Published online Mar 14, 2018. doi: 10.3748/wjg.v24.i10.1152
Peer-review started: December 12, 2017
First decision: December 27, 2017
Revised: January 16, 2018
Accepted: January 24, 2018
Article in press: January 24, 2018
Published online: March 14, 2018
To investigate the clinicopathological significance of progesterone receptor membrane component 1 (PGRMC1) and PGRMC2 in hepatocellular carcinoma (HCC).
We performed immunohistochemical staining to evaluate the estrogen receptor (ER), progesterone receptor (PR), PGRMC1, and PGRMC2 in a clinical cohort consisting of 89 paired HCC and non-tumor liver samples. We also analyzed HCC data (n = 373) from The Cancer Genome Atlas (TCGA). We correlated the expression status of PGRMC1 and PGRMC2 with clinicopathological indicators and the clinical outcomes of the HCC patients. We knocked down or overexpressed PGRMC1 in HCC cell lines to evaluate its biological significance in HCC cell proliferation, differentiation, migration, and invasion.
We found that few HCC cases expressed ER (5.6%) and PR (4.5%). In contrast, most HCC cases expressed PGRMC1 (89.9%) and PGRMC2 (100%). PGRMC1 and PGRMC2 exhibited significantly lower expression in tumor tissue than in non-tumor tissue (P < 0.001). Lower PGRMC1 expression in HCC was significantly associated with higher serum alpha-fetoprotein expression (P = 0.004), poorer tumor differentiation (P = 0.045) and liver capsule penetration (P = 0.038). Low PGRMC1 expression was an independent predictor for worse disease-free survival (P = 0.002, HR = 2.384, CI: 1.377-4.128) in our cases, as well as in the TCGA cohort (P < 0.001, HR = 2.857, CI: 1.781-4.584). The expression of PGRMC2 did not relate to patient outcome. PGRMC1 knockdown promoted a poorly differentiated phenotype and proliferation of HCC cells in vitro, while PGRMC1 overexpression caused the opposite effects.
PGRMC1 is a non-classical hormonal receptor that negatively regulates hepatocarcinogenesis. PGRMC1 down-regulation is associated with progression of HCC and is a poor prognostic indicator.
Core tip: Neither estrogen receptor or progesterone receptor are commonly expressed in hepatocellular carcinoma (HCC), implying the existence of other hormone-related events in the pathogenesis of HCC. Most primary HCC cases expressed progesterone receptor membrane component 1 (PGRMC1) (89.9%) in our clinical cohort (n = 89). Down-regulation of PGRMC1 was associated with poor tumor differentiation and worse patient survival. The potential prognostic significance was independently validated by The Cancer Genome Atlas (TCGA) database (n = 373). Knockdown of PGRMC1 promoted proliferation and a poorly differentiated phenotype in vitro. Overexpression of PGRMC1 resulted in suppressed proliferation in response to progesterone treatment. PGRMC1 is a prognostic marker and a potential auxiliary therapeutic target for human HCC.
- Citation: Tsai HW, Ho CL, Cheng SW, Lin YJ, Chen CC, Cheng PN, Yen CJ, Chang TT, Chiang PM, Chan SH, Ho CH, Chen SH, Wang YW, Chow NH, Lin JC. Progesterone receptor membrane component 1 as a potential prognostic biomarker for hepatocellular carcinoma. World J Gastroenterol 2018; 24(10): 1152-1166
- URL: https://www.wjgnet.com/1007-9327/full/v24/i10/1152.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i10.1152
The major risk factors for hepatocellular carcinoma (HCC) are chronic liver diseases induced by hepatitis B (HBV) and hepatitis C (HCV), alcohol abuse, and non-alcoholic steatohepatitis. Regardless of etiology, the incidence of HCC is higher in males than in females, with a male to female ratio between 2:1 and 4:1[1]. However, the biological role of sex hormones and their receptors in HCC remains poorly understood. The androgen receptor appears to contribute to HCC development by acting as a tumor promoter[2], whereas the estrogen receptor (ER) appears to act as a tumor suppressor[3]. Oophorectomy performed during premenopausal years has been found to be a risk factor for HCC[4]. The human liver is important in the metabolism of progesterone. Progesterone is known to inhibit autophagy and to augment epirubicin-induced apoptosis in hepatoma cells by increasing oxidative stress and upregulating Fas/FasL[5,6]. In a clinical trial, HCC patients with variant ERs had a significantly longer median survival if megestrol acetate was given[7], suggesting that progesterone is protective against HCC.
Progesterone receptor membrane component 1 (PGRMC1) and PGRMC2, which belong to the membrane-associated progesterone receptor (MAPR) family, have been suggested to be non-classical progesterone receptors[8,9]. These proteins contain a cytochrome b5-like heme/steroid-binding domain. Both PGRMC1 and PGRMC2 are derived from a single gene. The structure of PGRMC1 contains two SH2 target sequences, a SH3 target sequence, a tyrosine kinase target site, two acidophilic kinase (CK2, casein kinase 2) target sites, and binding sites for ERK1 and PDK1. PGRMC2 differs from PGRMC1 in the following aspects: First, the transmembrane domain and N-terminals are different, resulting in diverse interaction partners in the lumen of sub-cellular organelles or on the cell membrane surface. Second, the SH3 target sequence of PGRMC1 with its consensus CK2 site is absent in PGRMC2, suggesting that PGRMC2 may not interact with SH3-containing proteins. Third, PGRMC2 has a predicted PDGFR or EGFR target and an additional potential CK2 site[8]. Therefore, these two proteins may have different interacting partners in terms of connecting with the cellular membrane, organelles and cell signaling molecules.
In the case of tumorigenesis, PGRMC1 expression is associated with advanced-stage disease and poor prognoses in both breast and ovarian cancer[10,11]. However, several studies have shown that PGRMC1 mediates the anti-mitotic actions of progesterone in endometrial and ovarian cancer cells[12,13]. With regard to PGRMC2, copy number loss has been correlated with nodal metastasis in uterine cervical adenocarcinoma, suggesting that PGRMC2 can function as a metastasis suppressor[14]. PGRMC2 negatively affects SKOV-3 ovarian cell migration[15]. Therefore, PGRMC1 and PGRMC2 may have multiple biological functions related to metabolism and carcinogenesis. A proteomic study showed that PGRMC1 was expressed in HCC[16] but there is no information regarding PGRMC1 or PGRMC2 expression patterns in HCC or their clinical significance in this disease. To address this issue, we examined PGRMC1 and PGRMC2 expression in a clinical cohort of paired HCC and non-tumor tissue samples (n = 89). We analyzed an independent HCC cohort (n = 373) from The Cancer Genome Atlas (TCGA cohort) to validate our findings. We also investigated the significance of PGRMC1 in cell proliferation, differentiation, migration, and invasion in vitro.
Eighty-nine patients who underwent surgical resection for HCC at National Cheng Kung University Hospital (NCKUH) from January 1995 to September 2000 were included in this study. Frozen tissue, serum samples and archival paraffin blocks were retrieved from the Human Biobank at NCKUH. HCC differentiation was categorized according to the World Health Organization (WHO) system[17]. Six paraffin samples and four frozen liver samples were retrieved from six normal non-hepatitis patients who underwent surgery for cavernous hemangioma and acted as controls.
We analyzed the TCGA provisional dataset (TCGA dataset, https://tcga-data.nci.nih.gov/tcga/), which contained 373 HCC patients with mRNA expression data (RNA Seq V2 RSEM), clinicopathological indicators, and follow-up information. Of these patients, 50 had expression data pertaining to HCC and matched adjacent non-tumor tissue samples. Disease-free survival (DFS) and overall survival (OS) were calculated based on PGRMC1 and PGRMC2 expression. Expression levels greater than the median were classified as high expression; otherwise, they were classified as low expression.
Protein lysates were prepared from either frozen tissue samples or HCC cell lines. Equal amounts of protein (50 micrograms) were separated via 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The proteins were then transferred to nitrocellulose membranes and stained with Ponceau S to assess transfer quality and ensure equal sample loading. The primary antibodies used were anti-PGRMC1 (Abnova Corporation, Walnut, CA, United States. PAB20135, 1:3000), anti-PGRMC2 (Abnova Corporation. H00010424-M04, 1:1000), anti-PR (Ventana Medical Systems, Inc. 790-2223, 1:100), anti-alpha-fetoprotein (Dako Cytomation, Inc., Carpinteria, CA, USA. A0008, 1:1000), anti-Glypican3 (BioMosaics, Burlington, VT, United States. 1G12, 1:1000) and anti-β-actin (GeneTex Inc. GTX109639, 1:10000). The indicated secondary antibodies (anti-rabbit and anti-mouse, IgG-HRP; Santa Cruz Biotechnology) were used to amplify the signals as appropriate.
Immunohistochemical staining was performed with primary antibodies against ER (Ventana Medical Systems, Inc., Arizona, United States), PR (Ventana Medical Systems, Inc.), PGRMC1 (Abnova Corporation, Walnut, CA, United States. PAB20135) or PGRMC2 (Abnova Corporation. H00010424-M04). ER, PR, PGRMC1 and PGRMC2 expression was graded independently by two pathologists (Tsai HW and Ho CL) according to the percentages of stained hepatocytes or HCC cells. Because PGRMC1 is found in the cytosol and subcellular organelles[8], cytoplasmic staining was considered to be positive. High PGRMC expression was defined as more than two-thirds of the cells exhibited positive staining. In the case of ER and PR, nuclear staining was considered to be positive.
Fifty micrograms of tissue extracts were resolved using 12% SDS-PAGE and were stained using Coomassie brilliant blue r-250. The protein spot located between 20-28 kDa was excised and then in-gel digested using 20 ng/μL of trypsin (Promega, San Luis Obispo, CA, United States, sequencing grade) in 10 mmol/L NH4HCO3 with an enzyme-to-substrate ratio of 1:100 at 37 °C overnight. Peptides were extracted using 50% acetonitrile in 1% formic acid followed by sonication.
A nanoflow high-performance liquid chromatography system (LC Packings, Amsterdam, The Netherlands) equipped with a C18 nano-precolumn cartridge (i.d. = 300 μm × 1 mm, 5 μm C18, P/N160458; LC Packings) and a C18 column (i.d. = 75 μm, o.d. = 280 μm × 15 cm, 3 μm C18, LC Packings) was coupled online to a Q-TOF micro-instrument (Micromass, Manchester, United Kingdom). Mobile phase A was 0.1% formic acid in a 5% acetonitrile solution, and mobile phase B was 0.1% formic acid in 80% acetonitrile. A linear gradient from 5% to 90% B over 60 min at a flow rate of 250 nL/min was applied. A survey MS spectrum with a mass-to-charge ratio ranging from 400 to 1600 was followed by a MS/MS scan at a mass-to-charge ratio ranging from 50 to 2000. The threshold to switch from MS to MS/MS was 10 counts. Raw data were processed into peak lists, and then a Mascot search on a Swiss-Prot (human) protein database was conducted.
The mass tolerance was set at 0.2 Da for both the precursor and product ions. Dimethyl labeling of both the N-terminal and lysine residues was chosen for variable modifications; Carbamidomethyl (C) was chosen for fixed modification, and one miss cleavage on Arg-C was allowed. A cutoff value of 20 was set for the ion score to eliminate proteins with low matches. The default significance threshold for protein identification was set at P < 0.05.
Tissue samples were minced and homogenized at a 1:10 (w:v) ratio with phosphate buffered saline at pH 7.4 using a homogenizer (PRO Scientific Inc., Oxford, CT, United States). The homogenates were centrifuged at 9000 × g at 4 °C for 30 min and the supernatants were immediately analyzed. The progesterone levels in the sera and tissue supernatants were analyzed using the Elecsys and Cobas e-immunoassay analyzer (Roche Diagnostics, Mannheim, Germany).
The HepG2 and Huh7 cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen Corp., Carlsbad, CA, United States) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL) in a humidified incubator at 37 °C with 5% CO2, whereas the PLC/PRF/5 and Hep3B cell lines were maintained in modified Eagle’s medium (MEM, Invitrogen Corp.) supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL) in a humidified incubator at 37 °C with 5% CO2. Huh-7 cells were obtained from JCRB. Hep3B, HepG2 and PLC/PRF/5 cells were obtained from BCRC.
pLKO.1 plasmids expressing small hairpin RNA (shRNA) were purchased from the National RNAi Core Facility (Academia Sinica, Taipei, Taiwan). Lentivirus particles were obtained from RNAi Core of the Research Center of Clinical Medicine, NCKUH. The following shRNAs were used to knock down PGRMC1 expression in HepG2 and Hep3B cells: sh-1: TRCN0000311671, target sequence: 5’-ACTGTGTACTCAGATGAGGAA-3’; sh-2: TRCN0000363644, target sequence: 5’-CGCCGACCCAAGCGATCTGGA-3’; and sh-3: TRCN0000349346, target sequence: 5’- AGGATGAGTACGATGACCTTT -3’. A pLKO_TRC005 plasmid was used as a negative control. PGRMC1 knockdown was performed as described previously[15].
The pMSCVpuro (BD Clontech), pMSCV-PGRMC1, and pSUPERretro vectors were co-transfected into GP2-293T packaging cells along with VSV-G plasmids for 48 h using the calcium phosphate method. Either PLC/PRF/5 or Huh7 cells (1 × 106 cells/well) were seeded in a 6-cm dish and incubated overnight under 5% CO2 at 37 °C. The retroviral supernatant was treated with 8 ng/mL polybrene (Sigma, St. Louis, MO, United States) to infect the cells. Pooled PLC/PRF/5 or Huh7 cells expressing either pMSCVpuro or pMSCV-PGRMC1 were selected using 0.7 μg/mL puromycin (Sigma-Aldrich).
Stable pools were seeded in 24-well plates for 96 h. Triplicate wells were plated at each time point and examined at 24-h intervals over 4 d. The number of viable cells for each time point was determined using the XTT reagent, according to standard protocols (Roche Diagnostics GmbH, Vienna, Austria). To evaluate the impact of progesterone treatment, control cells and PGRMC1-overexpressing cells were cultured to 50% confluence and treated with progesterone (0, 10, 100, or 1000 nmol/L) for 48 h before the XTT assay.
Transwell migration and invasion assays were performed using 24-well 8-micrometer pore Transwell plates, according to the manufacturer’s instructions (Corning, New York, NY, United States). A total of 5 × 104 cells in serum-free DMEM medium were split onto the upper Transwell chamber, the membrane of which was coated with (for invasion) or without (for migration) Matrigel (BD Biosciences, San Diego, CA, United States). The lower chamber was filled with DMEM containing 10% FBS as a chemoattractant. After incubation for 24 h at 37 °C, non-invading cells on the upper side of the chamber were removed from the surface of the membrane by scrubbing, and the membrane was fixed with 4% paraformaldehyde for 10 min. The cells on the membrane were stained using crystal violet solution and detected via microscopy. Mean cell numbers were calculated from five random fields.
The correlations between PGRMC1 expression, PGRMC2 expression, viral infection status, and clinicopathological indicators were assessed using the Wilcoxon rank sum test, the χ2 test, or Fisher’s exact test, as appropriate. Paired data were analyzed using paired Student’s t-tests or the Wilcoxon signed-rank test. For the in vitro experiments, a Student’s t-test was used for simple comparisons. Data are presented as the mean ± SEM. DFS and OS were calculated using the Kaplan-Meier method, and the log-rank test was used to assess the differences between groups. A Cox proportional hazards regression model was used to measure the independence of different factors. A Cox regression was performed via a forward stepwise analysis, and only the prognostic variables that were significant in the univariate analysis were included in the model. P values less than 0.05 were considered statistically significant.
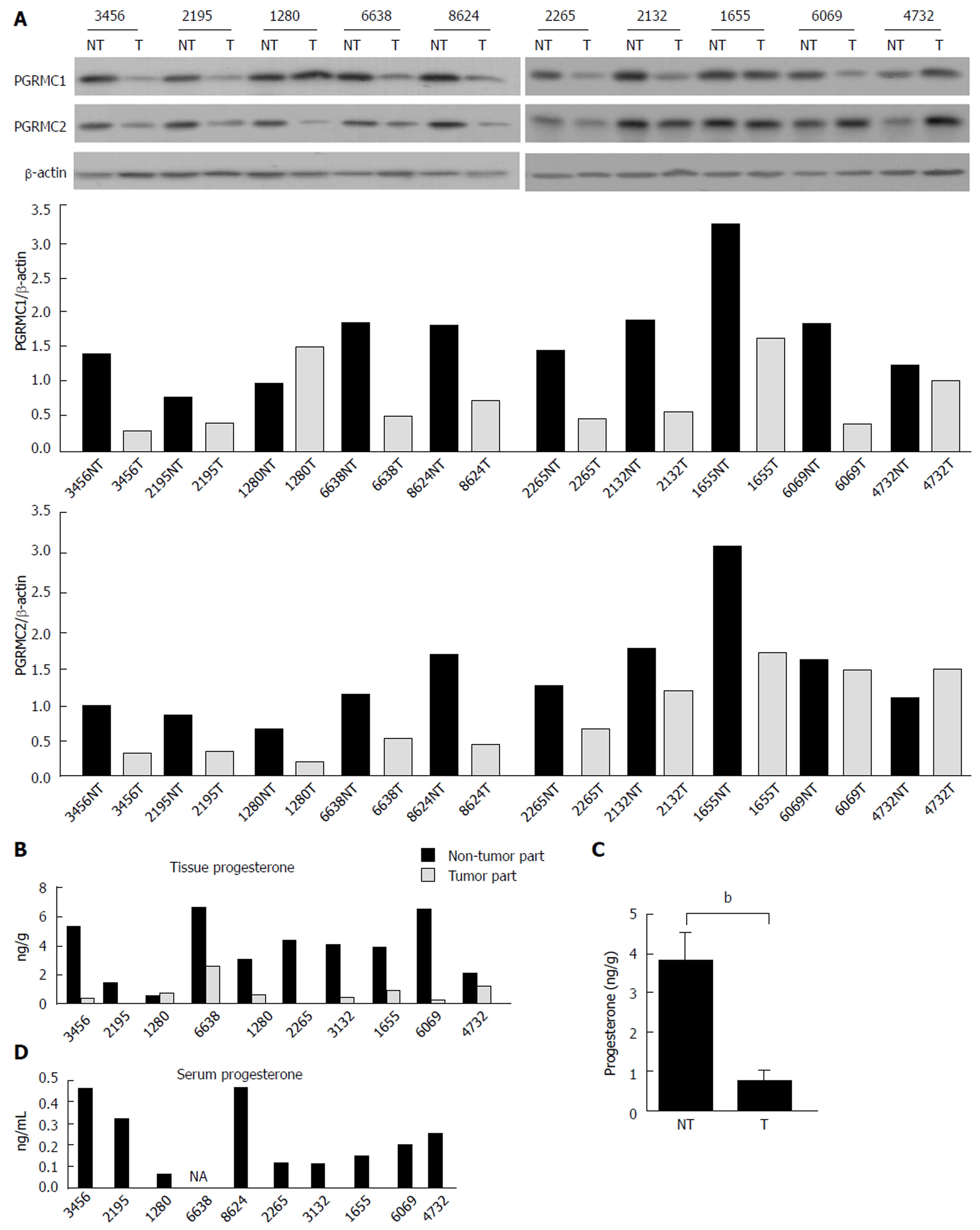
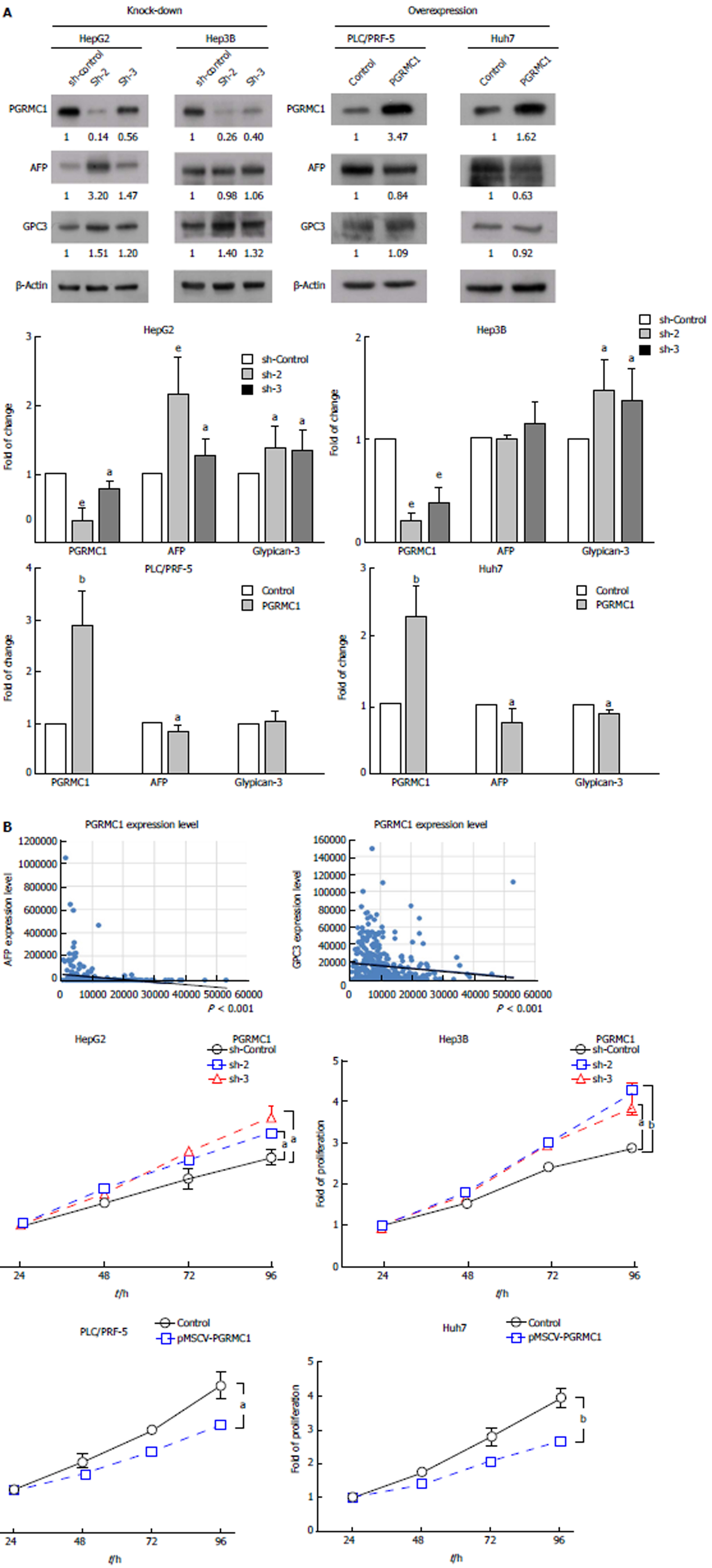
The HCC tissue extract was resolved using SDS-PAGE. The protein spot located between 20 to 28 kDa was in-gel trypsinized and subjected to mass spectrometry (MS). PGRMC1 was detected using MS (Supplementary Table 1). We subsequently examined the expression of PGRMC1 in 10 paired tumor and non-tumor liver samples (7 males and 3 females) using Western blotting. Another membrane-associated progesterone receptor family member, PGRMC2 was also examined for comparison. The age of the patients ranged from 57 to 77 years. Expression of PGRMC1 and PGRMC2 was lower in all HCC samples compared with the corresponding non-tumor liver samples (Figure 1A). Furthermore, both PGRMC1 and PGRMC2 were highly expressed in the normal liver as was the case in the non-tumor liver samples (Supplementary Figure 1A).
Progesterone expression was also evaluated in the HCC patients (n = 10). Serum progesterone levels (mean: 0.276 ng/mL for male patients and 0.167 ng/mL for female patients) were within the 5th-95th physical range in men (0.2-1.4 ng/mL) and postmenopausal women (0.1-0.8 ng/mL) (Figure 1D). However, progesterone levels in the HCC tissue were significantly lower than in the non-tumor liver tissue (P = 0.007, Figure 1B and C).
Immunohistochemical (IHC) staining for PGRMC1 and PGRMC2 was performed in the clinical cohort of 89 paired HCC and non-tumor liver samples. The profiles of the 89 patients are summarized in Supplementary Table 2. The patient population included 65 men and 24 women. The mean age was 55.2 years. The mean follow-up duration was 44.5 mo (range, 0.9-133.1 mo). Sixty-seven patients (75.3%) developed local recurrence at a mean of 21.8 mo after surgery (range, 0.2-115.1 mo). A total of 45 patients (50.1%) died of HCC after having survived for a mean of 35.4 mo after surgery (range, 1.3-123.4 mo). The profiles of the 373 patients in the TCGA cohort are summarized in Supplementary Table 3.
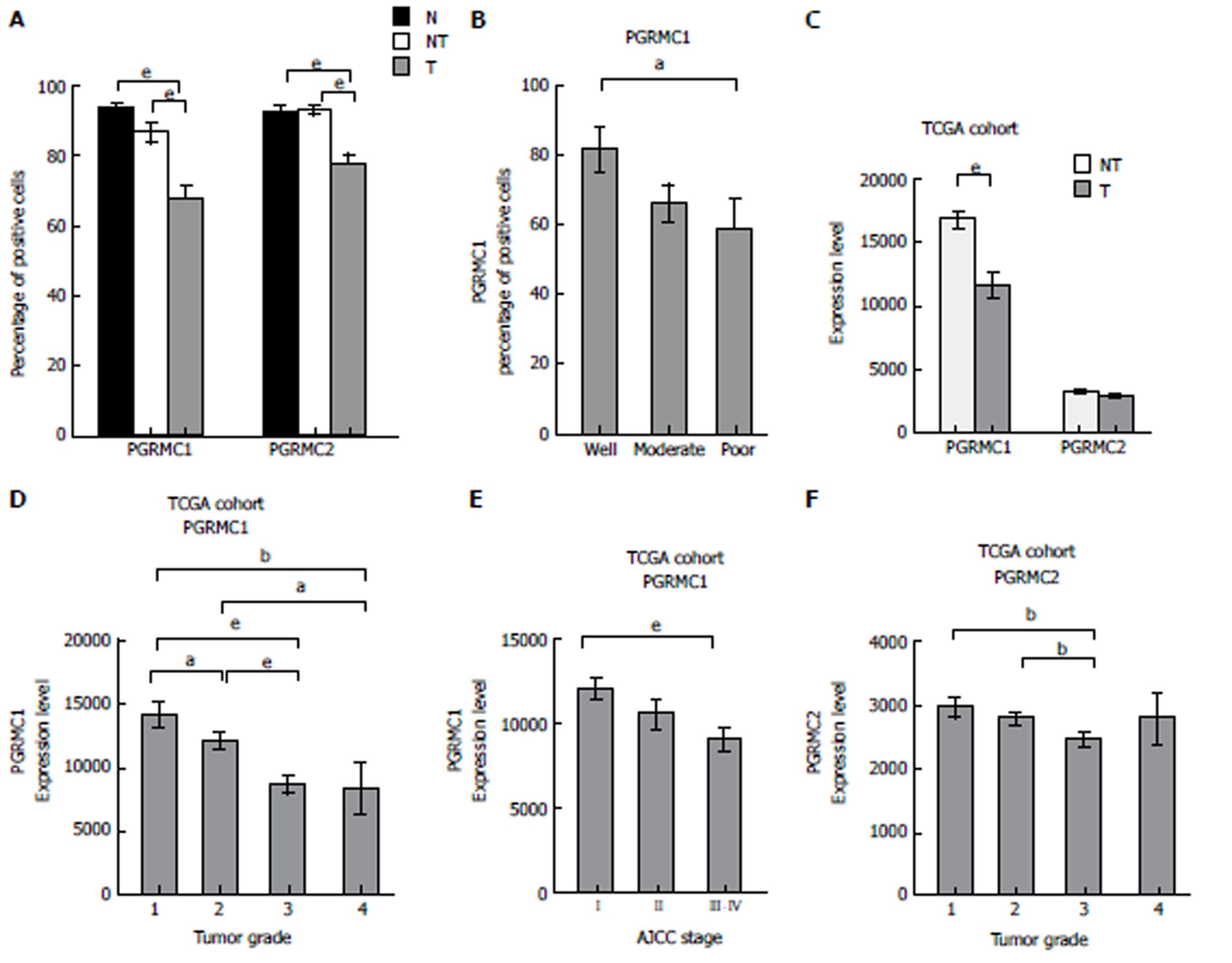
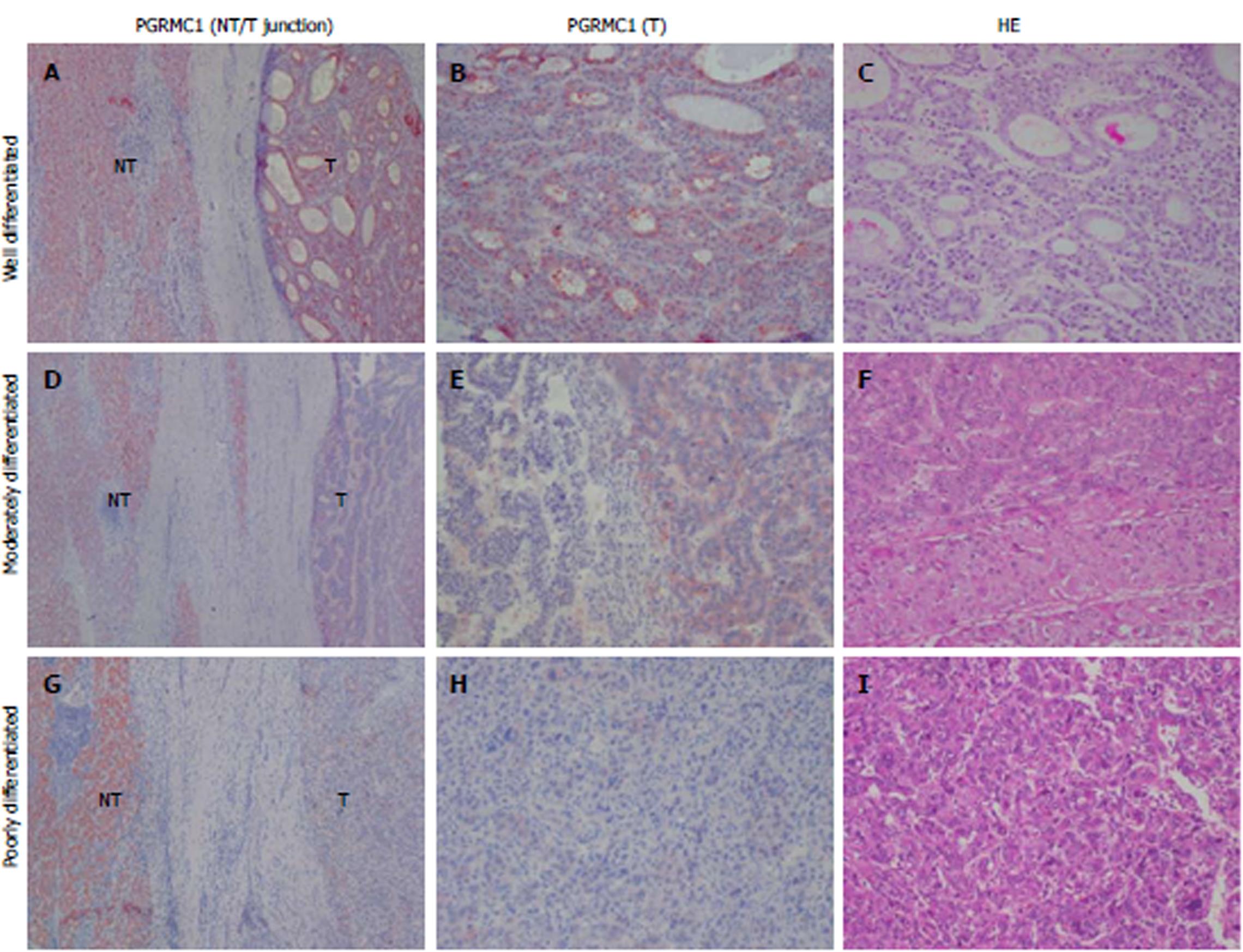
Few HCC cases expressed ER or PR (5.6% and 4.5%, respectively) (Supplementary Figure 2A-C). The mean cell percentage of ER expression was lower in the HCC tissue than in the non-tumor tissue (mean: 0.7% vs 12.4%, P < 0.001) (Supplementary Figure 2D). The mean cell percentage of PR expression was very low in the HCC tissue and non-tumor tissue (mean: 0.12% vs 0.01%, P = 0.172) (Supplementary Figure 2E). Positive IHC staining for PGRMC1 and PGRMC2 was observed in 80 (89.9%) and 89 (100%) cases of HCC, respectively. PGRMC1 (mean: 67.9% vs 86.9%, P < 0.001) and PGRMC2 (mean: 77.3% vs 92.6%, P < 0.001) were expressed at lower percentages in tumor cells than in non-tumor cells (Figure 2A, and 3, and Supplementary Figure 3). IHC expression of PGRMC1 and PGRMC2 in the normal liver tissue was not significantly different from that in the non-tumor liver samples, a result consistent with the Western blot experiment (Figure 2A and Supplementary Figure 1B and 1C). The PGRMC1 and PGRMC2 expression levels were compared among the normal livers of healthy persons, non-cirrhotic livers, and cirrhotic livers of HCC patients (Supplementary Figure 4A and B). There were no significant differences between the liver samples of healthy persons and those of HCC patients. In both non-cirrhotic patients and cirrhotic patients, the PGRMC1 and PGRMC2 expression levels were downregulated in the HCC tissue compared to non-tumor liver tissue (Supplementary Figure 4C-F). In the TCGA cohort, the HCC tissue samples exhibited lower PGRMC1 mRNA expression levels than in the non-tumor tissue samples (P < 0.001), while PGRMC2 levels between the HCC and non-tumor livers were not significantly different (Figure 2C).
Lower PGRMC1 expression in HCC was associated with poor HCC differentiation (P = 0.045) (Figure 2B and Figure 3), younger age (P = 0.016), higher serum alpha-fetoprotein (AFP) levels (P=0.004), and liver capsule penetration (P = 0.038) (Supplementary Table 4). Lower PGRMC2 expression in HCC was significantly associated with tumor capsular invasion (P = 0.048), liver capsule penetration (P = 0.035), and bile duct invasion (P = 0.032) (Supplementary Table 4). In the TCGA cohort, lower PGRMC1 expression in HCC was associated with higher tumor grade (P < 0.001) (Figure 2D), younger age (P = 0.014), female gender (P = 0.015), higher serum AFP expression (P < 0.001), vascular invasion (P = 0.007), and higher AJCC stage (Figure 2E) (P = 0.002) (Supplementary Table 5). Lower PGRMC2 expression in HCC was significantly associated with higher tumor grade (P = 0.022) (Figure 2F) and younger age (P = 0.006) (Supplementary Table 5). Female patients exhibited higher baseline non-tumor liver tissue PGRMC1 expression in the TCGA cohort (P < 0.001) (Supplementary Figure 5A-D). Using corresponding non-tumor liver tissue samples as a reference, it was determined that female HCC patients in the TCGA cohort exhibited greater PGRMC1 down-regulation than male patients (Supplementary Figure 5E-H).
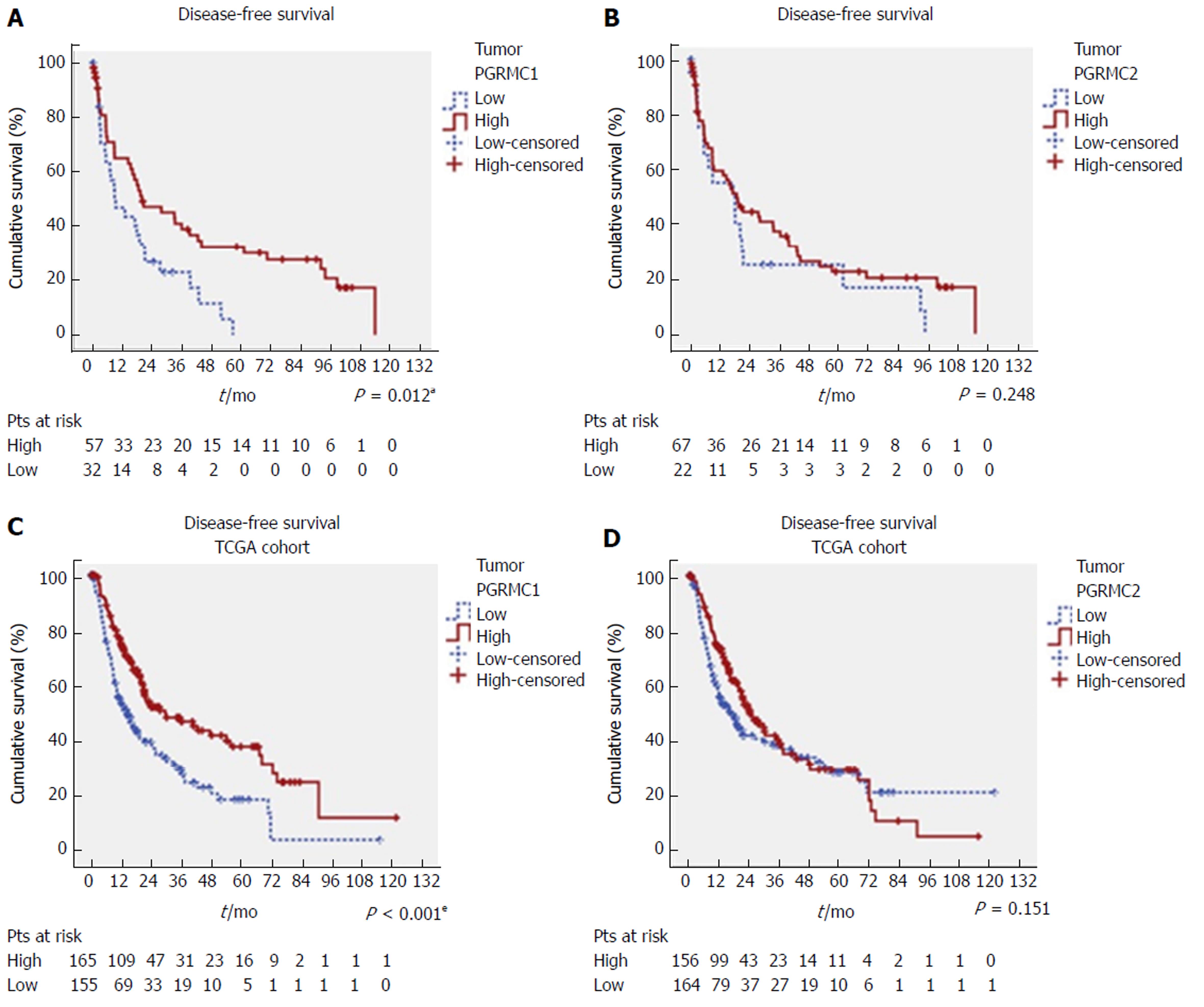
Low IHC PGRMC1 expression in HCC was significantly associated with worse DFS (P = 0.012) (Figure 4A), but was not significantly associated with OS (P = 0.395) (Supplementary Figure 6A). In contrast, PGRMC2 expression was not correlated with survival status (Figure 4B and Supplementary Figure 6B). The univariate analysis showed that the Child-Pugh scores (P = 0.003), satellite lesions (P = 0.003), tumor size (P < 0.001), vascular invasion (P < 0.001), liver capsule perforation (P = 0.047), insufficient surgical margins (P < 0.001), AJCC stage (P < 0.001), and low PGRMC1 expression (P = 0.013) were significant predictors of worse DFS (Table 1). The multivariate analysis showed that the Child-Pugh scores (P = 0.024, HR = 2.005, CI: 1.097-3.665), surgical margins (P < 0.001, HR = 4.720, CI: 2.458-9.063), AJCC stage (P < 0.001, HR = 3.262, CI: 1.895-5.617), and low PGRMC1 expression (P = 0.002, HR = 2.384, CI: 1.377-4.128) were independently associated with DFS (Table 1).
| Factor | DFS univariate | DFS multivariate | |||||
| Group | HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | < 60/≥ 60 | 0.997 | 0.609-1.632 | 0.989 | |||
| Sex | Male/female | 1.014 | 0.599-1.715 | 0.960 | |||
| Viral infection | 0.825 | ||||||
| B/C | 1.177 | 0.703-1.971 | |||||
| B/B + C | 1.068 | 0.448-2.547 | |||||
| Child-Pugh score | 5/≥ 6 | 2.407 | 1.346-4.302 | 0.003a | 2.005 | (1.097-3.665) | 0.024a |
| Cirrhosis | -/+ | 0.743 | 0.664-1.776 | 0.299 | |||
| Serum AFP | < 100/≥ 100 ng/ml | 1.357 | 0.823-2.238 | 0.231 | |||
| Differentiation | W/M-P | 1.682 | 0.943-2.999 | 0.078 | |||
| Multifocal tumor | -/+ | 0.985 | 0.514-1.888 | 0.965 | |||
| Satellite nodule | -/+ | 2.173 | 1.303-3.624 | 0.003a | NS | ||
| Tumor size | < 5/≥ 5 cm | 2.446 | 1.486-4.028 | < 0.001a | NS | ||
| Tumor capsular invasion | -/+ | 0.985 | 0.545-1.780 | 0.959 | |||
| Vascular invasion | -/+ | 2.551 | 1.553-4.190 | < 0.001a | NS | ||
| Liver capsule penetration | -/+ | 2.235 | 1.010-4.945 | 0.047a | NS | ||
| Bile duct invasion | -/+ | 3.200 | 0.986-10.385 | 0.053 | |||
| Margin status, mm | ≥ 1 /< 1 | 3.793 | 2.079-6.920 | < 0.001a | 4.720 | (2.458-9.063) | < 0.001a |
| AJCC stage | I-II/ IIIA-C | 2.907 | 1.738-4.861 | < 0.001a | 3.262 | (1.895-5.617) | < 0.001a |
| PGRMC1 | H/L | 1.918 | 1.145-3.213 | 0.013a | 2.384 | (1.377-4.128) | 0.002a |
| PGRMC2 | H/L | 1.377 | 0.798-2.379 | 0.251 | |||
| ER | -/+ | 0.528 | 0.128-2.173 | 0.376 | |||
| PR | -/+ | 0.777 | 0.243-2.484 | 0.670 | |||
To validate our findings, we analyzed the TCGA cohort data. Patients with low PGRMC1 expression consistently exhibited worse DFS (P < 0.001) and OS than those with high PGRMC1 expression (P = 0.013), as determined by the Kaplan-Meier and log-rank test analyses (Figure 4C and Supplementary Figure 6C). PGRMC2 expression was not correlated with survival status (Figure 4D and Supplementary Figure 6D). The univariate analysis showed that HCC risk factors (P = 0.014), vascular invasion (P < 0.001), AJCC stage (P < 0.001), and low PGRMC1 expression (P < 0.001) were significant predictors of worse DFS (Table 2), and that AJCC stage (P < 0.001) and low PGRMC1 expression (P = 0.014) were significant predictors of worse OS (Supplementary Table 6). The multivariate analysis showed that low PGRMC1 expression was an independent predictor of both worse DFS (P < 0.001, HR = 2.857, CI: 1.781-4.584) and worse OS (P = 0.020, HR = 1.556, CI: 1.072-2.260) (Table 2 and Supplementary Table 6).
| Factor | DFS univariate | DFS multivariate | |||||
| Group | HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | < 60/≥ 60 | 1.019 | 0.756-1.373 | 0.903 | |||
| Sex | Male/female | 1.157 | 0.845-1.585 | 0.364 | |||
| HCC risk factors | 0.014a | < 0.001a | |||||
| B/NAFLD | 1.433 | 0.601-3.414 | 0.417 | 2.903 | (1.130-7.461) | 0.027a | |
| B/Alcohol | 1.449 | 0.948-2.217 | 0.087 | 1.002 | (0.595-1.689) | 0.993 | |
| B/C | 2.421 | 1.422-4.121 | 0.001a | 3.368 | (1.773-6.395) | < 0.001a | |
| Child-Pugh score | A/B-C | 1.332 | 0.709-2.504 | 0.372 | |||
| Cirrhosis | -/+ | 1.117 | 0.761-1.641 | 0.572 | |||
| Serum AFP, ng/ml | < 100/≥ 100 | 1.097 | 0.752-1.599 | 0.632 | |||
| Tumor grade | 2/4/2001 | 1.330 | 0.868-2.038 | 0.191 | |||
| Vascular invasion | -/+ | 2.001 | 1.418-2.823 | < 0.001a | 3.040 | (1.889-4.891) | < 0.001a |
| Residual tumor | -/+ | 1.733 | 0.938-3.200 | 0.079 | |||
| AJCC stage | I-II/III -IV | 2.354 | 1.693-3.272 | < 0.001a | 1.899 | (1.107-3.255) | 0.020a |
| PGRMC1 | H/L | 1.834 | 1.359-2.475 | < 0.001a | 2.857 | (1.781-4.584) | < 0.001a |
| PGRMC2 | H/L | 1.242 | 0.923-1.671 | 0.152 | |||
As PGRMC1 was significantly associated with HCC prognosis, we examined its biological significance in vitro. HepG2 and Hep3B cells exhibited higher PGRMC1 expression than PLC/PRF/5 and Huh7 cells. Therefore, we knocked down PGRMC1 in HepG2 and Hep3B cells and overexpressed PGRMC1 in PLC/PRF/5 and Huh7 cells (Figure 5A). Both AFP and glypican-3 (GPC3) are well-known oncofetal proteins in malignant transformation and dedifferentiation of HCC. Higher expression of these markers has been associated with poor differentiation of HCC[18,19]. Knockdown of PGRMC1 resulted in increased expression of AFP and GPC3 in HepG2 cells, while GPC3 expression was increased in Hep3B cells (Figure 5A). In contrast, overexpression of PGRMC1 suppressed expression of AFP in PLC/PRF/5 cells and suppressed expression of AFP and GPC3 in Huh7 cells (Figure 5A). In addition, PGRMC1 expression was inversely correlated with AFP and GPC3 expression in the HCC tissue samples (Figure 5B) and serum AFP levels (Supplementary Tables 4 and 5). PGRMC1 knockdown resulted in significantly increased proliferation of HepG2 and Hep3B cells (Figure 5C). In contrast, PGRMC1 overexpression resulted in decreased proliferation of PLC/PRF/5 and Huh7 cells (Figure 5C). PGRMC1 did not have significant effects on cell migration and invasion (Supplementary Figure 7).
As PGRMC1 was downregulated in HCC and it has been suggested that it mediates the anti-proliferative effects of progesterone[12,13], we assessed if overexpression of PGRMC1 in HCC cells could increase the anti-proliferative effect under progesterone treatment. PGRMC1 overexpression resulted in a significant decrease in the proliferation of PLC/PRF-5 cells and Huh7 cells in response to progesterone treatment (10-6 mol/L) (Supplementary Figure 8A and B). Expression of PR was not increased in these cells (Supplementary Figure 8C). On this basis, it is suggested that a more plausible explanation for the significant progesterone treatment effect on PGRMC1-overexpressing PLC/PRF-5 and Huh7 cells may be the overexpression of PGRMC1 per se rather than upregulated PR in these cells.
In this study, few HCC cases expressed ER and PR (5.6% and 4.5%, respectively), implying that an alternative hormone-related event may be involved in this sexually dimorphic malignancy. Both PGRMC1 and PGRMC2 were expressed in normal liver and non-tumor liver, but were down-regulated in HCC. In addition, the level of progesterone was lower in the HCC as compared to the non-tumor liver, suggesting that progesterone-related signaling is down-regulated in the pathogenesis of HCC. As down-regulated PGRMC1 and PGRMC2 were correlated with poorer differentiation and higher tumor grading, PGRMC down-regulation may play an important role in the progression of hepatocarcinogenesis. PGRMC1 differs from PGRMC2 in the transmembrane domain, N-terminals and SH3 target sequence, resulting in different interaction partners in terms of connecting with the cellular membrane, organelles and cell signaling molecules[8]. PGRMC1 has been proposed as a sigma-2 receptor with capability to inhibit tumor growth[20-22]. In our study, PGRMC1 was inversely associated with AFP and AJCC stage compared with PGRMC2. In multivariate analysis, PGRMC1 was an independent parameter in predicting better patient survival in two different cohorts. Therefore, only PGRMC1 was proved to be a prognostic biomarker in HCC. All of our patients had HBV and/or HCV infections, while more than half of the patients in the TCGA cohort had alcoholic and/or non-alcoholic fatty liver disease, suggesting that the findings of this investigation are universal. Together, PGRMCs, especially PGRMC1, may play a protective role in liver tumorigenesis.
Experiments in vitro demonstrated that PGRMC1 knockdown results in a poorly differentiated phenotype of HCC cells and increased proliferation. PGRMC1 contains two SH2 target sequences, an SH3 target sequence, a tyrosine kinase target site, two acidophilic kinase target sites, and ERK1 and PDK1 consensus binding sites[8]. A prior report showed that PGRMC1 can interact with beta-tubulin and inhibit mitosis by increasing mitotic spindle stability[13]. PGRMC1 protein has also been proposed as a sigma-2 receptor binding site[20], and activation of the sigma-2 receptor can inhibit tumor growth[21,22]. Thus, PGRMC1 is speculated to inhibit HCC progression through activation of hormone-independent signaling events.
PGRMC1 contains a motif common to heme-binding proteins, suggesting a role in oxidative metabolism[23]. Free heme, i.e., heme not appropriately bound by hemoproteins or heme-binding proteins, is a powerful pro-oxidant agent and therefore potentially toxic[24]. Heme-binding proteins are required to maintain cellular stasis and to detoxify cells. Excessive heme iron has been reported to increase the risk of several types of cancer, such as colon cancer[25], gastric cancer[26], esophageal cancer[27], and HCC[28]. As a heme-binding protein, PGRMC1 has been reported to interact with P450 proteins and protect cells from DNA damage[29-31]. Therefore, PGRMC1 may protect hepatocytes from oxidative stress and suppress carcinogenesis by appropriate heme delivery or heme containment.
Most prior PGRMC1 studies have been focused on the female genital organs or female-related cancers. PGRMCs are involved in regulating the menstrual cycle and ovarian granulosa cell function by acting as progesterone receptors[9,32]. Endometrial expression of PGRMC1 in menstrual cycling is most abundant during the proliferative phase, while expression of PGRMC2 is highest during the secretory phase. These results highlight the differences between PGRMC1 and PGRMC2 in response to steroid hormones[32]. Previous reports showed that PGRMC1 mediates the anti-mitotic actions of progesterone in endometrial and ovarian cancer cells[12,13,33]. In immortalized granulosa cells, PGRMC1 suppresses cell cycle entry by binding to the GTPase activating protein binding protein 2[33]. PGRMC1 also suppresses the T-cell-specific transcription factor/lymphoid enhancer factor (Tcf/Lef) and its downstream c-myc activity in ovarian granulosa cells[9]. In this investigation, we provide evidence that overexpression of PGRMC1 may also activate the non-classical PR pathway in tumorigenesis. The potential of PGRMC1 being an alternative target for auxiliary anti-HCC treatment deserves further investigation.
Previous studies have shown that high levels of progesterone can be observed in patients with cirrhosis[34]. This is likely due to impairment of progesterone metabolism in the liver. It is controversial whether high levels of progesterone are associated with premalignant cirrhosis. Previous studies have shown that the occurrence of natural menopause at a younger age or oophorectomy performed at age 50 or younger is associated with increased risk of HCC[4,35]. PR expression in HCC has been correlated with a better prognosis[36]. These findings suggest that progesterone may be protective against HCC. Furthermore, progesterone can serve as the precursor for the major steroid hormones (androgens, estrogens, and corticosteroids). The oncogenic effects of androgen and the protective effects of estrogen and progesterone in liver may also depend on the hormonal receptors expressed on hepatocytes or cancer cells[4].
Sex hormones can exert different tumorigenic properties depending on the tissue type. For example, contrary to their hypothetical protective role in liver cancer development, chronic exposure to estrogens favors carcinogenesis in the breast and uterus. Expression of PGRMC1 was shown to be upregulated in breast cancer and ovarian cancer and was found to be associated with advanced stage or poor prognosis[10,11]. Its expression was more often detected in ER-negative breast cancers[10], and it may act via cross-talk with nuclear or extranuclear ER receptors[37]. PGRMC1 has been localized in hypoxic areas of breast cancer[10] and demonstrated to activate the expression of vascular endothelial growth factor in glial cells[38]. A D120G mutant of PGRMC1 increased the susceptibility of breast cancer cells to doxorubicin and camptothecin treatment[39]. However, PGRMC1 has been reported to be associated with EGFR in lung cancer cells and to enhance susceptibility to the EGFR inhibitor, erlotinib[40]. Overexpression of PGRMC1 in the MCF-7 breast cancer cell line sensitizes cancer cells to hydrogen peroxide treatment with corresponding hyperphosphorylation of Akt and IkB proteins[29]. These findings suggest that PGRMC1 plays a plethora of biological roles in human cancers. In contrast to breast and ovarian cancer, PGRMC1 is downregulated in HCC. PGRMC1 is located on chromosome Xq22-q24. A prior genomic study found a frequent loss of heterozygosity of Xq (43%) in HCC[41] with a progressive increase in fractional allelic imbalance from cirrhotic nodules at progressive stages (11%-57%) to HCC, suggesting its involvement in hepatocarcinogenesis. Furthermore, let-7/miR-98 was reported to repress PGRMC1[42,43], and gradually elevated miR-98 has been associated with the progression of liver cancer[44]. Therefore, microRNA could be an alternative regulatory mechanism in suppression of PGRMC1 expression. Further study is needed to clarify the mechanisms of PGRMC downregulation in HCC.
Overall, men are two to four times more likely to develop HCC than women[1]. Estrogen has been shown to inhibit IL-6 production[45]. Foxa1/a2 may interact with either the ER or AR to activate different hepatocyte target genes[46]. HBx can increase AR N-terminal transactivation domain activation through c-Src kinase and enhance AR dimerization by inhibiting GSK-3 activity[47]. This information may explain, in part, the molecular mechanisms underlying gender differences in HCC development. Female patients in the current study exhibited higher baseline non-tumor liver tissue PGRMC1 mRNA expression and greater HCC PGRMC1 down-regulation than male patients, suggesting that greater PGRMC1 down-regulation is needed to induce HCC transformation in female patients, thus causing the gender disparities associated with HCC development.
In conclusion, expression of PGRMC1 and PGRMC2 was suppressed in HCC, and PGRMC1 down-regulation promoted HCC progression. PGRMC1 may play a protective role in hepatocarcinogenesis by inhibiting cell proliferation and tumor dedifferentiation. Further study is necessary to evaluate the potential of targeting PGRMC1 in HCC treatment.
Hepatocellular carcinoma (HCC) is a sexually dimorphic disease with a significantly higher incidence in males than females. The androgen receptor appears to function as a tumor promoter, whereas the estrogen receptor appears to act as a tumor suppressor for HCC. Whether additional hormone-related events are implicated in the pathogenesis of HCC remain to be clarified.
The membrane-associated progesterone receptors, i.e. PGRMC1 and PGRMC2, have been investigated in female cancers of the breast, endometrium and ovary. PGRMC1 is thought to coordinate non-classical progesterone signaling. PGRMC1 was demonstrated to mediate the anti-mitotic actions of progesterone in endometrial and ovarian cancer cells. This study was performed to examine the significance of PGRMC1 and/or PGRMC2 in the progression of HCC.
The aim of this study was to clarify the potential significance of PGRMCs as prognostic biomarkers in HCC and their biological effects in vitro.
Immunohistochemical staining of the estrogen receptor (ER), progesterone receptor (PR), PGRMC1 and PGRMC2 was performed in a clinical cohort consisting of 89 cases of paired HCC and non-tumor liver. The clinical implications of PGRMCs in HCC (n = 373) from The Cancer Genome Atlas (TCGA) database were also analyzed. The expression of PGRMC1 and PGRMC2 was correlated with clinicopathological indicators and the clinical outcome of HCC patients. The impact of PGRMC1 on the biological effects of HCC was investigated by knocking down its expression in HepG2 and Hep3B cell lines, and overexpressing in PLC/PRF-5 and Huh7 cell lines. The analyzed cellular functions included proliferation, differentiation, migration, and invasion.
Primary HCC demonstrated a high incidence of PGRMC1 (89.9%) and PGRMC2 (100%) expression, respectively. Down-regulated PGRMC1 was significantly associated with higher serum alpha-fetoprotein levels, poor tumor differentiation, liver capsule penetration, and the risk of recurrence. Low PGRMC1 expression was an independent indicator of worse disease-free survival. Knock-down of PGRMC1 promoted a poorly differentiated phenotype and proliferation of HCC in vitro, while over-expression of PGRMC1 suppressed cell proliferation.
PGRMC1 is a prognostic marker for HCC. PGRMC1 may play a protective role in hepatocarcinogenesis by inhibiting cell proliferation and tumor dedifferentiation.
PGRMC1 could be a novel therapeutic target for human HCC, especially as a biotarget of chemoprevention.
We are grateful for the support from the Human Biobank, Research Center of Clinical Medicine and the Cancer Data Bank of National Cheng Kung University Hospital.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Elalfy H, Guan YS, Hashimoto N S- Editor: Gong ZM L- Editor: Webster JR E- Editor: Ma YJ
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3846] [Cited by in F6Publishing: 4102] [Article Influence: 241.3] [Reference Citation Analysis (2)] |
| 2. | Ma WL, Hsu CL, Yeh CC, Wu MH, Huang CK, Jeng LB, Hung YC, Lin TY, Yeh S, Chang C. Hepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikis. Hepatology. 2012;56:176-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Keng VW, Largaespada DA, Villanueva A. Why men are at higher risk for hepatocellular carcinoma? J Hepatol. 2012;57:453-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Yeh YT, Chang CW, Wei RJ, Wang SN. Progesterone and related compounds in hepatocellular carcinoma: basic and clinical aspects. Biomed Res Int. 2013;2013:290575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Chang WT, Hsieh BS, Cheng HL, Lee KT, Chang KL. Progesterone augments epirubicin-induced apoptosis in HA22T/VGH cells by increasing oxidative stress and upregulating Fas/FasL. J Surg Res. 2014;188:432-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Chang WT, Cheng HL, Hsieh BS, Chiu CC, Lee KT, Chang KL. Progesterone increases apoptosis and inversely decreases autophagy in human hepatoma HA22T/VGH cells treated with epirubicin. ScientificWorldJournal. 2014;2014:567148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Villa E, Ferretti I, Grottola A, Buttafoco P, Buono MG, Giannini F, Manno M, Bertani H, Dugani A, Manenti F. Hormonal therapy with megestrol in inoperable hepatocellular carcinoma characterized by variant oestrogen receptors. Br J Cancer. 2001;84:881-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105:16-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Peluso JJ, Pru JK. Non-canonical progesterone signaling in granulosa cell function. Reproduction. 2014;147:R169-R178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Neubauer H, Clare SE, Wozny W, Schwall GP, Poznanovic S, Stegmann W, Vogel U, Sotlar K, Wallwiener D, Kurek R. Breast cancer proteomics reveals correlation between estrogen receptor status and differential phosphorylation of PGRMC1. Breast Cancer Res. 2008;10:R85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Peluso JJ, Liu X, Saunders MM, Claffey KP, Phoenix K. Regulation of ovarian cancer cell viability and sensitivity to cisplatin by progesterone receptor membrane component-1. J Clin Endocrinol Metab. 2008;93:1592-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Friel AM, Zhang L, Pru CA, Clark NC, McCallum ML, Blok LJ, Shioda T, Peluso JJ, Rueda BR, Pru JK. Progesterone receptor membrane component 1 deficiency attenuates growth while promoting chemosensitivity of human endometrial xenograft tumors. Cancer Lett. 2015;356:434-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Lodde V, Peluso JJ. A novel role for progesterone and progesterone receptor membrane component 1 in regulating spindle microtubule stability during rat and human ovarian cell mitosis. Biol Reprod. 2011;84:715-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Hirai Y, Utsugi K, Takeshima N, Kawamata Y, Furuta R, Kitagawa T, Kawaguchi T, Hasumi K, Noda T. Putative gene loci associated with carcinogenesis and metastasis of endocervical adenocarcinomas of uterus determined by conventional and array-based CGH. Am J Obstet Gynecol. 2004;191:1173-1182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Albrecht C, Huck V, Wehling M, Wendler A. In vitro inhibition of SKOV-3 cell migration as a distinctive feature of progesterone receptor membrane component type 2 versus type 1. Steroids. 2012;77:1543-1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Lee NP, Chen L, Lin MC, Tsang FH, Yeung C, Poon RT, Peng J, Leng X, Beretta L, Sun S. Proteomic expression signature distinguishes cancerous and nonmalignant tissues in hepatocellular carcinoma. J Proteome Res. 2009;8:1293-1303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of Tumours of the Digestive System. Lyon: International Agency for Research on Cancer 2010; . [Cited in This Article: ] |
| 18. | Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol. 2008;21:1011-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Liu C, Xiao GQ, Yan LN, Li B, Jiang L, Wen TF, Wang WT, Xu MQ, Yang JY. Value of α-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J Gastroenterol. 2013;19:1811-1819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 89] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Xu J, Zeng C, Chu W, Pan F, Rothfuss JM, Zhang F, Tu Z, Zhou D, Zeng D, Vangveravong S. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat Commun. 2011;2:380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 21. | Hornick JR, Spitzer D, Goedegebuure P, Mach RH, Hawkins WG. Therapeutic targeting of pancreatic cancer utilizing sigma-2 ligands. Surgery. 2012;152:S152-S156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Huang YS, Lu HL, Zhang LJ, Wu Z. Sigma-2 receptor ligands and their perspectives in cancer diagnosis and therapy. Med Res Rev. 2014;34:532-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Mifsud W, Bateman A. Membrane-bound progesterone receptors contain a cytochrome b5-like ligand-binding domain. Genome Biol. 2002;3:RESEARCH0068. [PubMed] [Cited in This Article: ] |
| 24. | Correia MA, Sinclair PR, De Matteis F. Cytochrome P450 regulation: the interplay between its heme and apoprotein moieties in synthesis, assembly, repair, and disposal. Drug Metab Rev. 2011;43:1-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila). 2011;4:177-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 26. | Jakszyn P, Agudo A, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Navarro C, Palli D, Boeing H, Manjer J, Numans ME. Dietary intake of heme iron and risk of gastric cancer in the European prospective investigation into cancer and nutrition study. Int J Cancer. 2012;130:2654-2663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Ward MH, Cross AJ, Abnet CC, Sinha R, Markin RS, Weisenburger DD. Heme iron from meat and risk of adenocarcinoma of the esophagus and stomach. Eur J Cancer Prev. 2012;21:134-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Freedman ND, Cross AJ, McGlynn KA, Abnet CC, Park Y, Hollenbeck AR, Schatzkin A, Everhart JE, Sinha R. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH-AARP cohort. J Natl Cancer Inst. 2010;102:1354-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Hand RA, Craven RJ. Hpr6.6 protein mediates cell death from oxidative damage in MCF-7 human breast cancer cells. J Cell Biochem. 2003;90:534-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Hughes AL, Powell DW, Bard M, Eckstein J, Barbuch R, Link AJ, Espenshade PJ. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007;5:143-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 31. | Mallory JC, Crudden G, Johnson BL, Mo C, Pierson CA, Bard M, Craven RJ. Dap1p, a heme-binding protein that regulates the cytochrome P450 protein Erg11p/Cyp51p in Saccharomyces cerevisiae. Mol Cell Biol. 2005;25:1669-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Pru JK, Clark NC. PGRMC1 and PGRMC2 in uterine physiology and disease. Front Neurosci. 2013;7:168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Peluso JJ, Griffin D, Liu X, Horne M. Progesterone receptor membrane component-1 (PGRMC1) and PGRMC-2 interact to suppress entry into the cell cycle in spontaneously immortalized rat granulosa cells. Biol Reprod. 2014;91:104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Farinati F, De Maria N, Marafin C, Fagiuoli S, Della Libera G, Naccarato R. Hepatocellular carcinoma in alcoholic cirrhosis: is sex hormone imbalance a pathogenetic factor? Eur J Gastroenterol Hepatol. 1995;7:145-150. [PubMed] [Cited in This Article: ] |
| 35. | Yu MW, Chang HC, Chang SC, Liaw YF, Lin SM, Liu CJ, Lee SD, Lin CL, Chen PJ, Lin SC. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38:1393-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Vizoso FJ, Rodriguez M, Altadill A, González-Diéguez ML, Linares A, González LO, Junquera S, Fresno-Forcelledo F, Corte MD, Rodrigo L. Liver expression of steroid hormones and Apolipoprotein D receptors in hepatocellular carcinoma. World J Gastroenterol. 2007;13:3221-3227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Schneck H, Ruan X, Seeger H, Cahill MA, Fehm T, Mueck AO, Neubauer H. Membrane-receptor initiated proliferative effects of dienogest in human breast cancer cells. Gynecol Endocrinol. 2013;29:160-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Swiatek-De Lange M, Stampfl A, Hauck SM, Zischka H, Gloeckner CJ, Deeg CA, Ueffing M. Membrane-initiated effects of progesterone on calcium dependent signaling and activation of VEGF gene expression in retinal glial cells. Glia. 2007;55:1061-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Crudden G, Chitti RE, Craven RJ. Hpr6 (heme-1 domain protein) regulates the susceptibility of cancer cells to chemotherapeutic drugs. J Pharmacol Exp Ther. 2006;316:448-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Ahmed IS, Rohe HJ, Twist KE, Craven RJ. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J Biol Chem. 2010;285:24775-24782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Yeh SH, Chen PJ, Shau WY, Chen YW, Lee PH, Chen JT, Chen DS. Chromosomal allelic imbalance evolving from liver cirrhosis to hepatocellular carcinoma. Gastroenterology. 2001;121:699-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Panda H, Chuang TD, Luo X, Chegini N. Endometrial miR-181a and miR-98 expression is altered during transition from normal into cancerous state and target PGR, PGRMC1, CYP19A1, DDX3X, and TIMP3. J Clin Endocrinol Metab. 2012;97:E1316-E1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Wendler A, Keller D, Albrecht C, Peluso JJ, Wehling M. Involvement of let-7/miR-98 microRNAs in the regulation of progesterone receptor membrane component 1 expression in ovarian cancer cells. Oncol Rep. 2011;25:273-279. [PubMed] [Cited in This Article: ] |
| 44. | Sukata T, Sumida K, Kushida M, Ogata K, Miyata K, Yabushita S, Uwagawa S. Circulating microRNAs, possible indicators of progress of rat hepatocarcinogenesis from early stages. Toxicol Lett. 2011;200:46-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1362] [Cited by in F6Publishing: 1400] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 46. | Li Z, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 47. | Yang WJ, Chang CJ, Yeh SH, Lin WH, Wang SH, Tsai TF, Chen DS, Chen PJ. Hepatitis B virus X protein enhances the transcriptional activity of the androgen receptor through c-Src and glycogen synthase kinase-3beta kinase pathways. Hepatology. 2009;49:1515-1524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |