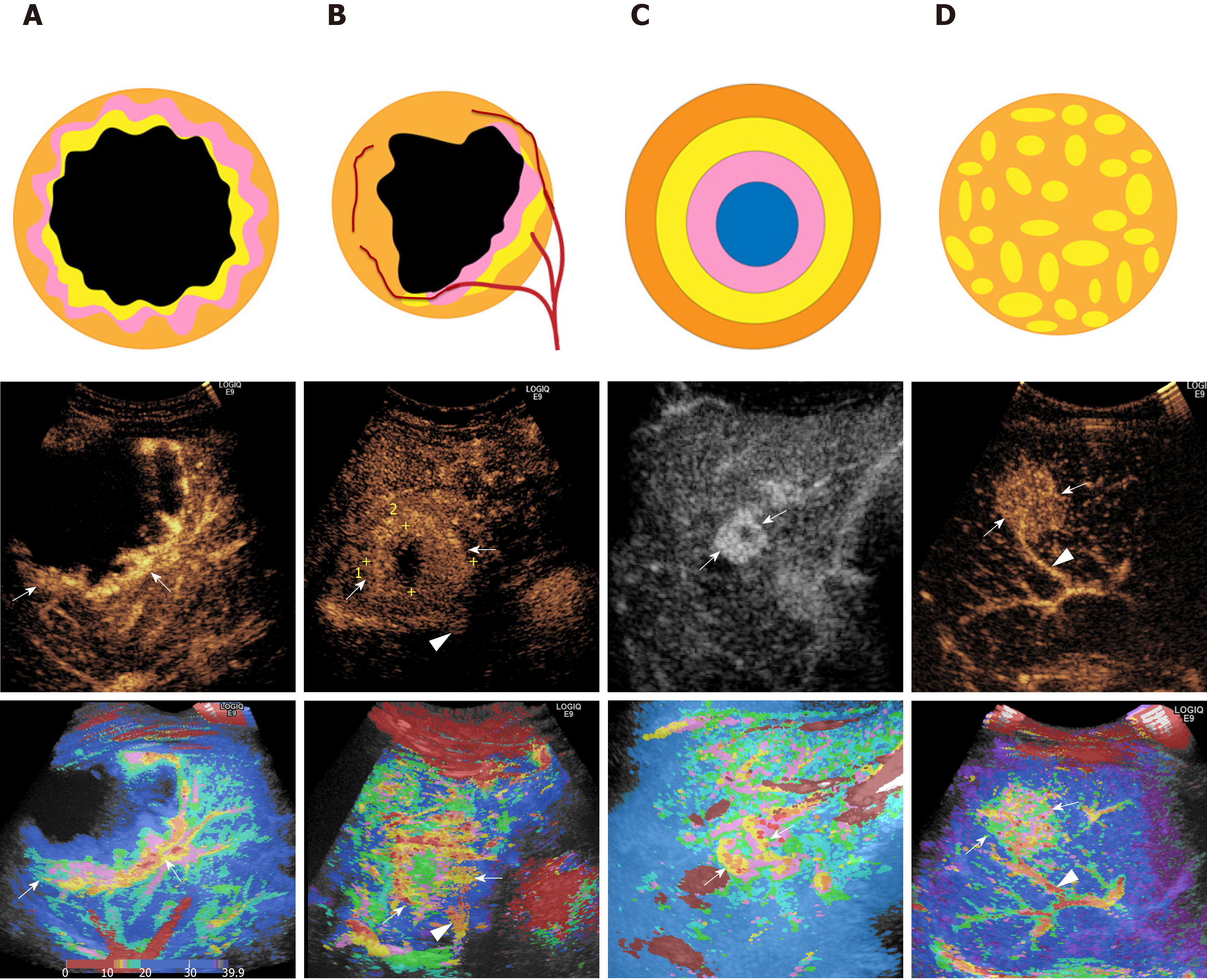
All of the CEUS images of the arterial phase were reconstructed with the Parametric Imaging program using a Logiq E9 XD Clear ultrasonic machine (GE Healthcare, Milwaukee, WI, United States). The CEUS video clips were reviewed to record the starting and ending points of lesion enhancement. In the CPI system, time zero was regarded as the point at which the contrast agent reached the liver, and the arrival time was then calculated between the current time and time zero. If the time zero was set at the time when contrast agent was injected, the arrival time of contrast agent at each pixel could be calculated. Thus, the color map consisted of individual pixels representing the arrival time of the contrast agent in the tumor. The residents and staff radiologists read all the CPI images blindly and independently. The CPI enhancement patterns of liver lesions were summarized and classified into four patterns: (1) Peripheral nodular enhancement (round or semicircle shaped enhancement visualized at the peripheral area of lesions and no enhancement in the center); (2) Peripheral rim-like enhancement (peripheral enhancement without nodular or regular shape); (3) Concentric circle enhancement (multiple circles with the same center); and (4) Mosaic enhancement (enhancing lines of the vascular tree, defined as one or more hypertrophic tortuous arteries that reached the edge of the lesion, partially encircling the nodule and penetrating internally with a basket or chaotic distribution) (Figure 2). The feeding artery, defined as a hypertrophic artery that was directed toward the lesion and was larger than the branches at the same depth during the arterial phase, was red in the central area, which was surrounded by yellow, or was a scattered distribution of red and yellow (Figure 2). In all cases, consensus agreement between the two radiologists was used to determine the CPI feature and possible diagnosis.
In the training, both staff and resident radiologists were required to review all 20 cases of hemangioma and liver metastasis with typical CEUS and CPI features. CEUS and CPI diagnoses were scored using a 5-point scale: 1 = hemangioma with strong evidence, 2 = possible hemangioma, 3 = undetermined, 4 = possible liver metastasis, and 5= liver metastasis with strong evidence.
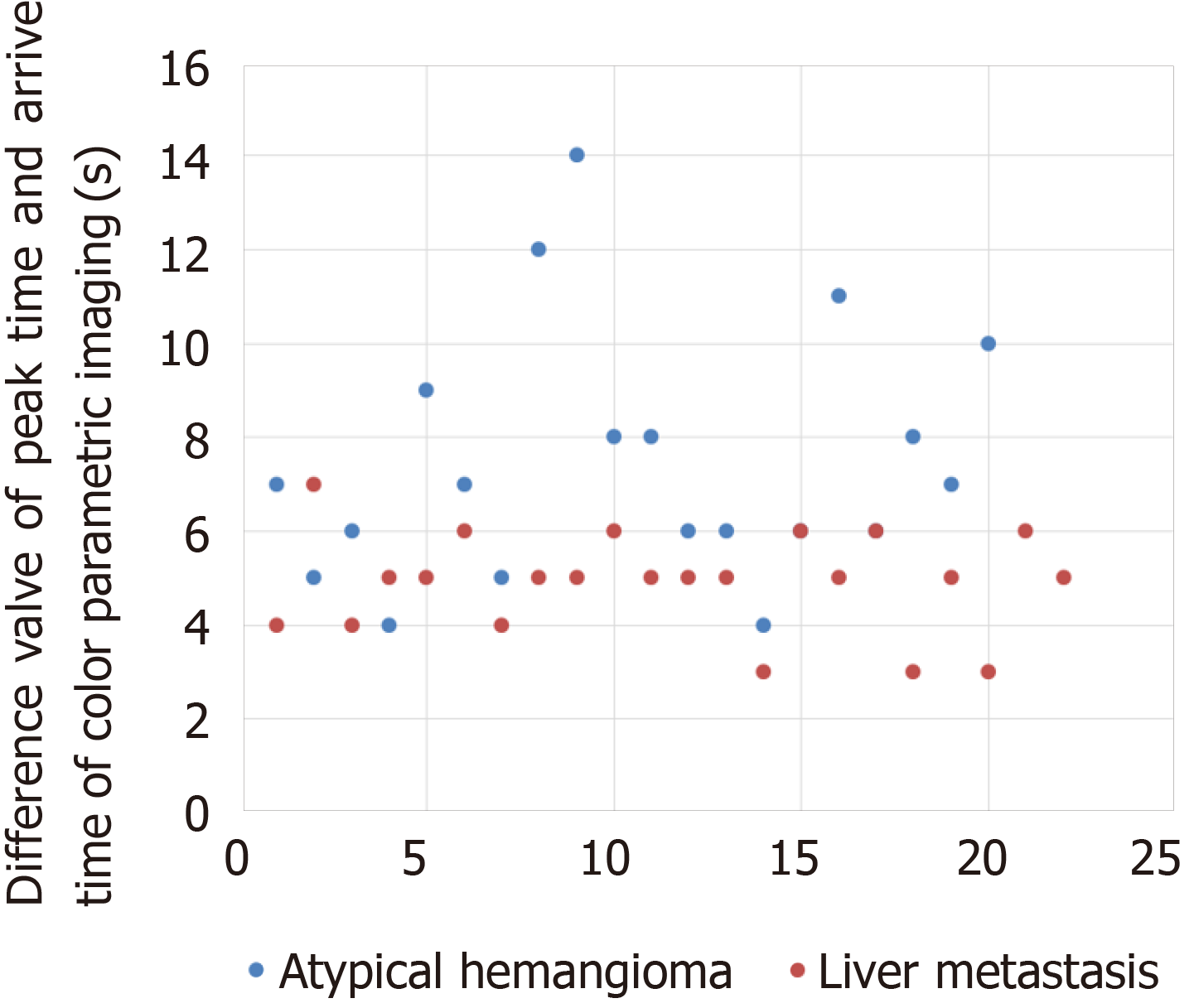
The arrival time of the contrast agent after injection could be displayed at any point of CPI. The arrival time of a lesion was defined as the arrival time of the earliest color point in the lesion, and the peak time was regarded as the arrival time of the last color point in the lesion. The peak time of CPI was regarded as the time point where the brightest color was in the lesion. △AT was regarded as the difference in peak time and arrival time of CPI of atypical hemangioma or liver metastasis.
SPSS 21.0 statistical software was used for statistical analysis. The quantitative data are displayed as the mean ± SD and were compared by t test. The Kappa test was used to analyze the interrater agreement between the staff and resident radiologists. The agreement was graded as follows: Moderate (0.2-0.39), fair (0.40-0.59), good (0.60-0.79), and perfect (0.80-1.0) agreement. The diagnostic sensitivity, specificity, and accuracy between CEUS and CPI patterns were compared by the McNemar test. The count data were analyzed using χ2 test and Fisher’s exact test. A P value less than 0.05 was considered statistically significant.
The routine CEUS features of liver atypical hemangioma and liver metastases analyzed by staff and resident radiologists are shown in Table 1.
| CEUS | Staff | Resident | ||||
| H (n = 20) | M (n = 22) | P value | H (n = 20) | M (n = 22) | P value | |
| Peripheral nodular | 10 (50) | 2 (9.1) | 0.003 | 8 (40) | 3 (13.6) | 0.052 |
| Peripheral rim-like | 6 (30) | 5 (22.7) | 0.592 | 8 (40) | 4 (18.2) | 0.118 |
| Homogenous hyper | 2 (10) | 6 (27.3) | 0.135 | 3 (15) | 9 (40.9) | 0.063 |
| Heterogeneous hyper | 2 (10) | 9 (40.9) | 0.023 | 1 (5) | 6 (27.3) | 0.096 |
| Feeding artery | 6 (30) | 10 (45.5) | 0.303 | 5 (25) | 8 (36.4) | 0.426 |
The CEUS features of peripheral nodular enhancement were observed during the arterial phase more frequently in patients with atypical hemangioma than in those with metastasis (P = 0.003) by staff radiologists. The CEUS features of heterogeneous hyperenhancement were observed during the arterial phase significantly more frequently in patients with metastasis than in those with atypical hemangioma by staff radiologists (P = 0.023). However, the perfusion patterns detected by resident radiologists were not as sensitive as those detected by staff radiologists (Table 1). The features of peripheral nodular enhancement and heterogeneous hyperenhancement for resident radiologists were not significantly different between atypical hemangioma and metastasis (P = 0.052, P = 0.096). The feeding arteries for the staff and resident radiologists were at a power of 25%–45.5%.
The CPI features of liver atypical hemangioma and liver metastases analyzed by staff and resident radiologists are shown in Table 2.
| CPI | Staff | Resident | ||||
| H (n = 20) | M (n = 22) | P value | H (n = 20) | M (n = 22) | P value | |
| Peripheral nodular1 | 14 (70.0) | 1 (4.5) | < 0.001 | 13 (65.0) | 3 (13.6) | 0.001 |
| Peripheral rim-like | 2 (10.0) | 6 (27.3) | 0.152 | 3 (15.0) | 4 (18.2) | 0.556 |
| Concentric circles1 | 3 (15.0) | 0 (0) | 0.099 | 2 (10.0) | 1 (4.5) | 0.463 |
| Mosaic/ chaotic. | 1 (5.0) | 15 (68.2) | < 0.001 | 2 (10.0) | 14 (63.6) | < 0.001 |
| Feeding artery | 4 (20) | 13 (59.1) | 0.010 | 4 (20) | 12 (54.5) | 0.021 |
The CPI features of peripheral nodules were observed during the arterial phase more frequently in patients with atypical hemangioma than in those with metastasis by both groups of radiologists (65%-70.0% vs 4.5%-13.6%, P < 0.001, P = 0.001). In addition, the CPI features of mosaic enhancement (5%-10% vs 68.2%-63.6%, P < 0.001, P < 0.001) and feeding artery (20% vs 59.1%-54.5%, P = 0.010, P = 0.021) were found during the arterial phase more frequently in patients with metastasis than in those with atypical hemangioma by both groups of radiologists. The feeding arteries for the staff and resident radiologists were at a power of 20%–59.1%. CPI imaging offered significant improvements in detection rates compared with routine CEUS signs in both groups.
The consistency of CEUS and CPI between staff and resident radiologists was analyzed (Table 3). With regard to CEUS features found by staff and resident radiologists, the diagnostic consistency in homogenous hyperenhancement and heterogeneous enhancement was perfect (k = 0.835, k = 0.804), and the diagnostic consistency in peripheral nodular enhancement and rim-like enhancement was good (k = 0.602, k = 0.692).
| Feature | Kappa value |
| CEUS | |
| Peripheral nodular enhancement | 0.602 ± 0.142 |
| Peripheral Rim-like enhancement | 0.692 ± 0.127 |
| Homogenous hyper-enhancement | 0.835 ± 0.064 |
| Heterogeneous enhancement | 0.804 ± 0.132 |
| CPI | |
| Peripheral nodular enhancement | 0.885 ± 0.079 |
| Peripheral rim-like enhancement | 0.713 ± 0.177 |
| Concentric circles enhancement | 0.760 ± 0.112 |
| Mosaic enhancement | 0.803 ± 0.134 |
With regard to CPI features found by staff and resident radiologists, the diagnostic consistency in peripheral nodular enhancement and mosaic enhancement was perfect (k = 0.885, k = 0.803), and the diagnostic consistency in peripheral rim-like enhancement and concentric circle enhancement was good (k = 0.713, k = 0.760).
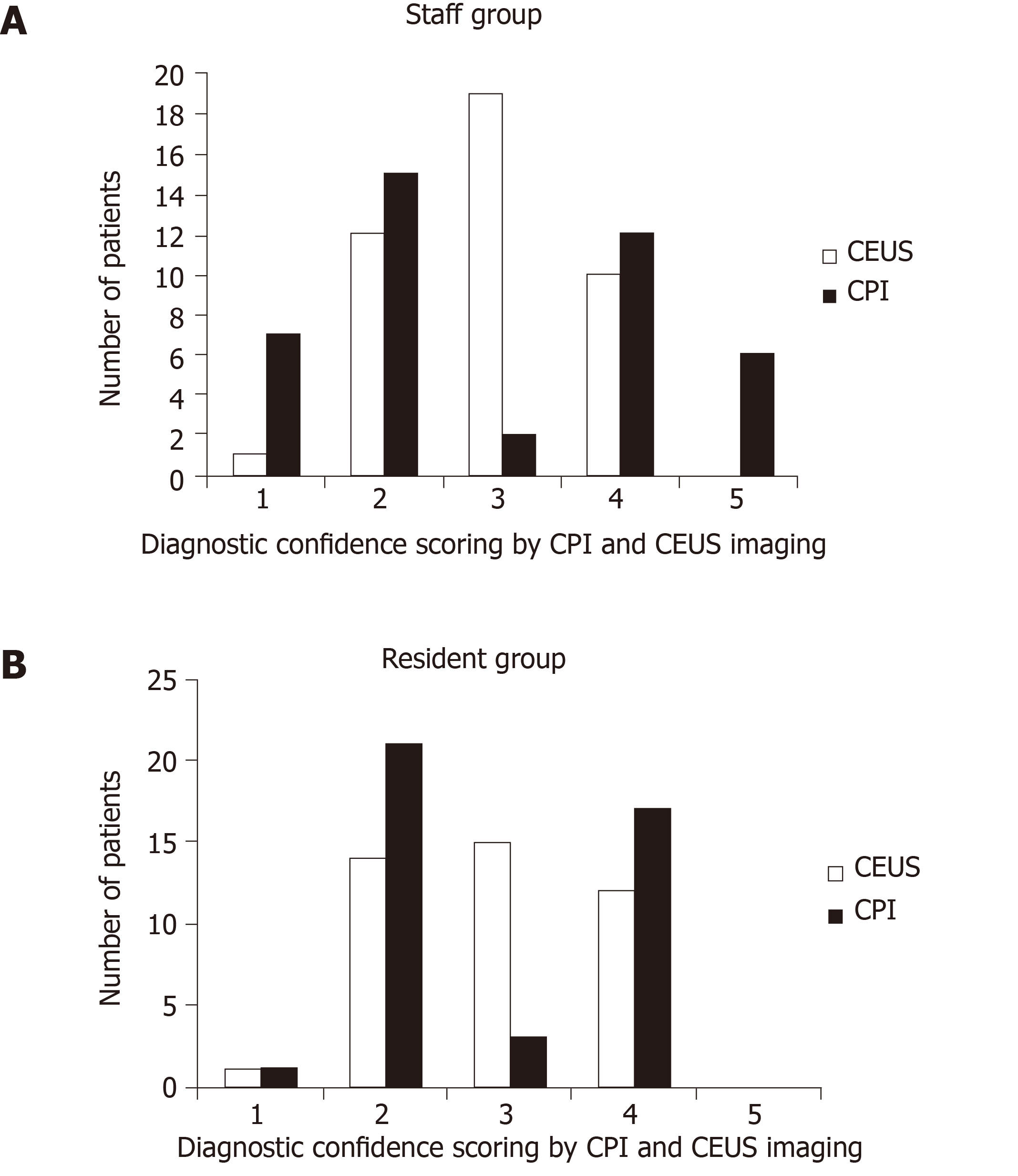
The diagnostic confidence scoring of lesions by staff and resident radiologists is summarized in Figure 3. There were significant differences in the final score distribution between the two methods. The number of 3-score (undetermined diagnosis) in CEUS was obviously higher than that in CPI by both the groups of staff (45.2% vs 4.8%, P < 0.001) and resident radiologists (35.7% vs 7.1%, P = 0.001).
The diagnostic performance of CEUS and CPI by staff and resident radiologists is shown in Table 4.
| Criteria | Sensitivity | P value | Specificity | P value | Accuracy | P value | AUC | P value |
| Resident | ||||||||
| CEUS | 55.0 (11/20) | 0.456 | 45.5 (10/22) | 0.030 | 50.0 (21/42) | 0.006 | 0.757 | 0.036 |
| CEUS+CPI | 80.0 (16/20) | 77.3 (17/22) | 78.6 (33/42) | 0.803 | ||||
| Staff | ||||||||
| CEUS | 65.0 (13/20) | 0.144 | 54.5 (12/22) | 0.112 | 54.8 (23/42) | 0.010 | 0.825 | 0.013 |
| CEUS+CPI | 85.0 (17/20) | 77.3 (17/22) | 81.0 (34/42) | 0.890 |
By resident radiologists, the specificity of CEUS+CPI was significantly increased compared with that of CEUS (77.3% vs 45.5%, P = 0.030). The sensitivity of CEUS+CPI was higher than that of CEUS, but the differences were not significant (80.0% vs 55.0%, P = 0.456). The accuracy of CEUS+CPI was significantly higher than the accuracy of CEUS (78.6% vs 50%, P = 0.006). Additionally, the AUC of CEUS+CPI was significantly higher than that of CEUS (AUC = 0.803 vs AUC = 0.757, P = 0.036).
By staff radiologists, accuracy was improved in CEUS+CPI (81.0% vs 54.8%, P = 0.010), whereas no significant differences in specificity and sensitivity were found (P = 0.144, P = 0.112). The AUC of CEUS+CPI was significantly higher than the AUC of CEUS (0.890 vs 0.825, P = 0.013) by staff radiologists.
The comparison of the △AT of CPI between atypical hemangioma and liver metastasis is summarized in Figure 4. The difference in peak time and arrival time of CPI of atypical hemangioma was significantly longer than that of liver metastasis (8.31 ± 3.05 s vs 5.13 ± 0.99 s, P < 0.001).
Ultrasonography is useful for the diagnosis of hepatic focal lesions, which is based on their distinctive echogenicities—the grayscale morphologic features. The development of ultrasound contrast agents provided us with more information about tissue perfusion and helped to improve diagnostic accuracy, particularly in focal liver lesions (FLLs)[15-17]. Compared with first-generation agents, the second-generation ultrasound contrast agent SonoVue consisting of sulfur hexafluoride microbubbles (Bracco, Milan, Italy) has a high flexibility shell and is more stable to acoustic pressure. SonoVue microbubbles produce a longer duration and stable continuous nonlinear harmonic signal when insonated with low acoustic power. With SonoVue, we could acquire important information on both the macrovasculature and microvasculature and then evaluate the flow dynamic features of FLLs in real time[18-21]. Many studies have reported the typical or atypical features of different liver tumors in CEUS performance, and many clinical centers and guidelines have recommended a diagnostic criterion of FLLs using CEUS in clinical practice[18,22,23].
It was reported that the incidence of hemangioma in the general population varies from 0.4% to 20%[24], the latter resulting from a thorough prospective search of the liver in an unselected autopsy series. CEUS imaging of hemangioma can be performed during the vascular phase assessing the dynamic enhancement pattern and the vascular morphology of the lesion[25]. Moreover, the majority of hemangiomas presents as peripheral nodular or rim enhancement at arterial phase with centripetal progression in portal venous and late phase on CEUS. However, some cases had the atypical pattern of CEUS for hemangioma as hyperenhancement during the arterial phase or have nonenhancement area at portal and late phases, which often caused various misdiagnoses, including malignant liver tumors. When the case had a history of malignant tumors, it was a challenge to differentiate the atypical hemangioma from liver metastasis. As the treatments for these diseases are completely different, misdiagnosis might result in the unnecessary traumatic resection of benign lesions or miss the opportunity for radical resection of malignant lesions. In our center, 7 cases of atypical hemangioma with a history of colon-rectal cancer had been misdiagnosed as liver metastasis on CEUS and received unnecessary surgical resection of liver lesions before we started this study. These misdiagnosed cases encouraged us to carry out the present study. This study mainly assessed the value of the combination of CEUS and CPI for differentiating hemangioma and liver metastases.
In our study, the CEUS feature of atypical hemangioma included homogenous hyperenhancement/rim-like enhancement during the arterial phase and nonenhancement during the late phase, and these appearances might also be found in liver metastasis. Thus, the similar manifestations made it difficult for radiologists to differentiate them. The newly developed technique of CPI offered a more objective color-coded map to display FLL dynamic perfusion, which can provide more information for differential diagnosis. Parametric images could quantify the dynamic procedure of CEUS and then evaluate the vascular architecture in lesions more objectively than the regular CEUS review[23]. One of the CEUS shortcomings was that CEUS could not detect tiny changes in contrast agent dynamics due to rapid monochromatic enhancement of small lesions or lesions that were enhanced very shortly. In this line, CPI could overcome the disadvantage of conventional CEUS because CPI has higher ability to demonstrate temporal changes in contrast-enhanced imaging findings. Therefore, CPI has better potential for detecting concentric circles or peripheral nodular enhancement of hemangioma and mosaic enhancement of liver metastasis.
Since the application period was short after the CPI technique was put to market, there were only a few clinical reports[26-28]. Some researchers indicated that CPI using Sonazoid as a contrast agent was better for detecting spoke-wheel patterns of FNH less than 3 cm in size[13]. In Li et al[28]’s study, the diagnostic sensitivity, specificity, accuracy, PPV, and NPV of CPI for atypical hepatocellular carcinoma from focal nodular hyperplasia were higher than those of CEUS only. They concluded that the newly developed technique of CPI offered a more objective color-coded map to display focal liver lesion dynamic perfusion and greatly improved the differentiation of focal liver lesions, especially in the resident radiologist group. To our knowledge, our study is the first report on the role of CPI in the differential diagnosis of atypical hemangioma and liver metastasis. In our study, CPI improved the diagnostic performance in the resident group. The diagnostic specificity and accuracy rate of the combination CPI and CEUS (77.3% and 78.6%, respectively) were significantly higher than those of CEUS alone (45.5% and 50.0%, respectively) in the resident group. The accuracy rate of the combination (81.0%) was also higher than that of CEUS alone (54.8%) in the staff group. Our data indicated that the differential diagnosis of atypical hemangioma and liver metastasis was still challenging, even for experienced radiologists, and CPI provided a very useful tool to improve diagnosis.
Our study showed that the 3-score (undetermined diagnosis) in CEUS was obviously higher than that in CPI+CEUS in both the staff group (45.2% vs 4.8%, P < 0.001) and in the resident group (35.7% vs 7.1%, P = 0.001). Our data confirmed that the application of CPI significantly increased the diagnostic confidence of focal liver lesions compared with CEUS, and the number of undetermined cases decreased greatly compared with CEUS. CPI can display the color-coded imaging of regional flow dynamics based on the arrival time parameter. The color map emphasized the difference in perfusion viscosity of lesions and revealed the features of blood flow perfusion and pathological structures. On CPI imaging, we found that the flow perfusion gradually filled from the peripheral area to the center of the hemangioma. Additionally, the visualization of tumor vessels, such as mosaic enhancement, was made possible by CPI. The clear depiction of tumor microvascular structures provides a clue for the diagnosis of liver metastasis.
In addition to consensus review by two readers from each group, we found that inter-reader agreement between the staff and resident radiologists for CEUS and CPI imaging was good. With regard to CEUS features by staff and resident radiologists, the diagnostic consistency in homogenous hyperenhancement and heterogeneous enhancement was perfect (k = 0.835, k = 0.804), and the diagnostic consistency in peripheral nodular enhancement and rim-like enhancement was good (k = 0.602, k = 0.692). For CPI features by staff and resident radiologists, the diagnostic consistency in peripheral nodular enhancement and mosaic enhancement was perfect (k = 0.885, k = 0.803) and the diagnostic consistency in concentric circle enhancement and peripheral rim-like enhancement was good (k = 0.760, k = 0.713). These findings showed that CPI imaging could provide an objective tool to improve the learning curve.
This study has several limitations. First, the comparative analysis was from retrospective research. Second, the sample size was small to show the benefits in some of the subgroup analyses. Because patients with atypical CEUS patterns of hemangioma accounted for a small percentage of the regular patient population, only 20 cases were enrolled in this study during the 3-year period. Third, a score of 3 was classified as undetermined diagnosis or diagnosed errors, reducing the diagnostic performance of CEUS and CPI. Finally, the application of CPI requires specific hardware, and software standardization of the conditions of examination needs further improvement in the future.
In the present study, we used SonoVue as a contrast agent together with color parameter imaging of the liver lesion. Compared to CT imaging, low-MI CEUS has several advantages, including no ionizing radiation, real-time imaging and low cost[29,30]. However, multiple imaging modalities and referring to the results of laboratory examinations and needle biopsy are still required for final diagnosis when necessary.
In conclusion, compared with CEUS, CPI could provide specific information on the hemodynamic features of liver lesions and help to differentiate atypical hemangioma from liver metastases, for both staff and resident radiologists. CPI is useful especially for radiologists with less CEUS experience. It is anticipated that in the future, new methods of contrast ultrasonography will gain importance.
In clinical practice, the diagnosis is sometimes difficult with contrast-enhanced ultrasound (CEUS) when the case has an atypical perfusion pattern. Color parametric imaging (CPI) is an analysis software for CEUS with better detection of temporal differences in CEUS imaging using arbitrary colors. It measures the differences in arrival time of the contrast agent in lesions so that the perfusion features of atypical hemangioma and colorectal cancer liver metastasis can be distinguished.
The motivation of this study was to evaluate the role of CPI in the differential diagnosis of atypical hemangioma from liver metastases and the diagnostic performance by staff and resident radiologists. The patients with atypical hemangioma would be benefited by avoiding invasive test or even surgical resection. Furthermore, a junior radiologist can be more confident in the differential diagnosis of liver lesions by CPI.
To evaluate the role of a novel type of CPI of CEUS in the differential diagnosis of atypical hemangioma from liver metastases in patients with a history of colorectal cancer.
All enrolled patients received ultrasound, CEUS and CPI examinations. Resident and staff radiologists independently and retrospectively reviewed CEUS and CPI images. Two sets of criteria were assigned: (1) Routine CEUS alone; and (2) CEUS and CPI. The diagnostic sensitivity, specificity, accuracy and receiver operating characteristic (ROC) curve of resident and staff radiologists were analyzed.
The following CPI features were significantly different between liver hemangioma and liver metastases analyzed by staff and resident radiologists: Peripheral nodular enhancement (65%-70.0% vs 4.5%-13.6%, P < 0.001, P = 0.001), mosaic/chaotic enhancement (5%-10% vs 68.2%-63.6%, P < 0.001, P < 0.001) and feeding artery (20% vs 59.1-54.5%, P = 0.010, P = 0.021). CPI imaging offered significant improvements in detection rates compared with routine CEUS signs in both resident and staff groups.
CPI could provide specific information on the hemodynamic features of liver lesions and help to differentiate atypical hemangioma from liver metastases, for both staff and resident radiologists. CPI is useful especially for radiologists with less CEUS experience.
In this study, a novel type of color contrast enhanced ultrasound provided supplemental information for differential diagnosis between atypical hemangioma and liver metastasis. This technique is safe and effective in clinical practice. However, to confirm the performance of this new imaging method, studies on a larger sample set are required.
We thank Dan Yan for her assistance with software technique.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rolle U, Sporea I S-Editor: Ma YJ L-Editor: A MedE-Ma JY E-Editor: Liu MY
| 1. | Jang JY, Kim MY, Jeong SW, Kim TY, Kim SU, Lee SH, Suk KT, Park SY, Woo HY, Kim SG, Heo J, Baik SK, Kim HS, Tak WY. Current consensus and guidelines of contrast enhanced ultrasound for the characterization of focal liver lesions. Clin Mol Hepatol. 2013;19:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | D'Onofrio M, Romanini L, Serra C, Magnolfi F, Bertolotto M, Quaia E, Puntel G, Colleoni A, Fiorini E, Cenci C, Santi E, Ciaravino V, Laffranchi F, Catalano O, Cantisani V, Calliada F, Derchi L. Contrast enhancement ultrasound application in focal liver lesions characterization: a retrospective study about guidelines application (SOCEUS-CEUS survey). J Ultrasound. 2016;19:99-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Fan ZH, Chen MH, Dai Y, Wang YB, Yan K, Wu W, Yang W, Yin SS. Evaluation of primary malignancies of the liver using contrast-enhanced sonography: correlation with pathology. AJR Am J Roentgenol. 2006;186:1512-1519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Fang L, Zhu Z, Huang B, Ding H, Mao F, Li C, Zeng M, Zhou J, Wang L, Wang W, Chen Y. A comparative study of contrast enhanced ultrasound and contrast enhanced magnetic resonance imaging for the detection and characterization of hepatic hemangiomas. Biosci Trends. 2015;9:104-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Sirli R, Sporea I, Săndulescu DL, Popescu A, Dănilă M, Săftoiu A, Spârchez Z, Badea R. Contrast enhanced ultrasound for the diagnosis of liver hemangiomas - results of a Romanian multicentre study. Med Ultrason. 2015;17:444-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Dietrich CF, Mertens JC, Braden B, Schuessler G, Ott M, Ignee A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology. 2007;45:1139-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Jang H, Kim TK, Lee JS, Sim JS, Kim EA. Hepatic hemangioma: typical and atypical appearances on various ultrasound imaging techniques. Ultrasound Med Biol. 2003;29:166-167. [DOI] [Cited in This Article: ] |
| 8. | Zviniene K, Zaboriene I, Basevicius A, Jurkiene N, Barauskas G, Pundzius J. Comparative diagnostic value of contrast-enhanced ultrasonography, computed tomography, and magnetic resonance imaging in diagnosis of hepatic hemangiomas. Medicina (Kaunas). 2010;46:329-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Huang M, Zhao Q, Chen F, You Q, Jiang T. Atypical appearance of hepatic hemangiomas with contrast-enhanced ultrasound. Oncotarget. 2018;9:12662-12670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Giannetti A, Franci L, Grechi C, Giangregorio F. Contrast-enhanced sonography in the diagnosis of hepatic hemangiomas: atypical appearance due to the washout of microbubbles. J Clin Ultrasound. 2013;41:361-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Shiozawa K, Watanabe M, Takayama R, Kudo T, Maruyama K, Sumino Y. Hepatic parenchymal hemodynamics of cholangitis with portal trunk thrombus using contrast-enhanced ultrasonography with Sonazoid: delineation of so-called central and peripheral zonal differentiation by arrival-time parametric imaging. J Med Ultrason (2001). 2013;40:73-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Watanabe M, Shiozawa K, Takahashi M, Wakui N, Otsuka Y, Kaneko H, Tanikawa K, Shibuya K, Kamiyama N, Sumino Y. Parametric imaging using contrast-enhanced ultrasound with Sonazoid for hepatocellular carcinoma. J Med Ultrason (2001). 2010;37:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Wakui N, Takayama R, Kamiyama N, Kobayashi K, Matsui D, Matsukiyo Y, Kanekawa T, Ikehara T, Ishii K, Sumino Y. Arrival time parametric imaging using Sonazoid-enhanced ultrasonography is useful for the detection of spoke-wheel patterns of focal nodular hyperplasia smaller than 3 cm. Exp Ther Med. 2013;5:1551-1554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, Chaubal NG, Chen MH, Clevert DA, Correas JM, Ding H, Forsberg F, Fowlkes JB, Gibson RN, Goldberg BB, Lassau N, Leen EL, Mattrey RF, Moriyasu F, Solbiati L, Weskott HP, Xu HX; World Federation for Ultrasound in Medicine; European Federation of Societies for Ultrasound. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 479] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 15. | Dill-Macky MJ, Burns PN, Khalili K, Wilson SR. Focal hepatic masses: enhancement patterns with SH U 508A and pulse-inversion US. Radiology. 2002;222:95-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Klibanov AL. Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest Radiol. 2006;41:354-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 273] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 17. | Ta CN, Kono Y, Eghtedari M, Oh YT, Robbin ML, Barr RG, Kummel AC, Mattrey RF. Focal Liver Lesions: Computer-aided Diagnosis by Using Contrast-enhanced US Cine Recordings. Radiology. 2018;286:1062-1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 19. | Dai Y, Chen MH, Yin SS, Yan K, Fan ZH, Wu W, Wang YB, Yang W. Focal liver lesions: can SonoVue-enhanced ultrasound be used to differentiate malignant from benign lesions? Invest Radiol. 2007;42:596-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Huang-Wei C, Bleuzen A, Bourlier P, Roumy J, Bouakaz A, Pourcelot L, Tranquart F. Differential diagnosis of focal nodular hyperplasia with quantitative parametric analysis in contrast-enhanced sonography. Invest Radiol. 2006;41:363-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Mandai M, Koda M, Matono T, Nagahara T, Sugihara T, Ueki M, Ohyama K, Murawaki Y. Assessment of hepatocellular carcinoma by contrast-enhanced ultrasound with perfluorobutane microbubbles: comparison with dynamic CT. Br J Radiol. 2011;84:499-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Ding H, Wang WP, Huang BJ, Wei RX, He NA, Qi Q, Li CL. Imaging of focal liver lesions: low-mechanical-index real-time ultrasonography with SonoVue. J Ultrasound Med. 2005;24:285-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Catalano O, Nunziata A, Lobianco R, Siani A. Real-time harmonic contrast material-specific US of focal liver lesions. Radiographics. 2005;25:333-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Karhunen PJ. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol. 1986;39:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 212] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Strobel D, Seitz K, Blank W, Schuler A, Dietrich CF, von Herbay A, Friedrich-Rust M, Bernatik T. Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS). Ultraschall Med. 2009;30:376-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Sugimoto K, Moriyasu F, Kamiyama N, Metoki R, Iijima H. Parametric imaging of contrast ultrasound for the evaluation of neovascularization in liver tumors. Hepatol Res. 2007;37:464-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Van Beers BE, Daire JL, Garteiser P. New imaging techniques for liver diseases. J Hepatol. 2015;62:690-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Li W, Wang W, Liu GJ, Chen LD, Wang Z, Huang Y, Liu JY, Xie XY, Lu MD. Differentiation of Atypical Hepatocellular Carcinoma from Focal Nodular Hyperplasia: Diagnostic Performance of Contrast-enhanced US and Microflow Imaging. Radiology. 2015;275:870-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Sandrose SW, Karstrup S, Gerke O, Rafaelsen S. Contrast Enhanced Ultrasound in CT-undetermined Focal Liver Lesions. Ultrasound Int Open. 2016;2:E129-E135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography. 2015;34:3-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |