Published online May 7, 2020. doi: 10.3748/wjg.v26.i17.2119
Peer-review started: January 15, 2020
First decision: February 29, 2020
Revised: March 31, 2020
Accepted: April 10, 2020
Article in press: April 10, 2020
Published online: May 7, 2020
Fistulas are common complications of Crohn’s disease (CD). Gastrocolic fistulas (GFs) are rare, occult and potentially life-threatening complications. Few cases of GFs have been reported. Oral agent contrast-enhanced ultrasound (OA-CEUS) is a novel technique of ultrasound (US) for gut. Contrast agent made by Chinese yam is taken orally to dilate the lumen of the upper gastrointestinal tract. Thus, the impediment of gas inside gastrointestinal tract is removed and a good acoustic window is provided for gastroin-testinal tract scanning. This paper describes a case of GF secondary to CD detected by OA-CEUS when it was missed by endoscopy and computed tomography (CT). To our knowledge, this is the first report of GF secondary to CD detected by OA-CEUS up to date.
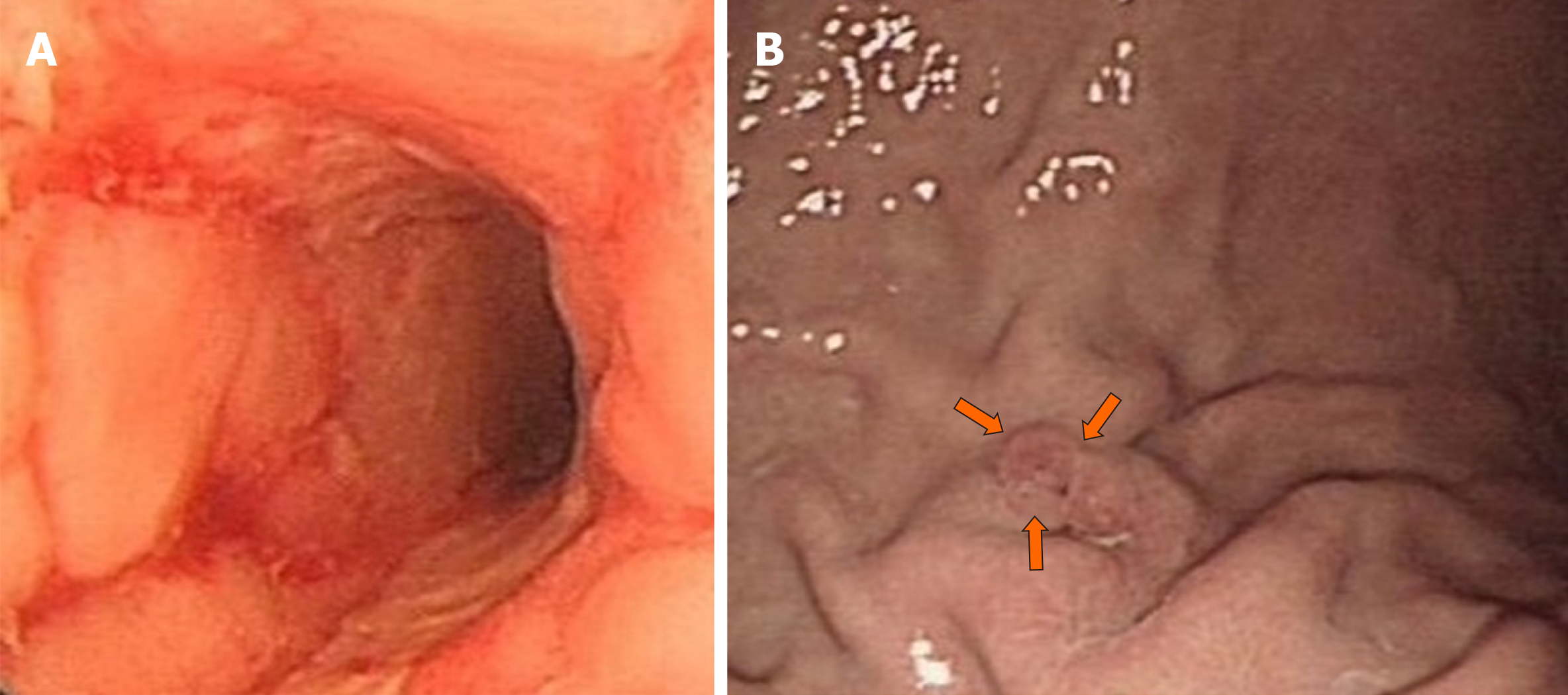
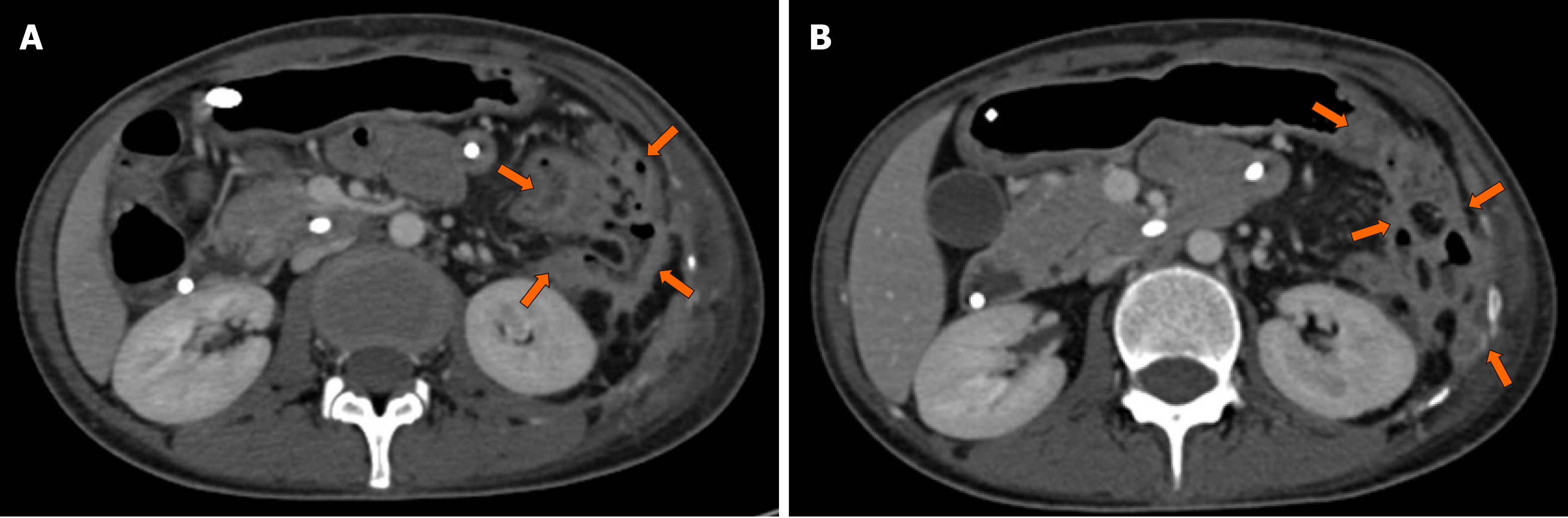
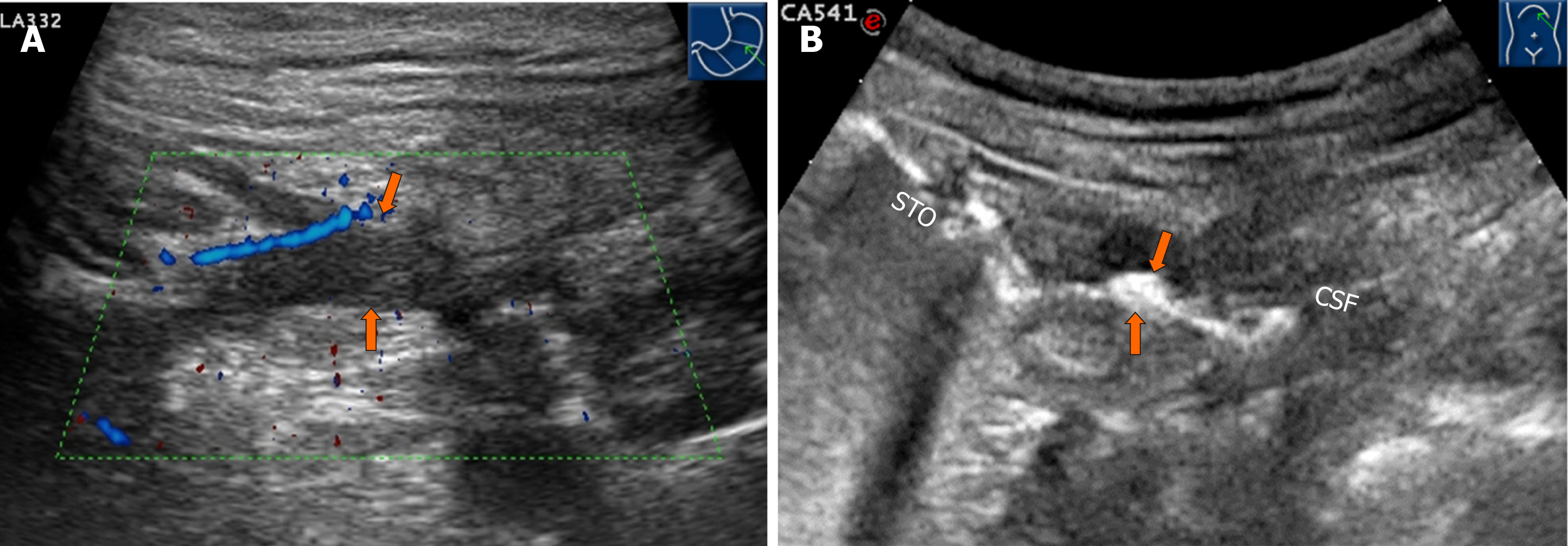
A 29-year-old woman with a 6-year history of CD was admitted to our hospital for abdominal pain and diarrhea for 5 months without obvious predisposing causes. Initial gastroscopy failed to show any evidence of lesions. Colonoscopy revealed multiple erosions, mucosal nodularity, linear ulcers and a cobblestone appearance. A CT scan of her abdomen showed a complex multilocular structure adherent to the greater curvature of the stomach in her left lower abdomen, with fluid, gas and significant surrounding inflammation. CT also demonstrated an abdominal abscess, which was later treated with US-guided drainage. Colonoscopy, gastroscopy and CT missed the presence of a GF. OA-CEUS was performed. A contrast agent made from Chinese yam was taken orally to dilate the lumen of the gastrointestinal tract. A good acoustic window was provided for gastrointestinal tract scanning and the impediment of gas inside the gastrointestinal tract was removed. With the aid of the “window”, a canal with hypoechoic wall was identified connecting the greater curvature of stomach to the splenic colon flexure in free sections. We also observed the hyperechoic gas flowing dynamically inside the canal. Thus, a GF was suspected. US is the first imaging modality taking GF into account. At the same time, OA-CEUS identified the site of the fistula and its two orifices. Gastroscopy was performed again, revealing a small ulcer approximately 5 mm in diameter, which was considered as an orifice. On the basis of OA-CEUS and other examinations, the patient was diagnosed with a GF secondary to CD. Then, laparoscopic exploration, partial stomach resection, transverse colostomy and abdominal abscess drainage were performed. The patient recovered uneventfully.
GFs are rare, occult and potentially life-threatening complications in CD. US is one of the first-line modalities to evaluate CD and its complications. OA-CEUS, a novel technique of US for gut, may be helpful in reducing the possibility of a missed diagnosis of GF.
Core tip: Gastrocolic fistulas (GFs) are rare, occult and potentially life-threatening complications in Crohn’s disease (CD), which can indicate the active status of CD and predict a future surgery within a short period. Ultrasound is one of the first-line modalities to evaluate CD. Oral agent contrast-enhanced ultrasound (OA-CEUS) may be helpful in reducing the possibility of a missed diagnosis of GF. Only a few cases of GF have been reported in the current literature. Surgery is the definitive treatment for GF. It is important to identify and manage GF in a timely manner. This paper describes a GF secondary to CD detected by OA-CEUS when endoscopy and computed tomography missed the diagnosis.
- Citation: Wu S, Zhuang H, Zhao JY, Wang YF. Gastrocolic fistula in Crohn’s disease detected by oral agent contrast-enhanced ultrasound: A case report of a novel ultrasound modality. World J Gastroenterol 2020; 26(17): 2119-2125
- URL: https://www.wjgnet.com/1007-9327/full/v26/i17/2119.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i17.2119
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) characterized by discontinuous skip lesions affecting any part of the gastrointestinal tract from the mouth to the anus[1]. The exact pathogenesis of CD is not clear, but many genetic and environmental factors have been shown to increase the risk of CD and induce an abnormal intestinal immune response[2]. CD can also lead to the cumulative damage to intestinal tissue, and serious complications can occur, such as stenosis, abscesses and fistulas[3]. The above-mentioned complications suggest active and extensive CD, and the treatment plan will be altered[4-6].
Intra-abdominal fistulas occur in approximately 20%-35% of patients with CD[7], which can predict a future surgery within a short period. GFs are rare. As their manifestations are atypical, they cannot be identified easily. As a novel technique of ultrasound (US) for gut , oral agent contrast-enhanced ultrasound (OA-CEUS) was used and detected a GF in our case. This report describes the OA-CEUS to evaluate fistulas secondary to CD.
A 29-year-old woman presented to the gastroenterology department of our hospital complaining of abdominal pain and diarrhea without obvious predisposing causes.
The patient had a 6-year history of CD and gradual worsening abdominal pain and diarrhea over 5 months before admission.
Six years ago, she had the above symptoms, and was diagnosed with CD based on the combination of all clinical presentations and endoscopic, radiologic and pathologic findings at a local hospital. She was treated with azathioprine, with a lack of response to infliximab previously.
Physical examination on admission revealed tachycardia (116/min), an arterial blood pressure of 12.8/8.7 kPa, a temperature of 36.2 °C and a respiratory rate of 16/min.
Blood analysis revealed moderate anemia (hemoglobin of 8.2 g/dL). Serum C-reactive protein content was increased at 110 mg/L (normal range < 5 mg/L) and the erythrocyte sedimentation rate was 74 mm/h. The blood biochemistry results showed hypoalbuminemia at 32 g/L. Prothrombin and partial thromboplastin times, electrocardiogram and urine analyses were normal. Tests for toxoplasma, rubivirus, herpesvirus and cytomegalovirus (TORCH) and Epstein-Barr virus (EBV) were normal.
Initial gastroscopy failed to show any evidence of lesions. A colonoscopy revealed multiple erosions, mucosal nodularity, linear ulcers and cobblestone appearance (Figure 1A). A computed tomography (CT) scan of her abdomen showed a complex multilocular structure adherent to the greater curvature of the stomach in her left lower abdomen (Figure 2), with fluid, gas and significant surrounding inflammation. The CT also demonstrated an abdominal abscess, which was later treated with an US-guided drainage. However, the CT showed only inflammatory changes and failed to show definite evidence of a fistula. The conventional US demonstrated an unevenly-thickened left colonic wall with its thickest part being 9 mm in the spleen flexure using Esaote Mylab Twice ultrasound system. The lumen was characterized by stenosis to varying degrees (the narrowest luminal diameter was approximately 2 mm). Dotted blood signals were revealed in the thickened wall of the fistula canal by color Doppler flow imaging . Then, the patient took an oral intraluminal contrast agent (Tianxia, Huzhou, China) and OA-CEUS was performed. Contrast agent dilated the lumen of the upper gastrointestinal tract. A good acoustic window was provided for gastrointestinal tract scanning and the impediment of gas inside the gastrointestinal tract was removed. With the aid of the ”window”, a canal with hypoechoic wall was identified connecting the greater curvature of stomach to the splenic colon flexure in free sections (Figure 3A). We also observed the hyperechoic gas flowing dynamically inside the canal (Figure 3B). Thus, a GF was suspected. In order to alleviate the patient's physical pain and avoid radiation accumulation, Barium enema or meal was not performed. Another gastroscopy was performed again, revealing an ulcer approximately 5 mm in diameter with purulent secretion, suggesting an orifice of the GF (Figure 1B).
The final diagnosis of the presented case was a GF secondary to CD (Montreal A2L3B3).
Laparoscopic exploration, partial stomach resection, transverse colostomy and abdominal abscess drainage were performed. The diagnosis of GF was confirmed by the operation.
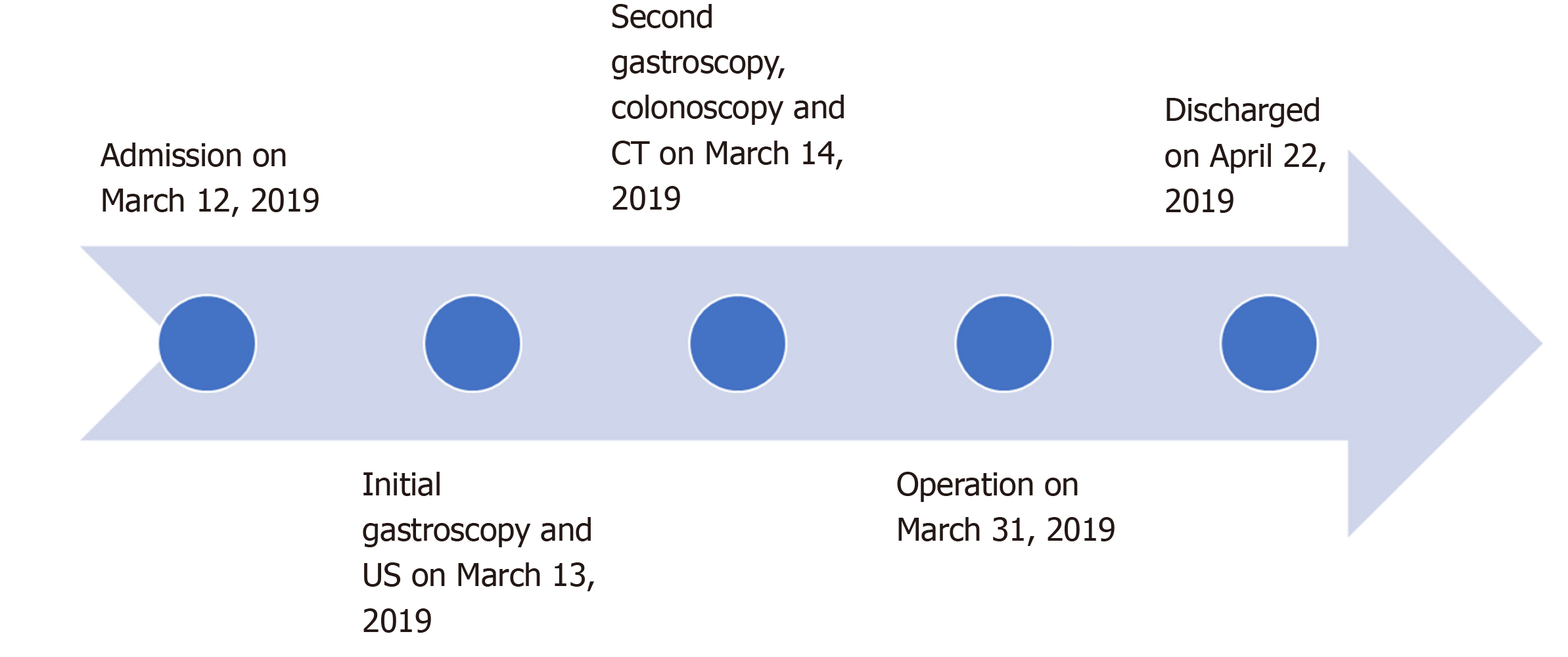
Postoperatively, the patient recovered uneventfully. She was treated with methotrexate and gained weight. The timeline information in this case report is shown in Figure 4.
Fistulas are common complications of CD, resulting from full-thickness inflammation connecting to other organs or through the abdominal wall. Stenosis and chronic inflammatory infiltration are common findings surrounding fistulas[8]. Intra-abdominal fistulas occur in approximately 20-35% of patients with CD[7]. The most common types of internal fistulas in CD are entero-colonic fistulas (29%), followed by enteroenteric fistulas (18%) and enterosigmoid fistulas (17%)[9,10]. GFs secondary to CD are rare[11], with an estimated incidence of 0.6 %[12,13]. Only 40 cases of GF in CD have been reported in the literature[14,15]. Fistulas can be indicators of the active status of CD and contribute to the prediction of short-term intestinal surgery[16,17]. For symptomatic major fistulas (such as those from the stomach to ileum, or mid or proximal small bowel to colon), surgery is advocated, and secondary abscesses need to be drained[5].
The classic triad of GF, including fecal vomiting, weight loss, and diarrhea, only occurs in one-third of cases[12,18]. The typical symptoms of the classic triad did not occur in all patients. The other frequent but less specific manifestations are abdominal pain, emaciation, and ascites. Actually, it is difficult for clinicians to distinguish this entity from an uncomplicated exacerbation of CD. Clinicians should be reminded of the possibility of GF, especially for patients who have above worsening symptoms and signs.
When a fistula secondary to CD is suspected, cross-sectional image examinations are recommended. Cross-sectional imaging techniques are first-line modalities for assessment of complications of CD[19], such as intestinal US and magnetic resonance imaging (MRI)[20]. According to the ECCO-ESGAR Guideline for Diagnostic Assessment in IBD published in 2019, extramural complications in CD, such as fistula and abscesses, should be monitored by cross-sectional imaging, including intestinal ultrasound or MRI (or both), in combination with clinical and laboratory parameters[21]. Endoscopy is not a first-line examination for the diagnosis, because unless the orifice is large enough to be visualized, a fistula between the gastric folds or the colonic haustra can be easily missed[22,23], and tracts are difficult to visualize endoscopically[15]. Due to radiation safety, CT should not usually be used for monitoring disease, particularly in young and child-bearing age female patients and in those who may require repetitive follow-up assessments[21]. The absence of radiation, along with very high soft-tissue contrast, low incidence of adverse events related to intravenous contrast and high diagnostic accuracy in the evaluation of luminal and extraluminal abnormalities prioritize the application of MRI. However, MRI also has some shortcomings in the evaluation of CD: It is noisy, time consuming, space confined, expensive, relatively low availability and prone to motion artifacts due to intestinal peristalsis[24]. On the other hand, CT and endoscopy are useful in evaluating surgical planning, assessing extent of CD and identifying the subsequent etiology, as well as ruling out an alternate cause. Therefore, the combination of various imaging modalities is needed for diagnosis[25]. An accurate preoperative assessment of associated complications is required to plan the surgical approach and intervention[26]. According to a systematic review published in 2011, US, CT and MRI have a high specificity and a relatively low sensitivity for the diagnosis of intra-abdominal fistulas, with similar diagnostic accuracies[19]. The sensitivity of US for the diagnosis of fistulas is 67-87%, and the specificity is 90-100%. The sensitivity of CT is 20-100% and the specificity is 91-100%; the sensitivity of MRI is 40-100%, and the specificity is 93-100%[19]. Intestinal US is not superior to MRI and CT in detection of fistulas. Traditional US was performed without oral contrast agent. The low sensitivity of intestinal US was believed to be due to the interference of intestinal gas. This report documents a GF detected by OA-CEUS that most likely originated from Crohn's colitis. In all reported previously cases, the GFs were all first discovered by barium enema, CT, MRI or endoscopy. To our knowledge, none of them was discovered by US. Because the fistula was too small, with a diameter of 2 mm, and hidden between gastric and colonic folds, neither CT nor endoscopy found the fistula successfully in our case. In addition, instead of being in a standard transverse section, the fistula ran obliquely in our case. This might be another reason for the missed diagnosis by CT. Although CT showed a thickened wall of the stomach and colon, with disordered surrounding inflammation, it failed to show the fistula clearly and indicated only that an orifice was located in the splenic flexure of the colon but not in the gastric wall. Actually, with bowel under distention, the CT modality may miss the diagnosis[27]. OA-CEUS was the first modality taking the diagnosis of GF into account. A contrast agent made from Chinese yam was taken orally to dilate the lumen of the upper gastrointestinal tract. A good acoustic window was provided for the gastrointestinal tract scanning and the impediment of gas inside the gastrointestinal tract was removed[20]. With the aid of the “window”, a canal with hypoechoic wall was identified connecting the greater curvature of stomach to the splenic colon flexure in free sections. We also observed the hyperechoic gas flowing dynamically inside the canal. Thus, a GF was suspected.
In a recent retrospective study including 76 patients that compared the number of stenoses, fistulas, and abscesses on CT enterography (CTE) and/or MR enterography (MRE) before surgery with operative findings, the results showed that CTE and MRE missed many fistulas[28]. The sensitivity of detection of fistulas in CD still needs to be improved.
Being noninvasive, convenient, inexpensive and radiation free, US can dynamically observe abdominal organs from different angles in real time[3]. In our case, OA-CEUS indicated narrow and small fistula and its two orifices according to the low echo of fistula wall and the high echo of flowing gas inside the fistula. It was more sensitive than other imaging modalities. It was reported that OA-CEUS is superior to conventional US in monitoring fistulas[20].
GFs are rare, occult and potentially life-threatening complications in CD. US is one of the first-line modalities to evaluate CD and its complications. OA-CEUS, a novel technique of US for gut, may be helpful in reducing the possibility of a missed diagnosis of GF.
Manuscript source: Unsolicited Manuscript
Corresponding Author's Membership in Professional Societies: Member of the Expert Group on Digestive Imaging Collaboration of Chinese Medical Association Gastroenterology Branch; Member of Reproductive Health and Eugenics Ultrasound Branch of Chinese Association of Ultrasound in Medicine and Engineering; Member of the Ultrasound Professional Committee of Association of Medical Exchanges across the Taiwan Straits.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sandhu DS S-Editor: Gong ZM L-Editor: MedE-Ma JY E-Editor: Ma YJ
| 1. | Feuerstein JD, Cheifetz AS. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin Proc. 2017;92:1088-1103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 2. | Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, Tysk C, O'Morain C, Moum B, Colombel JF; Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD). Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 403] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 3. | Novak K, Tanyingoh D, Petersen F, Kucharzik T, Panaccione R, Ghosh S, Kaplan GG, Wilson A, Kannengiesser K, Maaser C. Clinic-based Point of Care Transabdominal Ultrasound for Monitoring Crohn's Disease: Impact on Clinical Decision Making. J Crohns Colitis. 2015;9:795-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010;105:289-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 654] [Cited by in F6Publishing: 686] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 5. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 705] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 6. | Tun GS, Cripps S, Lobo AJ. Crohn's disease: management in adults, children and young people - concise guidance. Clin Med (Lond). 2018;18:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Nagai S, Nagayoshi K, Sadakari Y, Fujita H, Ohuchida K, Ohtsuka T, Nakamura M. Application of a linear stapler to the laparoscopic treatment of gastrocolic fistula in patients with Crohn's disease. Tech Coloproctol. 2018;22:981-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Scharl M, Rogler G, Biedermann L. Fistulizing Crohn's Disease. Clin Transl Gastroenterol. 2017;8:e106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Ruffolo C, Angriman I, Scarpa M, D'Odorico A, Polese L, Barollo M, Bertin M, Pagano D, D'Amico DF. A gastrocolic fistula in Crohn's disease. Dig Dis Sci. 2005;50:933-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Michelassi F, Stella M, Balestracci T, Giuliante F, Marogna P, Block GE. Incidence, diagnosis, and treatment of enteric and colorectal fistulae in patients with Crohn's disease. Ann Surg. 1993;218:660-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 108] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Zhou B, Li W. A case of gastrocolic fistula secondary to adenocarcinoma of the colon. Int J Surg Case Rep. 2015;15:46-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Pichney LS, Fantry GT, Graham SM. Gastrocolic and duodenocolic fistulas in Crohn's disease. J Clin Gastroenterol. 1992;15:205-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Greenstein AJ, Present DH, Sachar DB, Slater G, Heimann T, Lachman P, Aufses AH. Gastric fistulas in Crohn's disease. Report of cases. Dis Colon Rectum. 1989;32:888-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Lockhart-Mummery HE, Morson BC. Crohn's disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut. 1960;1:87-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 359] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Sheen E, Huang RJ, Triadafilopoulos G. Two evils: gastrocolic fistula and heart failure. Dig Dis Sci. 2014;59:928-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Sato Y, Matsui T, Yano Y, Tsurumi K, Okado Y, Matsushima Y, Koga A, Takahashi H, Ninomiya K, Ono Y, Takatsu N, Beppu T, Nagahama T, Hisabe T, Takaki Y, Hirai F, Yao K, Higashi D, Futami K, Washio M. Long-term course of Crohn's disease in Japan: Incidence of complications, cumulative rate of initial surgery, and risk factors at diagnosis for initial surgery. J Gastroenterol Hepatol. 2015;30:1713-1719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Rigazio C, Ercole E, Laudi C, Daperno M, Lavagna A, Crocella L, Bertolino F, Viganò L, Sostegni R, Pera A, Rocca R. Abdominal bowel ultrasound can predict the risk of surgery in Crohn's disease: proposal of an ultrasonographic score. Scand J Gastroenterol. 2009;44:585-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Logio T, Chaiken B, Roth J, Newman E, Siegel T. The management of Crohn's colitis with colonogastric fistula. Report of a case. Dis Colon Rectum. 1987;30:699-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Panés J, Bouzas R, Chaparro M, García-Sánchez V, Gisbert JP, Martínez de Guereñu B, Mendoza JL, Paredes JM, Quiroga S, Ripollés T, Rimola J. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn's disease. Aliment Pharmacol Ther. 2011;34:125-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 20. | Pallotta N, Vincoli G, Montesani C, Chirletti P, Pronio A, Caronna R, Ciccantelli B, Romeo E, Marcheggiano A, Corazziari E. Small intestine contrast ultrasonography (SICUS) for the detection of small bowel complications in crohn's disease: a prospective comparative study versus intraoperative findings. Inflamm Bowel Dis. 2012;18:74-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Maaser C, Sturm G, Montesani A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, KrustinÅ¡ E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 633] [Cited by in F6Publishing: 805] [Article Influence: 161.0] [Reference Citation Analysis (0)] |
| 22. | Kumar GK, Razzaque MA, Naidu VG, Barbour EM. Gastrocolic fistulae in benign peptic ulcer disease. Ann Surg. 1976;184:236-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Huttenhuis JM, Kouwenhoven EA, van Zanten RA, Veneman TF. Malignant Gastrocolic Fistula: Review of the Literature and Report of a Case. Acta Chir Belg. 2015;115:423-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Kumar S, Hakim A, Alexakis C, Chhaya V, Tzias D, Pilcher J, Vlahos J, Pollok R. Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn's disease: correlation with intraoperative findings and magnetic resonance enterography. J Gastroenterol Hepatol. 2015;30:86-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Bhatnagar G, Von Stempel C, Halligan S, Taylor SA. Utility of MR enterography and ultrasound for the investigation of small bowel Crohn's disease. J Magn Reson Imaging. 2017;45:1573-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Roses RE, Rombeau JL. Recent trends in the surgical management of inflammatory bowel disease. World J Gastroenterol. 2008;14:408-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Hara AK, Alam S, Heigh RI, Gurudu SR, Hentz JG, Leighton JA. Using CT enterography to monitor Crohn's disease activity: a preliminary study. AJR Am J Roentgenol. 2008;190:1512-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Seastedt KP, Trencheva K, Michelassi F, Alsaleh D, Milsom JW, Sonoda T, Lee SW, Nandakumar G. Accuracy of CT enterography and magnetic resonance enterography imaging to detect lesions preoperatively in patients undergoing surgery for Crohn's disease. Dis Colon Rectum. 2014;57:1364-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |