Published online Dec 7, 2017. doi: 10.3748/wjg.v23.i45.8017
Peer-review started: July 20, 2017
First decision: September 6, 2017
Revised: September 22, 2017
Accepted: October 26, 2017
Article in press: October 26, 2017
Published online: December 7, 2017
To investigate the rates of pretransplantation fetal-maternal microchimerism (MC) and its effect on rejection in children receiving maternal liver grafts.
DNA or blood samples before liver transplantation (LT) were available in 45 pediatric patients and their mothers. The presence of pretransplantation MC to non-inherited maternal antigens (NIMAs) (NIMA-MC) in the peripheral blood was tested using nested PCR-single-strand conformation polymorphism analysis for the human leukocyte antigen (HLA)-DRB1 alleles. NIMA-MC was successfully evaluated in 26 of the 45 children. Among these 45 pediatric LT recipients, 23 children (51.1%) received transplants from maternal donors and the other 22 from non-maternal donors.
Among these 26 children, pretransplantation NIMA-MC was detected in 23.1% (n = 6), 6.1 (range, 0.8-14) years after birth. Among the children with a maternal donor, the rate of biopsy-proven cellular rejection (BPCR) was 0% in patients with NIMA-MC positivity (0/3) and those with HLA-DR identity with the mother (0/4), but it was 50% in those with NIMA-MC negativity (5/10). Patients with NIMA-MC positivity or HLA-DR identity with the mother showed significantly lower BPCR rate compared with NIMA-MC-negative patients (0% vs 50%, P = 0.04). NIMA-MC-positive patients tended to show lower BPCR rate compared with NIMA-MC-negative patients (P = 0.23).
The presence of pretransplantation NIMA-MC or HLA-DR identity with the mother could be associated with BPCR-free survival in pediatric recipients of LT from maternal donors.
Core tip: We firstly investigated the rates of pretransplantation microchimerism (MC) to non-inherited maternal antigens (NIMAs) (NIMA-MC) in pediatric liver transplantation (LT) patients and its effect on biopsy-proven cellular rejection (BPCR) in those receiving maternal grafts. NIMA-MC for HLA-DRB1 alleles was successfully evaluated in 26 of the 45 children, and pretransplantation NIMA-MC was detected in 23.1% of the patients. Our study demonstrated that none of the patients with NIMA-MC positivity or HLA-DR identity with the mother developed BPCR. The presence of pretransplantation NIMA-MC or HLA-DR identity with the mother could be associated with BPCR-free survival in pediatric LT recipients from maternal donors.
- Citation: Yi NJ, Park MS, Song EY, Ahn HY, Byun J, Kim H, Hong SK, Yoon K, Kim HS, Ahn SW, Lee HW, Choi Y, Lee KW, Suh KS, Park MH. Pretransplantation fetal-maternal microchimerism in pediatric liver transplantation from mother. World J Gastroenterol 2017; 23(45): 8017-8026
- URL: https://www.wjgnet.com/1007-9327/full/v23/i45/8017.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i45.8017
Liver transplantation (LT) represents the gold standard of treatment for liver failure. Over 70% of pediatric recipients of LT exhibit 10-year patient and graft survival, which is higher than that observed in adults[1,2]. However, there are several challenges specifically associated with pediatric LTs: the implantation of relatively small grafts in children undergoing growth and development, the possibility of rare disease entities and accompanied congenital anomalies, and the requirement for life-long immunosuppression. In particular, a critical consideration in pediatric LT is the management of risks associated with life-long immunosuppression. Tolerance induction is utilized in order to enable discontinuation of immunosuppressant use, or the use of low-dose immunosuppressant in pediatric LT recipients.
Donor-specific microchimerism (MC), which develops following solid organ transplantations, especially LT, is considered to underlie tolerance induction and graft acceptance[3-5]. MC, which is defined as the presence of less than 1% of foreign cells in peripheral blood or tissues, may develop following transfusion, solid organ transplantation, and pregnancy[6]. Bidirectional cellular trafficking between mother and fetus during pregnancy results in postpartum fetal-maternal MC, which may persist in mothers and children for decades, and potentially over the course of a lifetime[7]. Exposure of the fetus to maternal cells results in MC to non-inherited maternal antigens (NIMAs) (NIMA-MC). Conversely, exposure of the mother to fetal cells results in MC to inherited paternal antigens (IPAs) (IPA-MC). The presence of maternal cells in the fetus generates fetal regulatory T cells that suppress the fetal response to NIMA and the presence of fetal cells in the mother suppresses the maternal response to IPAs; however, the underlying immune mechanism in the latter is far more complex.
Recent clinical studies investigating human leukocyte antigen (HLA)-haploidentical hematopoietic stem cell transplantation have suggested the association of NIMA-MC with acquired immunologic hyporesponsiveness to NIMAs; this has been termed the “NIMA effect”[8-10]. The NIMA effect on allograft survival in renal transplant recipients is controversial[11-14]. Reports of this effect in LT are scarce; the NIMA effect has been observed only in pediatric patients with biliary atresia (BA), with lower rates of graft failure reported in children receiving maternal grafts compared with those receiving paternal or deceased donor grafts[15]. Most studies on the NIMA effect in organ transplant recipients have focused on the donor-recipient relationship in the family or the presence or absence of NIMAs in the donor. Investigation of donor-specific MC in pretransplantation blood samples has rarely been performed[14].
In the present study, we investigated the rate of NIMA-MC in pretransplantation blood samples from pediatric recipients of LT, and examined the effect of NIMA-MC on rejection-free graft survival in patients with a maternal donor.
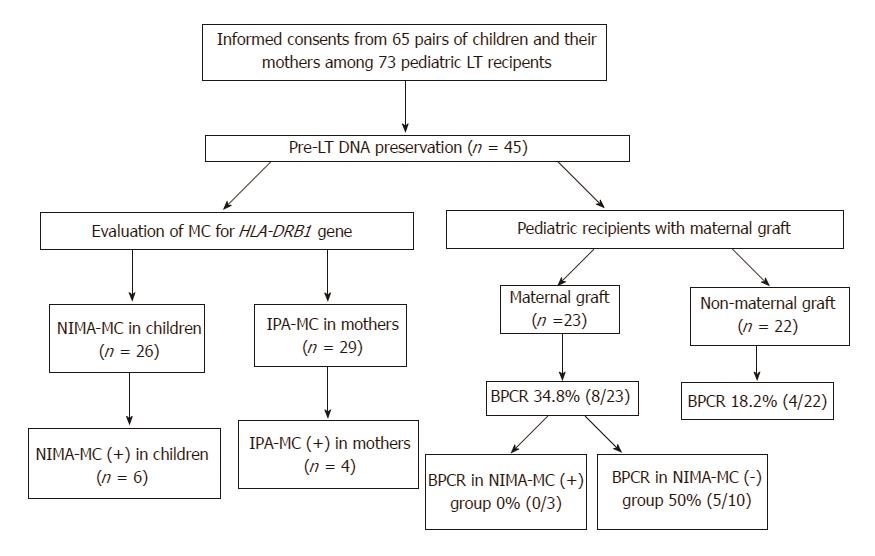
A total of 73 children below the age of 18 years, who underwent pediatric LT at the Seoul National University Hospital between 1999 and 2012, were retrospectively reviewed. None underwent ABO-incompatible LT. Informed consent was obtained from guardians of the 65 pediatric patients before commencing the investigation, and the study protocol was reviewed and approved by the Institutional Review Board of the Seoul National University Hospital (H-1210-134-438 and H-1208-030-121). Pretransplantation DNA was preserved according to protocol. A total of 45 patients for whom pretransplantation DNA or blood samples were available for investigation were included in this study (Figure 1). Among these 45 pediatric LT recipients, 23 (51.1%) received transplants from maternal donors and the other 22 from non-maternal donors; the latter from either deceased donors (n = 10) or other family members (n = 12).
Only one of the 45 recipients (2.2%) showed preoperative positive results for complement-dependent cytotoxicity crossmatch of T cells; a titer of 1: 8 was obtained via the antihuman globulin-augmented method. As the titer for the crossmatch test was not sufficiently high for preoperative desensitization[16], desensitization was not performed in this patient. Induction therapy was not used in this study population. A routine dual immunosuppressant protocol in pediatric LT comprising tacrolimus and steroid was employed; steroid was tapered off over 6 mo, according to the protocol. The median follow-up period for the 45 patients after LT was 73 (range, 30-176) mo. At the time of examination, immunosuppressive monotherapy (tacrolimus) was administered to 44 patients (97.8%), except one, who was treated with tacrolimus and low-dose steroid, owing to side effects of tacrolimus. The median level of tacrolimus was 2.0 (2.0-5.0) ng/mL.
Out of the 73 pediatric patients who underwent LT between 1999 and 2012, HLA-DRB1 MC analysis was attempted in 45 patients and their mothers, for whom DNA or blood samples were available for the study. Bidirectional HLA-DRB1 MC between the child and mother was analyzed, i.e., NIMA-MC in the child and IPA-MC in the mother (Figure 1). Genomic DNA was extracted from peripheral blood samples using the LaboPass Blood kit (Cosmo Genetech, Seoul, South Korea).
HLA-DRB1 typing: Pretransplantation HLA-DRB1 typing data were available for all 45 patients and 27 maternal donors, and blood sampling and HLA-DRB1 typing were performed later, at the time of the study, in 18 maternal donors. Low-to-intermediate resolution HLA-DRB1 typing was performed via the PCR-sequence specific oligonucleotide (SSO) typing method, using either the Dynal RELITM SSO HLA typing kit (Dynal Biotech Ltd., Wirral, United Kingdom) or the Luminex-based, WAKFlow HLA typing kit (Wakunaga, Hiroshima, Japan). For HLA-DRB1 MC analysis, high-resolution HLA-DRB1 allelic data are required for mismatched HLA-DRB1 alleles between the mother and child, i.e., NIMA for the child and IPA for the mother. Allele-level HLA-DRB1 typing was performed via the PCR-single-strand conformation polymorphism (SSCP) method, as previously described[17].
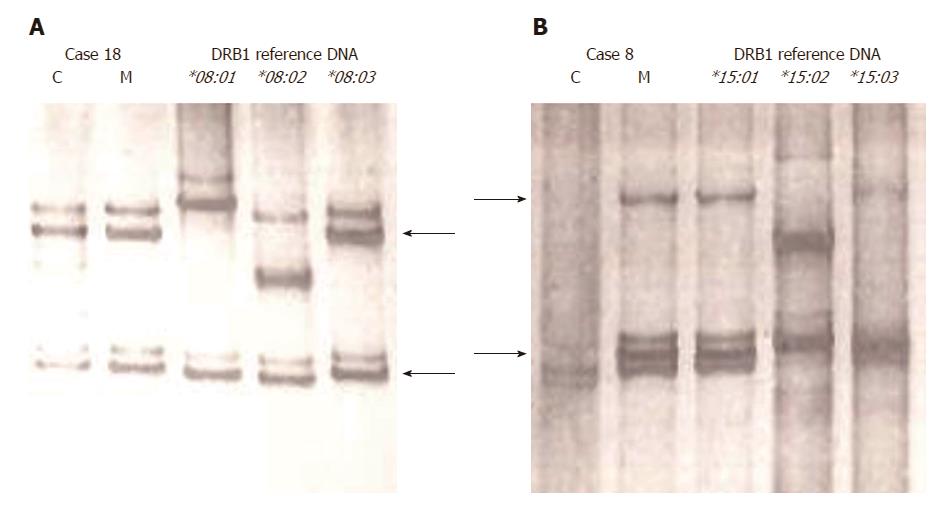
HLA-DRB1 MC analysis: HLA-DRB1 MC was analyzed via the nested PCR-SSCP method, as described previously[14,18]. For nested PCR amplification of the HLA-DRB1 gene, we used primers to amplify exon 2 of the HLA-DRB1/B3/B4/B5 genes[19] for the first-round PCR, and HLA-DR group-specific primers (for eight different groups) to amplify exon 2 of HLA-DRB1[17] for the second-round PCR. The first-round PCR products were diluted to 1:200 in water and used as templates for the second-round PCR. The nested PCR product was analyzed via SSCP analysis, and single-strand DNA fragments separated in the gel were visualized with silver staining. For the determination of the presence or absence of NIMA-MC, the band pattern of DNA from pediatric LT recipients was compared with those of maternal DNA and reference DNA with known DRB1 allele types (obtained from the University of California at Los Angeles DNA Exchange Program); these were simultaneously analyzed by gel electrophoresis (Figure 2).
Evaluation of NIMA-MC was possible in only 26 of the 45 children. NIMA-MC could not be tested in the following 19 cases: DR identical (n = 8), DR 0 mismatch (n = 8), and different DR alleles in the child and mother amplified by the same group-specific PCR (n = 3). Evaluation of IPA-MC was possible in only 29 of the 45 mothers; MC could not be tested in 16 cases: DR identical (n = 8), DR 0 mismatch (n = 6), and different DR alleles in the child and mother amplified by the same group-specific PCR (n = 2) (Figure 1).
The rate of pretransplantation NIMA-MC in the pediatric recipients and the rate of IPA-MC in the paired mothers were calculated. The factors associated with the presence or absence of NIMA-MC were evaluated in 26 recipients for whom NIMA-MC could be tested. The rate of biopsy-proven cellular rejection (BPCR) and associated factors were reviewed in order to investigate the impact of pretransplantation NIMA-MC and HLA-DR match status in 23 recipients with a maternal donor.
Continuous data are presented as median values with range, and categorical data as numbers with percentage. The categorical variables were compared using the Fisher’s exact test, and the continuous variables using the Mann-Whitney test. The level of significance was set at P < 0.05. Statistical analyses were performed using SPSS 19.0 statistical software (SPSS Inc. and Microsoft Corp., Chicago, IL, United States).
NIMA-MC was detected in 6 (23.1%) of 26 pretransplantation cases (Figure 1). The characteristics of these 26 patients, with respect to the presence or absence of NIMA-MC, are compared in Table 1. There was no definite factor associated with the presence of NIMA-MC: age at the time of transplantation, gender, recipient weight, original liver disease (BA vs non-BA), ABO compatibility with mother (identical vs non-identical) (each, P > 0.05).
| Factors1 | NIMA-MC (+) | NIMA-MC (-) | P value |
| (n = 6) | (n = 20) | ||
| Age at the time of transplantation in years (range) | 6.1 (1-14) | 6.7 (0-16) | 0.57 |
| Age at the time of transplantation (less than 12 mo) | 3 (50.0) | 7 (35.0) | 0.64 |
| Sex, male: female | 4 (66.7): 2 (33.3) | 6 (30.0): 14 (70.0) | 0.16 |
| Recipient weight, kg (range) | 28.9 (10.8-54.5) | 27.8 (8.1-56.3) | 0.92 |
| Original liver disease | 0.65 | ||
| Biliary atresia | 4 (66.7) | 9 (45.0) | |
| Others | 2 (33.3) | 11 (55.0) | |
| ABO identical to that of mother | 3 (50.0) | 13 (65.0) | > 0.99 |
IPA-MC was detected in 4 (13.8%) of 29 blood samples from paired mothers (Figure 1). Bidirectional MC was not observed in any of the child-mother pairs. There was no definite factor associated with the presence of IPA-MC: age of mothers at the time of study, gender mismatch with paired child, history of multi-parity, and ABO compatibility (data not shown).
BPCR rates in patients receiving maternal grafts (34.8%, 8 of 23) and those receiving non-maternal grafts (18.2%, 4 of 22) were not significantly different (P = 0.31) (Figure 1). BPCR rates in the 23 patients that received maternal grafts, according to the presence or absence of NIMA-MC and HLA-DR match status, are shown in Table 2. BPCR rates in NIMA-MC-negative patients were as high as 50% (5 of 10). In comparison, none of the NIMA-MC-positive patients (n = 3) or those whose HLA-DR status was identical to that of their mother (n = 4) developed BPCR. Compared with NIMA-MC-negative patients, NIMA-MC-positive patients tended to show lower BPCR rates (P = 0.23).
| NIMA-MC and HLA-DR match status | Biopsy-proven cellular rejection | P valuea |
| NIMA-MC (-) (n = 10) | 5 (50.0) | |
| NIMA-MC (+) (n = 3) | 0 (0) | 0.23 |
| NIMA-MC (+) or HLA-DR 0 mismatch with mother (n = 11) | 2 (18.2) | 0.18 |
| NIMA-MC (+) or HLA-DR identity with mother (n = 7) | 0 (0) | 0.04 |
| Total transplantation with maternal graft (n = 23) | 8 (34.8) |
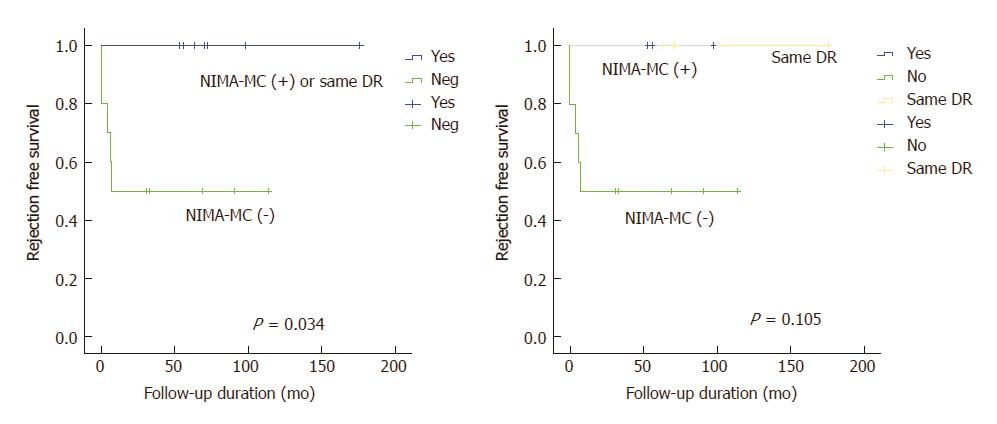
In addition, patients with NIMA-MC positivity or HLA-DR identity with mother exhibited significantly lower BPCR rates (P = 0.04). All BPCRs occurred within 7 mo after transplantation (Figure 3). Rejection activity indexes varied from 3 to 5; rejection activity index of one of the recipients was 5, while that of the other 4 recipients was 3.
In order to investigate other factors associated with lower BPCR rates, various factors were compared in these two groups of patients (Table 3). No significant differences were found in the two groups of patients when compared by sex, sex mismatch, age and weight at the time of transplantation, liver disease, crossmatch positivity, ABO compatibility, donor age, PELD score, and graft versus recipient weight ratio (each, P > 0.05).
| Factors1 | NIMA-MC (+) or HLA-DR identity with mother | NIMA-MC (-) | P value |
| (n = 7) | (n = 10) | ||
| Age at the time of transplantation in years, median (range) | 5.6 (0.8-10) | 7.3 (0.8-16) | 0.42 |
| Age at the time of transplantation (less than 12 mo) | 3 (42.9) | 4 (40.0) | > 0.99 |
| Sex, male: female | 3 (42.9): 4 (57.1) | 4 (40.0): 6 (60.0) | > 0.99 |
| Recipient weight, kg, median (range) | 27.4 (6.2-54.5) | 26.0 (9.6-56.3) | > 0.99 |
| Original liver disease | 0.64 | ||
| Biliary atresia | 4 (57.1) | 4 (40.0) | |
| Others | 3 (42.9) | 6 (60.0) | |
| Crossmatch positivity | 0 (0) | 1 (10.0) | > 0.99 |
| ABO identical to that of mother | 7 (100) | 7 (70.0) | 0.23 |
| PELD score, median (range) | 11.2 (-7.7-39) | 2.3 (-10.0-14.1) | 0.27 |
| The year of LT (before 2007) | 2 (28.6) | 3 (30.0) | > 0.99 |
| Donor age in years, median (range) | 34 (26-39) | 38 (33-43) | 0.16 |
| Sex match, yes: no | 4:3 | 6:4 | 0.65 |
| Operation time, median, min, median (range) | 443 (300-595) | 492 (285-710) | 0.46 |
| Graft vs recipient weight ratio, %, median (range) | 2.68 (1.62-3.23) | 2.15 (1.23-2.96) | 0.40 |
| Follow-up duration, mo, median (range) | 84 (63-176) | 66 (30-114) | 0.32 |
| Graft failure | 0 (0) | 0 (0) | > 0.99 |
| Biopsy-proven cellular rejection | 0 (0) | 5 (50) | 0.04 |
In order to examine the long-term effect of NIMA-MC positivity on graft function, we compared the levels of aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase at 1, 2, 3 and 5 years after LT. However, none of these markers were significantly different between the NIMA-MC-positive and -negative groups of patients who received a maternal graft (data not shown).
In this study, we investigated the incidence of NIMA-MC (maternal MC) in pretransplantation peripheral blood samples of pediatric LT recipients, and its effect on BPCR rates in recipients of maternal grafts. To our knowledge, this is the first study demonstrating the possible beneficial effect of pretransplantation fetal-maternal MC on LT outcomes, with verification of the presence or absence of MC in recipients.
The positive rate of pretransplantation NIMA-MC was 23.1% in the present LT recipients, which is similar to that in mother-to-child kidney transplant recipients (22.7%) as reported in our previous study[14]. We were unable to identify specific factors, such as age, sex, liver disease, and mother-child ABO blood group identity, which were significantly associated with the presence of NIMA-MC in the LT recipients (Table 1).
As to the liver disease, patients with BA have been reported to have increased maternal MC in their livers[20-22], suggesting increased maternal MC in their peripheral blood as well; however, this has rarely been studied. In the present LT recipients, the positive rate of NIMA-MC in the peripheral blood was higher in BA patients (4/13, 30.8%) than in non-BA patients (2/13, 15.4%). However, this difference was not statistically significant as the number of patients in the present study was small. Further investigation in a larger number of patients is therefore needed.
We evaluated the clinical effect of NIMA-MC in 23 LT recipients with maternal grafts in terms of BPCR rate (Table 2). BPCR rate in recipients with NIMA-MC negativity was as high as 50%, whereas none of the recipients with NIMA-MC positivity or mother-child HLA-DR identity showed BPCR (P = 0.04). Nijagal et al[15] reported beneficial effects of maternal graft in patients with BA, in terms of decreased risk of hepatic graft failure. Furthermore, it was suggested that these patients with BA exhibit allograft tolerance to maternal grafts[15,23,24]. Sanada et al[24] described the importance of sex matching of parental grafts; in particular, the authors showed that maternal grafts were associated with lower incidence of acute cellular rejection than paternal grafts in daughters. This finding represents important clinical evidence of the beneficial impact of preexisting NIMA-MC on post-transplantation outcomes in patients with BA.
As patients with BA were found to exhibit increased number of maternal cells and inflammation of the biliary tree in their livers, the disease was viewed as an inflammatory disease of fetal-maternal circulation; further, graft-versus-host reaction was proposed to represent the underlying pathophysiology of BA[20-22]. Accordingly, beneficial effects of maternal liver grafts have been demonstrated in patients with BA, but not in non-BA LT recipients[15,23,24]. However, in these studies, donors were analyzed by parental relationship (maternal vs paternal) to the recipient, and the presence or absence of maternal MC was not tested. As only a proportion of children exhibit maternal MC (23.1% in this study), beneficial effects of maternal graft recipients that do not have BA may not be detectable by simple analysis of the donor-recipient relationship. Via determination of the presence or absence of fetal-maternal MC in renal transplantation from family donors, we reported beneficial effects on rejection-free graft survival in pretransplantation MC-positive patients in a previous study[14]. Further investigation of clinical outcomes, with determination of maternal MC, in patients with conditions other than BA is needed. Future studies should aim to determine whether such patients experience beneficial effects from maternal grafts when positive for the presence of pretransplantation maternal MC. Findings should be of significance for counseling in cases of pediatric LT, particularly with regard to donor selection when both parental donors are available. On the other hands, we can consider less immunosuppressant during life-long follow-up, if the patient has NIMA positivity or HLA-DR identity with mother and can get a maternal graft.
Apart from studies of pretransplantation MC, most reports on MC in solid organ transplantations (i.e., kidney, liver, heart, and lung) investigated the development of MC following transplantation[5,25-30]. The overall positive rate of donor-specific MC detected at various intervals after transplantation has been reported to be high (> 50%)[20-23,25]; this is much higher than that of the pretransplantation fetal-maternal MC detected in the present LT recipients. However, posttransplantation MC exhibits fluctuation and variable patterns over time[20-23,25,26], and is generally detected at higher frequencies during the early period and at lower frequencies in the late period[20-23,25,30]. Posttransplantation MC has been considered to be associated with tolerance induction, as this phenomenon was initially detected in long-term hepatic and renal allograft recipients receiving minimal immunosuppressive therapy[3]. However, this association was not confirmed in a meta-analysis of the association of posttransplantation MC with graft survival in solid organ transplantations[31]. Posttransplantation MC was found to be generally associated with a higher incidence of acute rejection for heart, lung, and kidney transplants and a lower incidence for liver transplants. Privileged tolerogenicity of the liver compared with other organs may be explained by the large population of migratory cells in, and their migration from, hepatic grafts, resulting in balanced lymphodendritic cell chimerism[4].
This study has some limitations. First, MC detection via the amplification of HLA-DRB1 gene is not a robust method applicable to all transplant cases, especially between one haplotype-matched mother and child pairs: in the present work, HLA-DRB1 NIMA-MC could be tested only in 26 (58%) of the 45 children. Second, this was a retrospective study and posttransplantation follow-up of the persistence of NIMA-MC or the development of de novo donor-specific MC was not performed. NIMA-MC detected before transplantation is expected to persist for a long time following transplantation as fetal-maternal MC, when present, has been shown to persist for decades[7]. However, this has to be verified in a prospective study. Third, the number of cases examined in this study was small, and further studies on a larger number of patients are warranted.
In conclusion, we investigated the effects of pretransplantation NIMA-MC on allograft outcomes in pediatric LT recipients of maternal grafts, but failed to show that pretransplantation NIMA-MC was associated with a lower risk of cellular rejection. Only the presence of pretransplantation NIMA-MC or HLA-DR identity with the mother could be associated with BPCR-free survival in pediatric recipients of LT from maternal donors. This study was limited in terms of the small number of patients examined; future studies on a larger number of patients are warranted. The confirmation of the present beneficial effects of pretransplantation NIMA-MC on allograft outcomes via larger scale multi-center studies should have implications for donor selection and the development of protocols, including reduced immunosuppression, for managing pediatric LT recipients.
A critical consideration in pediatric liver transplantation (LT) is the management of risks associated with life-long immunosuppression. Tolerance induction is utilized in order to enable discontinuation of immunosuppressant use in pediatric LT recipients. Donor-specific microchimerism (MC) is considered to underlie tolerance induction and graft acceptance. During pregnancy, exposure of the fetus to maternal cells results in natural MC to non-inherited maternal antigens (NIMAs) (NIMA-MC). Recent clinical studies have suggested the association of NIMA-MC with acquired immunologic hyporesponsiveness to NIMAs; this has been termed the “NIMA effect”. However, reports of this effect in LT are scarce.
To investigate the pretransplantation status of NIMA-MC and its effect on cellular rejection in children receiving maternal liver grafts, HLA-DRB1 MC between the child and mother was analyzed. This blood DNA test was already reported by this research team, and we found that the presence of pretransplantation MC was associated with beneficial effects on rejection-free survival in family donor renal transplantation.
In the present study, we aimed to investigate the rate of NIMA-MC in pretransplantation blood samples from pediatric recipients of LT, and examine the effect of NIMA-MC on rejection-free graft survival in patients with a maternal donor.
The presence of pretransplantation NIMA-MC in the peripheral blood was tested using nested PCR-single-strand conformation polymorphism (SSCP) analysis for the HLA-DRB1 alleles. At first, low-to-intermediate resolution HLA-DRB1 typing was performed via the PCR-sequence specific oligonucleotide (SSO) typing method, using either the Dynal RELITM SSO HLA typing kit (Dynal Biotech Ltd., Wirral, UK) or the Luminex-based, WAKFlow HLA typing kit (Wakunaga, Hiroshima, Japan). For HLA-DRB1 MC analysis, high-resolution HLA-DRB1 allelic data are required for mismatched HLA-DRB1 alleles between the mother and child. Allele-level HLA-DRB1 typing was performed via the PCR- SSCP method. For nested PCR amplification of the HLA-DRB1 gene, we used primers to amplify exon 2 of the HLA-DRB1/B3/B4/B5 genes for the first-round PCR, and HLA-DR group-specific primers (for eight different groups) to amplify exon 2 of HLA-DRB1 for the second-round PCR. The first-round PCR products were diluted to 1:200 in water and used as templates for the second-round PCR. The nested PCR product was analyzed via SSCP analysis, and single-strand DNA fragments separated in the gel were visualized with silver staining. For the determination of the presence or absence of NIMA-MC, the band pattern of DNA from pediatric LT recipients was compared with those of maternal DNA and reference DNA with known DRB1 allele types; these were simultaneously analyzed by gel electrophoresis. The rate of pretransplantation NIMA-MC in the pediatric recipients was calculated. To evaluate the effect of NIMA-MC on transplantation outcome, the rate of biopsy-proven cellular rejection (BPCR) among different groups of NIMA-MC and/or HLA-DR match status was compared in patients with a maternal donor using the Fisher’s exact test.
In this small cohort study, pretransplantation NIMA-MC tended to be associated with a lower risk of cellular rejection in pediatric LT recipients with maternal donors. BPCR rate in the patients with pretransplantation NIMA-MC or HLA-DR identity with the mother was significantly lower than those with NIMA-MC negativity (0% vs 50%). This is a novel finding, and further study for large cohort validation is required.
The presence of pretransplantation NIMA-MC or HLA-DR identity with the mother could be associated with BPCR-free survival in pediatric recipients of LT from maternal donors. NIMA effect has been validated in HLA-haploidentical hematopoietic stem cell transplantation. Pretransplantation NIMA-MC could also exert a beneficial effect on rejection-free graft survival in pediatric LT with a maternal graft. Most studies on the NIMA effect in organ transplant recipients have focused on the donor-recipient relationship in the family or the presence or absence of NIMAs in the donor. By actual testing of the presence or absence of NIMA-MC in peripheral blood of the patients, the NIMA effect on outcomes of solid organ transplantations, including LT can be better investigated. Investigation of donor-specific MC in pretransplantation blood samples has rarely been performed. Presence of pretransplantation NIMA-MC could have a beneficial effect on the rejection-free survival in pediatric LT recipients with maternal grafts. Laboratory testing of pretransplantation NIMA-MC in the peripheral blood using nested PCR-single-strand conformation polymorphism analysis for the HLA-DRB1 alleles was previously reported by this research team in renal transplantation (Transplantation 2013; 95(11): 1375-1382). Pretransplantation NIMA-MC for HLA-DRB1 alleles was detected in 23.1% of the pediatric LT recipients; the presence of pretransplantation NIMA-MC or HLA-DR identity with the mother could be associated with BPCR-free survival in pediatric recipients of LT from maternal donors. The presence of pretransplantation NIMA-MC could be associated with BPCR-free survival in pediatric recipients of LT from maternal donors. If the beneficial effect of pretransplantation NIMA-MC on allograft outcomes can be confirmed via larger scale multi-center studies, it would have implications for donor selection when both parental donors are available and the development of protocols, including reduced immunosuppression, for managing pediatric LT recipients.
Presence of microchimerism (MC) to non-inherited maternal antigen (NIMA) is known to be associated with acquired immunologic hyporesponsiveness to NIMA, which is called “NIMA effect”. NIMA effect has been suggested in pediatric liver transplant patients with biliary atresia receiving maternal grafts. However, actual presence or absence of NIMA-MC has never been verified. This is the first study showing possible beneficial effect of NIMA-MC on rejection-free survival in pediatric liver transplant patients receiving maternal grafts with actual laboratory determination of the presence or absence of NIMA-MC. This study is based on a small number of patients and could not get a strong evidence of beneficial effect of NIMA-MC in pediatric liver transplant patients receiving maternal grafts. Further studies using a larger number of patients, and preferentially multicenter studies are warranted. If beneficial effects of NIMA-MC in pediatric liver transplant patients is verified, it will have useful clinical implications of selecting family donors and modifying life-long immunosuppression. MC detection via the amplification of HLA-DRB1 gene was used in this study, which is not a robust method applicable to all transplant cases, especially between one haplotype-matched mother and child pairs. MC detection methods using robust genetic markers other than HLA-DRB1 gene with good enough sensitivities had better be developed and used in the future research. In the present study, only the presence or absence of pretransplant NIMA-MC has been investigated and future studies are required to investigate the effect of posttransplant persistence of NIMA-MC on clinical outcome.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cerwenka H, Sanada Y S- Editor: Qi Y L- Editor: A E- Editor: Huang Y
| 1. | Kasahara M, Umeshita K, Inomata Y, Uemoto S; Japanese Liver Transplantation Society. Long-term outcomes of pediatric living donor liver transplantation in Japan: an analysis of more than 2200 cases listed in the registry of the Japanese Liver Transplantation Society. Am J Transplant. 2013;13:1830-1839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Kim JM, Kim KM, Yi NJ, Choe YH, Kim MS, Suh KS, Kim SI, Lee SK, Lee SG. Pediatric liver transplantation outcomes in Korea. J Korean Med Sci. 2013;28:42-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579-1582. [PubMed] [Cited in This Article: ] |
| 4. | Starzl TE, Demetris AJ, Trucco M, Ramos H, Zeevi A, Rudert WA, Kocova M, Ricordi C, Ildstad S, Murase N. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876-877. [PubMed] [Cited in This Article: ] |
| 5. | Araújo MB, Leonardi LS, Leonardi MI, Boin IF, Magna LA, Donadi EA, Kraemer MH. Prospective analysis between the therapy of immunosuppressive medication and allogeneic microchimerism after liver transplantation. Transpl Immunol. 2009;20:195-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Pujal JM, Gallardo D. PCR-based methodology for molecular microchimerism detection and quantification. Exp Biol Med (Maywood). 2008;233:1161-1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Ichinohe T, Teshima T, Matsuoka K, Maruya E, Saji H. Fetal-maternal microchimerism: impact on hematopoietic stem cell transplantation. Curr Opin Immunol. 2005;17:546-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | van Rood JJ, Loberiza FR Jr, Zhang MJ, Oudshoorn M, Claas F, Cairo MS, Champlin RE, Gale RP, Ringdén O, Hows JM, Horowitz MH. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572-1577. [PubMed] [Cited in This Article: ] |
| 9. | Ichinohe T, Uchiyama T, Shimazaki C, Matsuo K, Tamaki S, Hino M, Watanabe A, Hamaguchi M, Adachi S, Gondo H. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation between noninherited maternal antigen (NIMA)-mismatched family members linked with long-term fetomaternal microchimerism. Blood. 2004;104:3821-3828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Stern M, Ruggeri L, Mancusi A, Bernardo ME, de Angelis C, Bucher C, Locatelli F, Aversa F, Velardi A. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112:2990-2995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Opelz G. Analysis of the “NIMA effect” in renal transplantation. Collaborative Transplant Study. Clin Transpl. 1990;63-67. [PubMed] [Cited in This Article: ] |
| 12. | Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, Brennan DC, de Fijter H, van Gelder T, Pirsch JD. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 230] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Mahanty HD, Cherikh WS, Chang GJ, Baxter-Lowe LA, Roberts JP. Influence of pretransplant pregnancy on survival of renal allografts from living donors. Transplantation. 2001;72:228-232. [PubMed] [Cited in This Article: ] |
| 14. | Joo SY, Song EY, Shin Y, Ha J, Kim SJ, Park MH. Beneficial effects of pretransplantation microchimerism on rejection-free survival in HLA-haploidentical family donor renal transplantation. Transplantation. 2013;95:1375-1382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Nijagal A, Fleck S, Hills NK, Feng S, Tang Q, Kang SM, Rosenthal P, MacKenzie TC. Decreased risk of graft failure with maternal liver transplantation in patients with biliary atresia. Am J Transplant. 2012;12:409-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Hong G, Yi NJ, Suh SW, Yoo T, Kim H, Park MS, Choi Y, Lee K, Lee KW, Park MH. Preoperative selective desensitization of live donor liver transplant recipients considering the degree of T lymphocyte cross-match titer, model for end-stage liver disease score, and graft liver volume. J Korean Med Sci. 2014;29:640-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Bannai M, Tokunaga K, Lin L, Kuwata S, Mazda T, Amaki I, Fujisawa K, Juji T. Discrimination of human HLA-DRB1 alleles by PCR-SSCP (single-strand conformation polymorphism) method. Eur J Immunogenet. 1994;21:1-9. [PubMed] [Cited in This Article: ] |
| 18. | Song EY, Chung HY, Joo SY, Roh EY, Seong MW, Shin Y, Park MH. Detection of HLA-DRB1 microchimerism using nested polymerase chain reaction and single-strand conformation polymorphism analysis. Hum Immunol. 2012;73:291-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Luo M, Blanchard J, Brunham K, Pan Y, Shen CX, Lu H, Brunham RC. Two-step high resolution sequence-based HLA-DRB typing of exon 2 DNA with taxonomy-based sequence analysis allele assignment. Hum Immunol. 2001;62:1294-1310. [PubMed] [Cited in This Article: ] |
| 20. | Kobayashi H, Tamatani T, Tamura T, Kusafuka J, Yamataka A, Lane GJ, Kawasaki S, Ishizaki Y, Mizuta K, Kawarasaki H. Maternal microchimerism in biliary atresia. J Pediatr Surg. 2007;42:987-91; discussion 991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Suskind DL, Rosenthal P, Heyman MB, Kong D, Magrane G, Baxter-Lowe LA, Muench MO. Maternal microchimerism in the livers of patients with biliary atresia. BMC Gastroenterol. 2004;4:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Irie N, Muraji T, Hosaka N, Takada Y, Sakamoto S, Tanaka K. Maternal HLA class I compatibility in patients with biliary atresia. J Pediatr Gastroenterol Nutr. 2009;49:488-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Nijagal A, Fleck S, MacKenzie TC. Maternal microchimerism in patients with biliary atresia: Implications for allograft tolerance. Chimerism. 2012;3:37-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Sanada Y, Kawano Y, Miki A, Aida J, Nakamura K, Shimomura N, Ishikawa N, Arai T, Hirata Y, Yamada N. Maternal grafts protect daughter recipients from acute cellular rejection after pediatric living donor liver transplantation for biliary atresia. Transpl Int. 2014;27:383-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Reinsmoen NL, Jackson A, McSherry C, Ninova D, Wiesner RH, Kondo M, Krom RA, Hertz MI, Bolman RM 3rd, Matas AJ. Organ-specific patterns of donor antigen-specific hyporeactivity and peripheral blood allogeneic microchimerism in lung, kidney, and liver transplant recipients. Transplantation. 1995;60:1546-1554. [PubMed] [Cited in This Article: ] |
| 26. | Hisanaga M, Hundrieser J, Böker K, Uthoff K, Raddatz G, Wahlers T, Wonigeit K, Pichlmayr R, Schlitt HJ. Development, stability, and clinical correlations of allogeneic microchimerism after solid organ transplantation. Transplantation. 1996;61:40-45. [PubMed] [Cited in This Article: ] |
| 27. | McSherry C, Jackson A, Hertz MI, Bolman RM 3rd, Savik K, Reinsmoen NL. Sequential measurement of peripheral blood allogeneic microchimerism levels and association with pulmonary function. Transplantation. 1996;62:1811-1818. [PubMed] [Cited in This Article: ] |
| 28. | Elwood ET, Larsen CP, Maurer DH, Routenberg KL, Neylan JF, Whelchel JD, O’Brien DP, Pearson TC. Microchimerism and rejection in clinical transplantation. Lancet. 1997;349:1358-1360. [PubMed] [Cited in This Article: ] |
| 29. | Wang J, Liu J, Guan D, Gao J. The study of peripheral blood microchimerism in kidney transplantation. Transplant Proc. 2001;33:177-178. [PubMed] [Cited in This Article: ] |
| 30. | Tajik N, Singal D, Pourmand G, Ebrahimi-Rad M, Radjabzadeh M, Tavasoli P, Khosravi F, Nikbin B. Prospective study of microchimerism in renal allograft recipients: association between HLA-DR matching, microchimerism and acute rejection. Clin Transplant. 2001;15:192-198. [PubMed] [Cited in This Article: ] |
| 31. | Sahota A, Gao S, Hayes J, Jindal RM. Microchimerism and rejection: a meta-analysis. Clin Transplant. 2000;14:345-350. [PubMed] [Cited in This Article: ] |