Published online Jul 7, 2020. doi: 10.3748/wjg.v26.i25.3517
Peer-review started: March 3, 2020
First decision: April 25, 2020
Revised: April 27, 2020
Accepted: June 10, 2020
Article in press: June 10, 2020
Published online: July 7, 2020
Totally implantable access port is a fully implantable drug delivery system that is implanted subcutaneously and can be retained for a long time. Advantages of ports include a simple nursing process, low risk of infection and embolism, and high patient comfort. In order to promote the standardized application of ports in the treatment of digestive tract tumors and reduce port-related complications, the Chinese Research Hospital Association Digestive Tumor Committee, the Chinese Association of Upper Gastrointestinal Surgeons, the Chinese Gastric Cancer Association, and the Gastrointestinal Surgical Group of Chinese Surgical Society Affiliated to Chinese Medical Association have organized multidisciplinary expert discussions at the General Hospital of the People’s Liberation Army and nation-wide expert letter reviews and on-site seminars, and formulated an expert consensus of the operation guidelines.
Core tip: Totally implantable access port is a fully implantable drug delivery system that is implanted subcutaneously and can be retained for a long time. Advantages of ports include a simple nursing process, low risk of infection and embolism, and high patient comfort. Currently no practice guideline is available. In order to reduce port-related complications, the Chinese Research Hospital Association Digestive Tumor Committee, the Chinese Association of Upper Gastrointestinal Surgeons, the Chinese Gastric Cancer Association, and the Gastrointestinal Surgical Group of Chinese Surgical Society Affiliated to Chinese Medical Association have, for the first time, organized multidisciplinary expert discussions at the General Hospital of the People’s Liberation Army and nation-wide expert letter reviews and on-site seminars, and formulated the present expert consensus of the operation guidelines.
- Citation: Zhang KC, Chen L, Chinese Research Hospital Association Digestive Tumor Committee; Chinese Association of Upper Gastrointestinal Surgeons; Chinese Gastric Cancer Association and Gastrointestinal Surgical Group of Chinese Surgical Society Affiliated to the Chinese Medical Association. Chinese expert consensus and practice guideline of totally implantable access port for digestive tract carcinomas. World J Gastroenterol 2020; 26(25): 3517-3527
- URL: https://www.wjgnet.com/1007-9327/full/v26/i25/3517.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i25.3517
Totally implantable access port is a fully implantable drug delivery system that is implanted subcutaneously and can be retained for a long time. It can be used for the infusion of various liquids, including chemotherapy drugs, parenteral nutrient solutions, and blood products. Some advantages of ports include a simple nursing process, low risk of infection and embolism, and high patient comfort. According to the infusion route, types of ports include venous[1], peritoneal[2], arterial[3] and intrathecal[4]. Venous access ports are widely used clinically. Therapies, such as chemotherapy and nutritional support, are often needed during the treatment of digestive tract tumors and require a reliable infusion channel. Ports are handled by various clinical personnel[5], and the standard operation needs to be improved. In order to promote the standardized application of ports in the treatment of digestive tract tumors and reduce port-related complications, the Chinese Research Hospital Association Digestive Tumor Committee, the Chinese Association of Upper Gastrointestinal Surgeons, the Chinese Gastric Cancer Association, and the Gastrointestinal Surgical Group of Chinese Surgical Society Affiliated to Chinese Medical Association have successively organized multidisciplinary expert discussions at the General Hospital of the People’s Liberation Army and nation-wide expert letter reviews and on-site seminars, and reached an expert consensus of the operation guidelines. The clinical questions included in this expert consensus were taken from the controversial points in the letter review by multidisciplinary experts. Corresponding suggestions are recommended based on the quality of the evidence using the evidence-based Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system[6]. The operation guidelines introduces the venous, peritoneal, and arterial implantation methods as there is a great deal of evidence supporting the use of these methods.
The main components of a port include the portal and the catheter. There are single-chamber and double-chamber portals[7], with various sizes. Single-chamber ports are used more often clinically[8]. Ports with the smallest number of chamber(s) that can meet the needs of the patient should be selected to reduce the risk of infection[9]. Double-chamber ports can be used for simultaneous infusion of chemotherapy drugs and parenteral nutrient solution[10]. Although some studies support that double-chamber ports do not increase the incidence of sepsis[11], the compatibility of different infusion fluids should be considered during application.
Port catheters are made of two different materials, silica gel and polyurethane, which are considered equally safe. However, silica gel catheters can easily be blocked, and polyurethane catheters can cause venous thrombosis[12,13]. Catheter ends with open and valvular designs are available. Compared with an open-end catheter, a valvular-end catheter can prevent blood from flowing back into the catheter lumen and does not require heparin washing, but a valvular-end catheter cannot be used to draw blood samples. A prospective randomized controlled study showed that the short-term and long-term complications caused by the two catheters were comparable[14]. Drug-coated catheters can significantly reduce the risk of blood infections[15,16], but there is no evidence of their application in ports.
Recommendation: Single-chamber ports are preferred to reduce the risk of drug interactions that may occur when a double-chamber port infuses liquids simultaneously. Silica gel and polyurethane catheters are safe when used for long-term central venous infusion. (Level of evidence: Medium; degree of recom-mendation: Strong; expert approval rate: 100%).
In 1982, Niederhuber et al[17] completed the first case of port implantation through the cephalic vein by surgical incision. In 1992, Morris et al[18] reported an ultrasound-guided percutaneous port implantation. Presently, the percutaneous puncture route is more commonly applied in clinical practice. The results of a systematic review show that the complications of the percutaneous puncture route are comparable to those after surgical incision, but the success rate of the percutaneous puncture route is higher[19]. The internal jugular vein, subclavian vein, basilic vein, and femoral vein can be selected for the percutaneous puncture route, and the literature reports that the internal jugular vein and subclavian vein are used most often[5]. The selection of the puncture vein should take into account patient-related specific factors (such as whether there is positive pressure ventilation or anatomical dysplasia), relative risks of complications during the operation (hemorrhage, pneumothorax, and thrombosis), and risk of infection. For experienced operators, there is no difference in postoperative complications or patient quality of life between different vein approaches for port implantation[20,21]. A randomized controlled study has confirmed that there are no differences in the incidence of catheter-related thrombotic events or catheter blockage between left-side and right-side port implantation[22]. Percutaneous puncture methods include blind puncture based on anatomical landmarks, real-time ultrasound guidance and venography, and the ultrasound-guided real-time method, which is currently the most common[23]. Research has shown that ultrasound real-time guidance can improve puncture efficiency and reduce venous catheter-related infections[24].
Recommendation: The port is recommended to be implanted through a percutaneous puncture of the internal jugular vein or subclavian vein using real-time ultrasound guidance. (Level of evidence: High; degree of recommendation: Strong; expert approval rate: 96%).
The position of catheter tip is very important for preventing complications and maintaining a smooth infusion. Data shows that the catheter tip position is an independent risk factor for venous thrombosis[25]. Catheter thrombosis can affect the patency of the infusion. When the catheter tip is located at the lower 1/3 of the superior vena cava, the incidence of venous thrombosis and abnormal catheter patency is the lowest[26]. Positioning the catheter parallel to the vessel wall helps to reduce the risk of catheter movement and the damage to the vessel wall. The position of the catheter tip can be confirmed by intraoperative fluoroscopy and postoperative chest radiograph. A study showed that intracardiac electrocardiogram technology can also accurately and conveniently confirm the position of the catheter tip[27].
Recommendation: The catheter tip is recommended to be placed at the lower 1/3 of the superior vena cava, and the location be confirmed by a postoperative chest radiograph. (Level of evidence: Medium; degree of recommendation: Strong; expert approval rate: 100%).
The time interval between the port implantation and first medication may be a risk factor for the occurrence of port-related infections[28]. The results of a prospective cohort study showed that when the intervals between the catheter implantation and first medication were 0-3 d, 4-7 d, and > 7 d, the total incidence rates of complications were 24.4%, 17.1%, and 12.1%, respectively, and the incidence rates of infections were 10.6%, 6.7%, and 2.0%, respectively[29]. The results of a single-center prospective study of 4045 cases also showed that a time interval between port implantation and first medication of > 6 d could significantly reduce the risk of complications and catheter removal rates[30]. However, scholars in China have not found any correlation between the timing of the first medication and complications.
Recommendation: In clinical practice, the timing of first medication should be considered and the first medication should be reasonable according to the regulations of each center. (Level of evidence: Medium; degree of recommendation: Strong; expert approval rate: 94%).
The results of a prospective randomized controlled study showed that the prophylactic use of antibiotics did not reduce the postoperative infection complications[31]. Recently, the results of systematic review showed that 5 (1.39%) out of 360 patients receiving prophylactic antibiotics experienced an infection, while 22 (1.23%) out of 1794 patients who did not receive an antibiotic experienced an infection; there were no statistical differences between the two groups. This indicates that prophylactic antibiotics had no effect on postoperative infection complications[32]. Currently, relevant guidelines do not recommend the routine prophylactic use of antibiotics before or during port implantation[33]. Unnecessary use of antibiotics increases the risk of anaphylaxis, leads to the growth of drug-resistant microorganisms, and increases medical expenses[32].
Recommendation: Routine prophylactic use of antibiotics is not recommended. (Level of evidence: High; degree of recommendation: Strong; expert approval rate: 92%).
Long-term indwelling catheters can easily be blocked by protein biological films generated by a cross-linking reaction between the biological proteins and the catheter. Flushing and sealing are effective measures to prevent these complications, but the specific protocols vary greatly[34]. To reduce the adhesion of injected substances on the inner surface of the catheter, it is generally recommended to flush the catheter with normal saline, infuse the drug, and flush the catheter with normal saline again[35]. The results of randomized controlled studies confirmed that flushing and sealing the catheter with normal saline is not inferior to using heparin[36,37]. A Cochrane systematic review also does not support any advantage of heparin in reducing catheter blockage[38]. During the non-treatment period, a valvular catheter can be sealed with 10 mL normal saline every 4 wk and an open catheter can be sealed with 10 mL normal saline or 5 mL (100 U/mL) heparin every 4 wk[39,40].
Recommendation: Under normal circumstances, the catheter can be flushed and sealed with normal saline. For maintenance, during the non-treatment period a valvular catheter can be sealed with normal saline every 4 wk and an open catheter can be sealed with normal saline or heparin (100 U/mL) every 4 wk. (Level of evidence: High; degree of recommendation: Strong; expert approval rate: 98%).
Port-related infections are caused by various factors. Standardized evidence-based strategies can significantly reduce catheter-related infections[41]. These measures include (operator) hand hygiene, skin disinfection, selection of optimal vascular access, and timely assessment of the necessity of catheter retention. It is not recommended to apply antibacterial ointment to the wound during port implantation[9]. There is no evidence to support the reduction of infections by routine antibiotic catheter sealing, but it is recommended for patients with a history of multiple catheter-related blood infections[42,43]. It is particularly important to provide the necessary training and education for catheter implantation operators and maintenance personnel[44].
Recommendation: The operation and maintenance of the port should be carried out by medical personnel trained on professional standards, and standardized measures should be adopted to prevent port-related infections. (Level of evidence: Medium; degree of recommendation: Strong; expert approval rate: 100%).
Indications for a venous port: (1) Long-term repeated infusion of corrosive and/or irritating drugs; (2) Long-term parenteral nutrition support is needed; (3) Long-term intermittent infusion and the transfusion of blood products is required; (4) It is difficult to establish peripheral venous access; (5) Frequent blood collection and monitoring are required; and (6) Contrast agent bolus injection (high pressure-resistant). Indications for a peritoneal port: Peritoneal metastasis requiring intraperitoneal perfusion chemotherapy[45]. Indications for an arterial port: Hepatic metastasis requiring hepatic artery perfusion chemotherapy[46]. Contraindications: (1) Patients with a massive pleural effusion who cannot tolerate and/or cooperate with surgery; (2) Uncontrolled bacteremia or local infection at the operation site (confirmed or suspected local infection by puncture, bacteremia or symptoms of septicemia); (3) Confirmed or suspected allergies to port materials; (4) Abnormal venous return, such as vena cava compression syndrome; (5) Obvious coagulation dysfunction; (6) No suitable implantable port size for the patient’s physique and/or body shape; (7) Severe pulmonary embolism; (8) Those who have received radiotherapy at the site to be punctured; (9) Signs of thrombosis at the site to be used for the catheter insertion or the patient has undergone a surgery; and (10) The puncture site is located on the same side as the only remaining normal lung tissue (there is a risk of fatal pneumothorax).
Before the port implantation, the patient needs to be evaluated in detail, including the medical history and physical, laboratory, and imaging examinations. The medical history inquiry should include the history of radiotherapy and chemotherapy, the history of central vein catheterization, relevant operation history of the proposed port implantation site, and history of anticoagulant and anti-angiogenic medication use. The physical examination should include an assessment of the anatomy of the proposed portal placement site to assure that it is abnormal and subcutaneous fat thickness/skin condition is acceptable. Laboratory examinations should include routine blood test, immunity and coagulation function. Imaging examinations should include electrocardiogram, erect chest radiograph, and B-mode ultrasonography of the proposed puncture site. The preset catheter insertion length should be estimated according to the chest radiograph.
During preparation for the port implantation operation, it is necessary to communicate with the patients and their family members to inform them of the risks related to the operation, the operation methods and process, as well as matters needing attention after the operation. The informed consent form should be signed and volume expansion should be performed fully before the operation. It is also recommended to take a shower the day before the operation.
Implantation should be completed in an operating room that meets aseptic requirements. Before operation, the integrity and patency of the port kit should be checked.
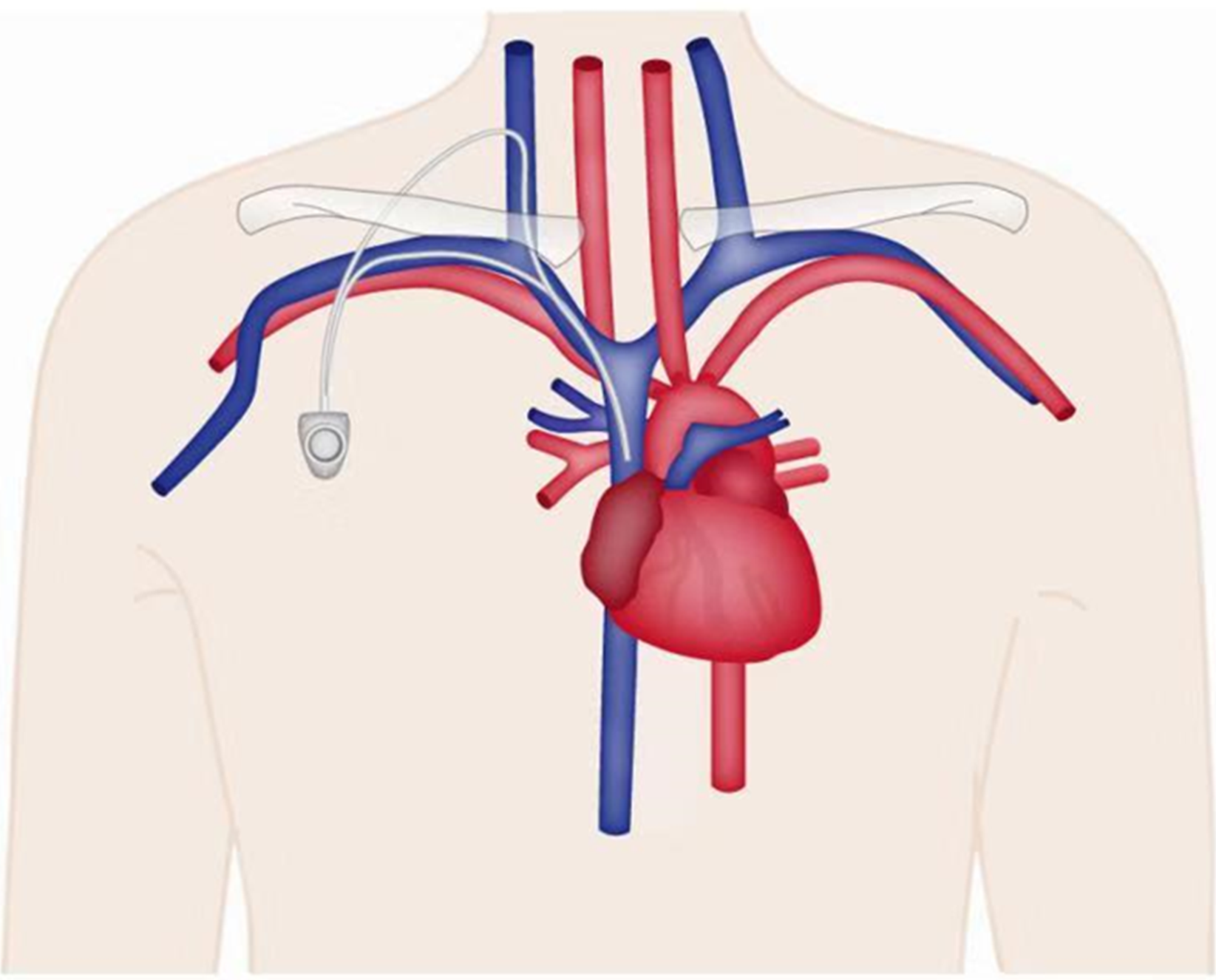
Venous port implantation: Internal jugular vein approach (Figure 1): The patient lies in the supine position with the head turned to the opposite side, exposing the operation area on the neck. The operation plane should be flat and the muscles relaxed. The operator stands on the cranial side of the patient, the B-mode ultrasonography machine is placed on the left side of the patient, and the assistants and console are on the right side of the patient. B-mode ultrasonography is used to probe and locate the internal jugular vein, and to determine the relationship between the carotid arteries and veins and the puncture point. The puncture point, subcutaneous tunnel course, and port pocket position are marked. The skin is disinfected with the collarbone as the center. The disinfection scope includes an area with the upper margin to the level of the ear roots and mandible, the lower margin to the connection line of the bilateral nipples, the margin on the operating side to the posterior axillary line, and the margin on the contralateral side to the clavicle midline. Routine draping is performed. Infiltration with 1% lidocaine is applied for a local anesthesia on the operating region. A small incision of 0.5 cm is made at the puncture site. The puncture needle is guided into the venous lumen layer by layer by holding a B-mode ultrasonography probe, and the needle tip with strong echo light spot in the venous center can be seen after the successful puncture. Blood is drawn to confirm again that the needle tip is in the blood vessel. The guide wire is inserted and slowly fed in by 10-15 cm through the puncture needle. The guide wire is confirmed to enter the superior vena cava by B-mode ultrasonography or fluoroscopy. The peel-away sheath is introduced along the guide wire, and the catheter is introduced through the sheath. The peel-away sheath is sent to the lower 1/3 of the superior vena cava according to the predicted length. Then, the peel-away sheath is removed, blood is drawn to confirm the catheter position, and the rear end of the catheter is clamped. A cutaneous incision is made 2-3 cm below the 1/3 clavicle on the same side. The incision is about 2-3 cm long. A blunt dissection is performed to reach the subcutaneous tissue. A port pocket with a depth of 0.5-1.0 cm is made according to the size of the portal. The upper edge of the portal body is about 1 cm away from the surgical incision site to prevent the atraumatic needle from puncturing on the incision. The tip of the tunneling needle is pushed from the puncture point to the incision of the port pocket to form a subcutaneous tunnel, and the catheter is slowly pulled through the subcutaneous tunnel. The catheter is trimmed to a suitable length and ensured that the trimming edge is flat. The catheter is connected to the portal. The portal is placed into the port pocket and the course and radian of the catheter are adjusted on the neck and in the subcutaneous tunnel to avoid corner folding. A 2-0 non-absorbable suture is used to fix the portal and the surrounding tissues, and suture the neck and port pocket incision, and finally the wound is bound with dressing.
Subclavian vein approach: The patient lies in a supine position with the neck and shoulders elevated with a pad, the head tilted back and turned to the opposite side. The implantation process is roughly the same as that of the internal jugular vein approach, with the following differences: The puncture point is 1-2 cm below the mid-point of the clavicle. The position of the port pocket can be selected by a transverse incision at the needle insertion point without the establishment of a tunnel. The port pocket can also be placed in other positions using tunneling technology. During the puncture, the puncture needle faces the sternoclavicular joint or suprasternal fossa. The insertion angle is horizontal, and the needle is withdrawn while being inserted. Generally, the needle is inserted about 3-4 cm. After blood is seen upon withdrawal, the needle is inserted 1-2 mm further to ensure that the needle tip completely entered the blood vessel. The needle end is fixed, the syringe is disconnected, and the guide wire is sent to the superior vena cava. The other steps are the same as in internal jugular vein implantation.
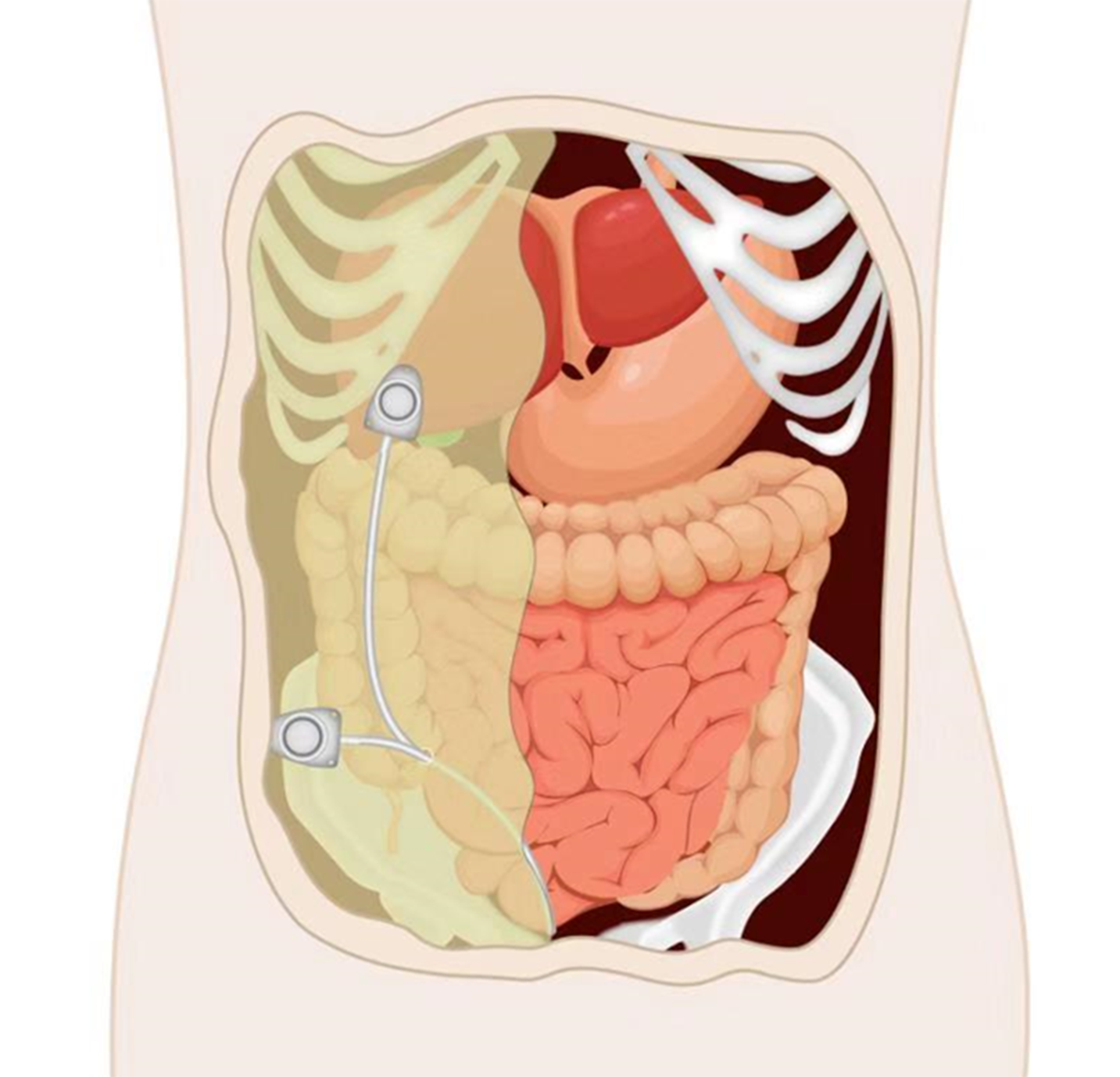
Peritoneal port implantation: A peritoneal port can be implanted by open surgery, laparoscopy, or ultrasound-guided percutaneous implantation (Figure 2). It is recommended to implant the peritoneal port via laparoscope because of its minimal invasiveness, ability to probe the peritoneal cavity to evaluate the lesions, and ability to separate peritoneal adhesions. Under general anesthesia, routine disinfection and draping are performed. Pneumoperitoneum is established through a transumbilical puncture, and the lens are placed in a 10-mm puncture hole. The peritoneal cavity is explored and a 12-mm hole is established to separate peritoneal adhesions if necessary. The catheter is implanted by a method similar to the venous puncture, and the puncture point 5-6 cm laterally of the umbilicus is selected. Under the direct vision of laparoscope, the puncture needle is guided into the peritoneal cavity, the guide wire is introduced, and the puncture needle is removed. The peel-away sheath is fed in along the guide wire and then the guide wire is withdrawn. The catheter is introduced through the sheath. At least 10 cm of the catheter should remain in the peritoneal cavity. Then, the peel-away sheath is removed. The port pocket can be placed near the anterior superior iliac spine on the same side as the puncture point or 2 cm below the costal margin of the medial clavicle on the same side (bony structure support). A longitudinal incision of about 3 cm is made near the anterior superior iliac spine and a transverse incision of about 3 cm is made below the costal margin. A tunneling needle is used to establish a subcutaneous tunnel from the puncture point to the port pocket, and the catheter is guided to the port pocket. The catheter is adjusted to the rectovesical or rectouterine pouch in the pelvic cavity via laparoscope. The catheter is trimmed to a suitable length, connected with the portal, and the patency is checked with an atraumatic needle. The edge of the portal is adjusted to about 1 cm away from the incision and the edge of the portal is fixed with a non-absorbable suture. The incision is sutured and the wound is bound with dressing.
Arterial port implantation: An arterial port can be implanted through the left subclavian or the right femoral artery. However, the right femoral artery approach is preferred. In the supine position, the skin on the groin and perineum is prepared, disinfected, draped, and 1% lidocaine is used for local anesthesia. The modified Seldinger method is used to puncture the right femoral artery and insert the appropriate guide wire and catheter. Using the catheter guide wire technology, the catheter is selectively inserted into the celiac trunk for angiography to determine the tumor location, size, and number of arteries that supply blood to the tumor. The catheter is super-selectively inserted into the artery that supplies blood to the tumor, the guide wire is replaced as appropriate along the catheter, the catheter is withdrawn, the guide wire is retained, the port catheter is fed in along the guide wire until reaching the target blood vessel, and the guide wire is slowly withdrawn. The port catheter is fed in along the super-smooth guide wire and then the guide wire is slowly withdrawn. A cavity for the port pocket is created by an incision at the peritoneal wall of the iliac fossa in the superior lateral direction of the puncture point. The catheter is guided to the port pocket cavity by a tunneling needle. The catheter is intercepted at a proper length and connected to the portal interface filled with heparin saline. The portal is embedded in the port pocket. The skin and subcutaneous tissue at the site of the port pocket are sutured layer by layer, and the wound is bound up with local pressure. The lower limb on the puncture side is straightened and strictly immobilized for 12 h.
Complications related to the implantation operation: Common complications during port implantation include pneumothorax, hemothorax, arterial injuries, thoracic duct injuries, hematoma, air embolisms, intestinal tract injuries, brachial plexus injuries, arrhythmias, and catheter insertion failure. The keys to prevention and management include: (1) Port implantation should be completed by or under the guidance of trained personnel; (2) The puncture should be guided by an ultrasound or digital subtraction angiography. For blind access without image guidance, the puncture should first be attempted with a fine needle to confirm the direction and depth of the needle insertion; (3) The puncture vessel should remain in a positive-pressure state, e.g., by keeping the head lowered and the feet elevated. Patients should be instructed to hold their breath and cooperate during the process of transporting the catheter through the sheath. At the same time, care should be taken to prevent the puncture needle and the opening of the peel-away sheath from being exposed to the air. Using a peel-away sheath with a one-way valve can also effectively prevent air embolism; (4) To prevent the catheter from entering too far into the atrium, intraoperative fluoroscopy or a real-time electrocardiogram can be used to accurately evaluate the catheter tip position; and (5) During peritoneal port implantation, the puncture should be performed with a curved puncture needle under the direct vision of the lens to avoid injuring the intestinal tract.
Portal related complications: Complications related to the portal include incision wound dehiscence, inversion of the portal, drug extravasation, port pocket hematoma, and infection. The keys to prevention and management include: (1) When the patient is malnourished or the possibility of cachexia is predicted, the portal can be implanted deeper to reduce the tension at the skin incision. Anti-angiogenic drugs should be avoided before the operation[47] and patients should be educated to avoid strenuous exercises that can increase wound tension after the operation; (2) The port pocket site should be selected as a location receiving relatively little traction from movements and the size should be matched with the portal, which should be sutured and fixed after being placed in the port pocket; (3) An atraumatic needle which is replaced every 7 d should be used to prevent the septum of the portal from being damaged. For peritoneal port implantation, a reinforced suture should be performed, or a catheter cuff should be used for the incision from which the catheter enters the peritoneal cavity. During intraperitoneal chemotherapy, the patient should adopt the supine position to reduce the risk of atraumatic needle prolapse; and (4) When creating the port pocket, bleeding should be minimized by dissection according to the anatomical layers, and the bleeding in the surgical field of view should be stopped completely. Care bundles should be used to prevent infections.
Catheter-related complications: Catheter-related complications include catheter displacement, thrombosis, pinch-off syndrome, breakage/fracture/detachment, deep vein thrombosis, fibrin sheath formation, superior vena cava erosion, and perforation. The prevention and management measures include: (1) Regular X-ray examinations should be performed for patients with a chronic cough or frequent movement of the limbs to eliminate the risk of catheter displacement; (2) The routine use of anticoagulant drugs is not recommended, but the prophylactic use of anticoagulants can be considered for tumor patients with a high risk of thrombosis and low risk of hemorrhage; the catheter flushing and sealing should be standardized to reduce the risk of thrombosis; (3) For a subclavian vein puncture, the lateral 1/3 of the clavicle should be selected as the puncture point to avoid an included angle between the clavicle and the first rib; and (4) Special care should be taken to ensure that the lock connection and catheter are not folded back during operation. If the patient suffers from pain and swelling around the catheter during the infusion, the infusion should be stopped immediately and an X-ray examination should be conducted to identify abnormalities such as catheter breakages as soon as possible. Patients should be instructed to avoid overstretching the catheter indwelling area during daily activities.
Most ports can perform their functions without complications, but if complications occur at the initial stage of implantation, it may be necessary to remove the device immediately and attempt to implant a new one during the removal operation. Most complications can be treated without removal, but sometimes removal is the only reasonable choice. The overall removal rate due to complications is 5.5%-18%. Most port-related complications occur after the first chemotherapy session. Common reasons for removal include: (1) Infections: Infections around the portal pocket and along the catheter tunnel can generally be treated with reasonably selected antibiotics, while bacteremia is an indication for port removal; (2) Thrombosis: Thrombosis is the second major cause of port removal; removal can be considered if anticoagulation is ineffective and thrombosis complicated with persistent infection is an absolute indication for port removal; (3) Catheter displacement: There are two path-ophysiological types of catheter displacement. One is the pinch-off sign on a chest radiograph, which indicates that the catheter leakage site is between the first rib and the collarbone; the other type occurs in obese patients when the catheter tip migrates to the periphery due to tissue traction in the upright position; (4) Catheter fragments and embolisms caused by catheter fractures: Detached fragments can be taken out through the femoral vein; the port does not need to be removed if there are no additional complications; and (5) Skin corrosion on the port surface: This is very rare. It is recommended to ensure that the skin thickness on the surface is 0.5-1 cm when implanting the port. When the port is removed, the catheter tunnel should be closed immediately to avoid an air embolism.
Health education for patients can reduce the risk of port-related complications. The specific recommendations include: (1) After the wound heals, patients can take showers or do low-intensity exercise such as walking, while strenuous exercises such as pull-ups should be avoided; (2) After the operation, if symptoms such as chest pain, abdominal discomfort, and fever and chills of an unknown cause occur, medical attention should be sought immediately; (3) Avoid local friction with clothes, bra straps, and knapsack shoulder straps to prevent skin rupture. Patients should go to the hospital every 4 wk for professional port maintenance; (4) During CT or MRI examinations, a contrast agent cannot be injected under high pressure unless a high pressure-resistant port is implanted; and (5) Keep the port maintenance manual.
We would like to thank the following participants in this study: Xinyu Qin, Yihong Sun, Fenglin Liu, Zhongshan Hospital, Fudan University; Shan Wang, Yinjiang Ye, Kewei Jiang, Peking University People’s Hospital; Jiafu Ji, Xiangqian Su, Ziyu Li, Peking University Cancer Hospital & Institute, Beijing, China; Huimian Xu, Zhenning Wang, the First Affiliated Hospital of China Medical University; Zhenggang Zhu, Minhua Zheng, Min Yan, Lu Zang, Shanghai Institute of Digestive Surgery, Shanghai Key Laboratory of Gastric Neoplasm, Ruijing Hospital, Shanghai Jiao Tong University School of Medicine; Xuming Bai, the Second Affiliated Hospital of Soochow University; Hui Cao, Gang Zhao, Renji Hospital; Huanqiu Chen, Jiangsu Province Tumor Hospital; Luchuan Chen, Fujian Cancer Hospital; Xiangdong Chen, the First Affiliated Hospital Zhejiang Chinese Medicine University; Pan Chi, Union Hospital Affiliated to Fujian Medical University; Guanghai Dai, Bo Wei, Jiandong Wang, Zhi Qiao, Niansong Qian, Fengyong Liu, Chaojun Zhang, Chinese PLA General Hospital; Xueyi Dang, Zefeng Gao, Shanxi Provincial Tumor Hospital; Jianhong Dong, Affiliated Tumor Hospital of Shanxi Medical University; Ming Dong, the First Hospital of China Medical University; Fanghai Han, Sun Yat-sen Memorial Hospital; Xianli He, Tangdu Hospital; Yulong He, the First Affiliated Hospital, Sun Yat-sen University; Jiankun Hu, West China Hospital; Hua Huang, Shanghai Cancer Center, Fudan University; Gang Ji, Xijing Hospital; Zhigang Jie, the First Affiliated Hospital of Nanchang Hospital; Leping Li, Changqing Jing, Shandong Provincial Hospital; Guoli Li, Nanjing Medical University; Guoxin Li, Nanfang Hospital; Dongbing Zhao, Xiao Li, Yantao Tian, Qian Liu, Cancer Hospital, Chinese Academy of Medical Sciences; Yong Li, Guangdong General Hospital; Yong Li, Qun Zhao, the Fourth Hospital of Hebei Medical University; Han Liang, Tianjin Medical University Cancer Institute & Hospital; Pin Liang, the First Affiliated Hospital, Dalian Medical University; Jian Suo, Quan Wang, the First Hospital of Jilin University; Liguo Tian, Chinese Journal of Practical Surgery; Jin Wan, Guangdong Provincial Hospital of Chinese Medicien; Xin Wang, Peking University First Hospital; Yi Xiao, Peking Union Medical College Hospital; Zekuan Xu, the First Affiliated Hospital of Nanjing Medical University; Su Yan, Affiliated Hospital of Qinghai University; Zhongtao Zhang, Hongwei Yao, Beijing Friendship Hospital; Jiren Yu, the First Affiliated Hospital of Zhejiang University; Yan Zhao, Zhichao Zheng, Liaoning Cancer Hospital & Institute; Yongliang Zhao, the First Affiliated Hospital of Army Medical University; Yanbing Zhou, Affiliated Hospital of Qingdao University; Jiaming Zhu, the Second Affiliated Hospital of Jilin University.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Muguruma N S-Editor: Ma RY L-Editor: MedE-Ma JY E-Editor: Ma YJ
| 1. | Peng JJ, Peng LF, Chen SL, Xu JB, He YL. Application of totally implantable venous access devices in patients with chemotherapy. J Digest Oncol (Elect Ver). 2013;1:56-60. [Cited in This Article: ] |
| 2. | Xue K, Li ZY, Yan GJ, Gao C, Li SX, Ren H, Ji JF. Safety analysis of implanted peritoneal ports in gastric cancer with peritoneal metastasis. Chin J Clin Oncol. 2019;1:34-38. [DOI] [Cited in This Article: ] |
| 3. | Ganeshan A, Upponi S, Hon LQ, Warakaulle D, Uberoi R. Hepatic arterial infusion of chemotherapy: the role of diagnostic and interventional radiology. Ann Oncol. 2008;19:847-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Song L, Lu F, Liu H. Implantation of intrathecal infusion system for intractable cancer pain therapy. Chin J Clin Oncol. 2016;8:339-343. [DOI] [Cited in This Article: ] |
| 5. | Di Carlo I, Pulvirenti E, Mannino M, Toro A. Increased use of percutaneous technique for totally implantable venous access devices. Is it real progress? A 27-year comprehensive review on early complications. Ann Surg Oncol. 2010;17:1649-1656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4091] [Cited by in F6Publishing: 4882] [Article Influence: 375.5] [Reference Citation Analysis (0)] |
| 7. | Kim SH, Oh JS, Chun HJ, Choi BG, Lee HG. Dual-Port versus Mono-Port Implantation for Intra-Arterial Chemoinfusion Therapy for Treatment of Hepatocellular Carcinoma in Patients with Anatomic Hepatic Artery Variation. J Vasc Interv Radiol. 2019;30:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Nezami N, Xing M, Groenwald M, Silin D, Kokabi N, Latich I. Risk Factors of Infection and Role of Antibiotic Prophylaxis in Totally Implantable Venous Access Port Placement: Propensity Score Matching. Cardiovasc Intervent Radiol. 2019;42:1302-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Loveday HP, Wilson JA, Pratt RJ, Golsorkhi M, Tingle A, Bak A, Browne J, Prieto J, Wilcox M, UK Department of Health. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2014;86 Suppl 1:S1-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 654] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 10. | Teichgräber UK, Nagel SN, Kausche S, Streitparth F, Cho CH. Double-lumen central venous port catheters: simultaneous application for chemotherapy and parenteral nutrition in cancer patients. J Vasc Access. 2010;11:335-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Johnson BH, Rypins EB. Single-lumen vs double-lumen catheters for total parenteral nutrition. A randomized, prospective trial. Arch Surg. 1990;125:990-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Seckold T, Walker S, Dwyer T. A comparison of silicone and polyurethane PICC lines and postinsertion complication rates: a systematic review. J Vasc Access. 2015;16:167-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Busch JD, Vens M, Mahler C, Herrmann J, Adam G, Ittrich H. Complication Rates Observed in Silicone and Polyurethane Catheters of Totally Implanted Central Venous Access Devices Implanted in the Upper Arm. J Vasc Interv Radiol. 2017;28:1177-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Biffi R, De Braud F, Orsi F, Pozzi S, Arnaldi P, Goldhirsch A, Rotmensz N, Robertson C, Bellomi M, Andreoni B. A randomized, prospective trial of central venous ports connected to standard open-ended or Groshong catheters in adult oncology patients. Cancer. 2001;92:1204-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1011] [Cited by in F6Publishing: 906] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 16. | Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dündar Y, Gamble C, McLeod C, Walley T, Dickson R. The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technol Assess. 2008;12:iii-iiv, xi-xii, 1-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982;92:706-712. [PubMed] [Cited in This Article: ] |
| 18. | Morris SL, Jaques PF, Mauro MA. Radiology-assisted placement of implantable subcutaneous infusion ports for long-term venous access. Radiology. 1992;184:149-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Hsu CC, Kwan GN, Evans-Barns H, Rophael JA, van Driel ML. Venous cutdown versus the Seldinger technique for placement of totally implantable venous access ports. Cochrane Database Syst Rev. 2016;CD008942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Biffi R, Orsi F, Pozzi S, Pace U, Bonomo G, Monfardini L, Della Vigna P, Rotmensz N, Radice D, Zampino MG, Fazio N, de Braud F, Andreoni B, Goldhirsch A. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol. 2009;20:935-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Biffi R, Orsi F, Pozzi S, Maldifassi A, Radice D, Rotmensz N, Zampino MG, Fazio N, Peruzzotti G, Didier F. No impact of central venous insertion site on oncology patients' quality of life and psychological distress. A randomized three-arm trial. Support Care Cancer. 2011;19:1573-1580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Lin WY, Lin CP, Hsu CH, Lee YH, Lin YT, Hsu MC, Shao YY. Right or left? Side selection for a totally implantable vascular access device: a randomised observational study. Br J Cancer. 2017;117:932-937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Shiono M, Takahashi S, Takahashi M, Yamaguchi T, Ishioka C. Current situation regarding central venous port implantation procedures and complications: a questionnaire-based survey of 11,693 implantations in Japan. Int J Clin Oncol. 2016;21:1172-1182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Karakitsos D, Labropoulos N, De Groot E, Patrianakos AP, Kouraklis G, Poularas J, Samonis G, Tsoutsos DA, Konstadoulakis MM, Karabinis A. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10:R162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 25. | Cadman A, Lawrance JA, Fitzsimmons L, Spencer-Shaw A, Swindell R. To clot or not to clot? That is the question in central venous catheters. Clin Radiol. 2004;59:349-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Caers J, Fontaine C, Vinh-Hung V, De Mey J, Ponnet G, Oost C, Lamote J, De Greve J, Van Camp B, Lacor P. Catheter tip position as a risk factor for thrombosis associated with the use of subcutaneous infusion ports. Support Care Cancer. 2005;13:325-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Walker G, Chan RJ, Alexandrou E, Webster J, Rickard C. Effectiveness of electrocardiographic guidance in CVAD tip placement. Br J Nurs. 2015;24:S4, S6, S8-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Pinelli F, Cecero E, Degl'Innocenti D, Selmi V, Giua R, Villa G, Chelazzi C, Romagnoli S, Pittiruti M. Infection of totally implantable venous access devices: A review of the literature. J Vasc Access. 2018;19:230-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Narducci F, Jean-Laurent M, Boulanger L, El Bédoui S, Mallet Y, Houpeau JL, Hamdani A, Penel N, Fournier C. Totally implantable venous access port systems and risk factors for complications: a one-year prospective study in a cancer centre. Eur J Surg Oncol. 2011;37:913-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Kakkos A, Bresson L, Hudry D, Cousin S, Lervat C, Bogart E, Meurant JP, El Bedoui S, Decanter G, Hannebicque K, Regis C, Hamdani A, Penel N, Tresch-Bruneel E, Narducci F. Complication-related removal of totally implantable venous access port systems: Does the interval between placement and first use and the neutropenia-inducing potential of chemotherapy regimens influence their incidence? A four-year prospective study of 4045 patients. Eur J Surg Oncol. 2017;43:689-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Karanlik H, Kurul S, Saip P, Unal ES, Sen F, Disci R, Topuz E. The role of antibiotic prophylaxis in totally implantable venous access device placement: results of a single-center prospective randomized trial. Am J Surg. 2011;202:10-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Johnson E, Babb J, Sridhar D. Routine Antibiotic Prophylaxis for Totally Implantable Venous Access Device Placement: Meta-Analysis of 2,154 Patients. J Vasc Interv Radiol. 2016;27:339-43; quiz 344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2225] [Cited by in F6Publishing: 2203] [Article Influence: 146.9] [Reference Citation Analysis (0)] |
| 34. | Sona C, Prentice D, Schallom L. National survey of central venous catheter flushing in the intensive care unit. Crit Care Nurse. 2012;32:e12-e19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Goossens GA. Flushing and Locking of Venous Catheters: Available Evidence and Evidence Deficit. Nurs Res Pract. 2015;2015:985686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Goossens GA, Jérôme M, Janssens C, Peetermans WE, Fieuws S, Moons P, Verschakelen J, Peerlinck K, Jacquemin M, Stas M. Comparing normal saline versus diluted heparin to lock non-valved totally implantable venous access devices in cancer patients: a randomised, non-inferiority, open trial. Ann Oncol. 2013;24:1892-1899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Dal Molin A, Clerico M, Baccini M, Guerretta L, Sartorello B, Rasero L. Normal saline versus heparin solution to lock totally implanted venous access devices: Results from a multicenter randomized trial. Eur J Oncol Nurs. 2015;19:638-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | López-Briz E, Ruiz Garcia V, Cabello JB, Bort-Martí S, Carbonell Sanchis R, Burls A. Heparin versus 0.9% sodium chloride locking for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst Rev. 2018;7:CD008462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | The Nebraska Medical Center. Standardizing central venous catheter care: hospital to home. National Guideline Clearinghouse 2013. Available from: URL: https://www.nebraskamed.com/central-line. [Cited in This Article: ] |
| 40. | Chinese Nursing Association Venous Infusion Therapy Committee. Expert consensus on venous catheter maintenance. Zhonghua Huli Zazhi. 2019;9:1334-1342. [DOI] [Cited in This Article: ] |
| 41. | Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725-2732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3000] [Cited by in F6Publishing: 2683] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 42. | O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39:S1-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 699] [Cited by in F6Publishing: 696] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 43. | Gorski LA. The 2016 Infusion Therapy Standards of Practice. Home Healthc Now. 2017;35:10-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 44. | Kolaček S, Puntis JWL, Hojsak I; ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Venous access. Clin Nutr. 2018;37:2379-2391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Chinese Gastric Cancer Association. Consensus of Chinese experts on prevention and treatment of peritoneal metastasis of gastric cancer. Zhonghua Weichang Waike Zazhi. 2017;5:481-490. [DOI] [Cited in This Article: ] |
| 46. | Zhang K, Chen L. Chinese consensus on the diagnosis and treatment of gastric cancer with liver metastases. Ther Adv Med Oncol. 2020;12:1758835920904803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Erinjeri JP, Fong AJ, Kemeny NE, Brown KT, Getrajdman GI, Solomon SB. Timing of administration of bevacizumab chemotherapy affects wound healing after chest wall port placement. Cancer. 2011;117:1296-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |