Published online Jul 21, 2017. doi: 10.3748/wjg.v23.i27.4978
Peer-review started: February 22, 2017
First decision: April 21, 2017
Revised: May 12, 2017
Accepted: June 1, 2017
Article in press: June 1, 2017
Published online: July 21, 2017
To find an accurate and simple predictor for postoperative short-term complications after gastrectomy.
Two hundred and twenty-three patients undergoing gastric cancer resection between October 1, 2015 and September 30, 2016 were enrolled in this study. Univariate and multivariate analyses were used to identify risk factors for complications after gastrectomy. The cutoff values and diagnostic accuracy were examined by receiver operating characteristic curves.
Sixty-two (27.8%) patients had short-term complications after gastric cancer resection. The postoperative decrease in serum albumin (∆ALB) was an independent risk factor for complications (OR = 17.957, 95%CI: 6.073-53.095, P < 0.001). The cutoff value was 14.0% and the area under the curve was higher than that of C-reactive protein on postoperative day 3 (area under the curve: 0.806 vs 0.709). Patients with ∆ALB ≥ 14.0% were more likely to have short-term complications after gastrectomy (46.7% vs 5.0%, P < 0.001), prolonged hospital stay (17.2 ± 10.8 d vs 14.1 ± 4.2 d, P = 0.007) and higher comprehensive complication index (P < 0.001) than those with ∆ALB < 14.0%.
Postoperative ∆ALB with a cutoff of 14.0% can be used to recognize patients who have high risk of short-term complications following gastric cancer resection.
Core tip: In this work, we investigated whether postoperative decrease of serum albumin can predict short-term complications following gastric cancer resection. Results indicate that the decrease of serum albumin could be more accurate than postoperative C-reactive protein in predicting complications after gastrectomy. Surgeons are warned of potential postoperative complications in patients whose serum albumin levels reduce by more than 14.0%. This is the first evaluation of the relationship between decrease in albumin and postoperative complications in gastric cancer resection.
- Citation: Liu ZJ, Ge XL, Ai SC, Wang HK, Sun F, Chen L, Guan WX. Postoperative decrease of serum albumin predicts short-term complications in patients undergoing gastric cancer resection. World J Gastroenterol 2017; 23(27): 4978-4985
- URL: https://www.wjgnet.com/1007-9327/full/v23/i27/4978.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i27.4978
Gastric cancer is one of the most common malignancies and is the third leading cause of cancer-related mortality in China. Surgery provides the only possibility of cure in patients with gastric cancer. Despite improvements in perioperative care and surgical procedures, postoperative complications remain a major impediment and threat to survival after gastric cancer surgery[1-3]. Therefore, it is crucial to specify a reliable and simple risk assessment index to indicate the possibility of postoperative complications, and the likelihood of early and safe patient discharge.
Many preoperative or postoperative indexes, such as C-reactive protein (CRP; a proinflammatory cytokine increasing in parallel with postoperative surgical stress), white blood cell count and albumin (ALB), have been identified as risk factors for complications after gastrectomy[4-8]. There are many studies revealing the association between serum ALB and postoperative outcomes. For example, preoperative hypoalbuminemia can predict surgical site infections[9]. Ryan et al[10] found that postoperative hypoalbuminemia on postoperative day (POD) 1 was associated with complications following esophagectomy. Sang et al[11] also found that hypoalbuminemia on POD 2 was an independent risk factor for acute kidney injury in patients with living donor liver transplantation. However, these studies mainly focused on the impact of serum ALB on nutritional status of patients[12,13].
ALB is also a negative acute phase protein and decreases after surgery, because of trauma and increased capillary leakage[14]. Norberg et al[15] observed an immediate and sharp decrease of serum ALB level (∆ALB) by 33% after major abdominal surgery, which occurred even earlier than the change in CRP. Unfortunately, few studies have identified the change in serum ALB level as a marker in predicting the outcomes of gastrectomy.
This study aimed to clarify whether the reduction of ALB level after surgery could be a predictor for short-term complications following gastric cancer resection. Therefore, the ∆ALB on POD 1 was examined and its diagnostic accuracy in gastric cancer was investigated.
Written informed consent was obtained from all the patients enrolled in the investigation. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and the Ethics Committee of Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, China.
The data of 317 consecutive patients who underwent surgery for gastric cancer between October 1, 2015 and September 30, 2016 at the Department of Gastrointestinal Surgery, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School were prospectively collected and standard ethnic audit was conducted. Patients with ALB infusion preoperatively or within POD 1, reoperation for postoperative complications, non-resection of the stomach, severe organ dysfunction or comorbidity, multivisceral resection, or incomplete laboratory data were excluded. Finally, a total of 223 patients were enrolled in the study.
Data were collected based on the following factors: age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) grade, initial clinical stage, comorbidity, surgical procedures (surgical approach, type of resection, degree of lymph node dissection), neutrophils, lymphocytes, hemoglobin, tumor makers such as carcinoembryonic antigen (CEA) and carbohydrate antigen (CA)19-9, preoperative CRP and ALB, CRP on POD 3[16], ALB on POD 1[11], operation time, estimated blood loss during surgery and intraoperative blood transfusion.
Relative ∆ALB was calculated as: (preoperative ALB level - ALB level on POD 1)/preoperative ALB level × 100%[17]. Receiver operating characteristic (ROC) curve was used to calculate the cutoff values of ∆ALB. The postoperative outcomes were analyzed which included length of postoperative hospital stay and postoperative complications before discharge or < 30 d after surgery. Postoperative complications was identified by Clavien--Dindo classification, which showed that the Grades I and II were mild complications and Grades III and IV were major complications in patients[18]. The comprehensive complication index (CCI) is a score developed to include all complications after surgery and is based on the Clavien-Dindo system[18,19]. The CCI of each patient was calculated using the Website http://www.assessurgery.com[20].
SPSS version 19.0 (SPSS Inc., Chicago, IL, United States) was used for the analysis. Categorical data were expressed by counts and percentages, while continuous data were expressed by mean ± SD or median (range). Fisher’s exact test or Pearson’s χ2 test was used to analyze categorical variables, while Student’s t-test was used to analyze continuous variables. P < 0.05 was considered statistically significant. ROC curve analysis was used to assess the predictive accuracy. Significant correlations (P < 0.05) on univariate analysis were used to verify independent predictors for postoperative complications by multivariate logistic regression analysis.
There were 159 men and 64 women, with a mean age of 62.3 ± 9.9 years. The clinical characteristics are summarized in Table 1. The preoperative mean BMI, and CRP and ALB level were 23.0 ± 3.4 kg/m2, 6.6 ± 10.3 g/L and 38.3 ± 3.2 g/L, respectively. One hundred and seventeen patients received total gastrectomy, 82 distal gastrectomy and 24 proximal gastrectomy. One hundred and eighty patients underwent D2 or more lymphadenectomy. The operation time was 239.1 ± 66.9 min, with blood loss of 228.6 ± 146.2 mL. The length of postoperative hospital stay was 15.8 ± 8.6. Sixty-two patients (27.8%) had postoperative complications. According to Clavien-Dindo classification, 40 patients (17.9%) had mild complications (Grade I or II), and 22 (9.9%) had major complications (Grade III or greater).
| Characteristic | n = 223 |
| Age in yr | 62.3 ± 9.9 |
| Sex, n | |
| Male | 159 |
| Female | 64 |
| BMI in kg/m2 | 23.0 ± 3.4 |
| Comorbidities, n | |
| Diabetes mellitus | 15 |
| Hypertension | 72 |
| Preoperative serum albumin in g/L | 38.3 ± 3.2 |
| Preoperative hemoglobin in g/L | 123.4 ± 24.9 |
| Preoperative CRP in g/L | 6.6 ± 10.3 |
| CA 19-9 in ng/mL | |
| ≥ 37 | 43 |
| < 37 | 180 |
| CEA in ng/mL | |
| ≥ 5 | 26 |
| < 5 | 197 |
| Lymphocyte count as × 109/L | |
| ≥ 3 | 9 |
| < 3 | 214 |
| CRP on POD 3 in mg/L | 99.7 ± 60.9 |
| ALB on POD 1 in g/L | 32.7 ± 3.6 |
| ASA ≥ 3, n | 122 |
| Clinical stage I/II/III/IV, n | 67/44/103/9 |
| Mode of surgical approach, n | |
| Laparoscopic | 18 |
| Open | 205 |
| Type of resection, n | |
| Distal gastrectomy | 82 |
| Proximal gastrectomy | 24 |
| Total gastrectomy | 117 |
| Degree of lymph node dissection ≥ 2, n | 180 |
| Operation time in min | 239.1 ± 66.9 |
| Blood loss in mL | 228.6 ± 146.2 |
| Postoperative complications as Clavien-Dindo grade, n | |
| I and II | 40 |
| ≥ III | 22 |
| Postoperative stay in d | 15.8 ± 8.6 |
The results of univariate analyses of various clinical factors are shown in Table 2, including age, sex, BMI, ASA grade, clinical stage, tumor makers (CA19-9, CEA), lymphocyte count, comorbidity, preoperative hemoglobin, operation time, intraoperative blood loss, surgical procedures (mode of approach, type of resection, degree of lymph node dissection), preoperative CRP and serum ALB, CRP on POD 3, and ∆ALB. Among these, diabetes mellitus (OR = 3.259, 95%CI: 1.128-9.414, P = 0.029), preoperative serum ALB (OR = 1.048, 95%CI: 1.018-1.109, P = 0.015), clinical stage (OR = 1.798, 95%CI: 1.255-2.686, P = 0.009), type of resection (OR = 1.291, 95%CI: 1.006-1.896, P = 0.013), CRP on POD 3 (OR = 4.653, 95%CI: 2.435-8.889, P < 0.001) and ∆ALB (OR = 16.837, 95%CI: 6.403-44.275, P < 0.001) were significantly associated with postoperative complications. Therefore, a multivariate analysis model was used to identify the independent predictive factors for complications after gastrectomy. As shown in Table 3, ∆ALB (OR = 17.957, 95%CI: 6.073-53.095, P < 0.001) remained as an independent risk factor in predicting complications after surgery. However, it was not strong enough for us to draw the conclusion that ∆ALB can be an accurate predictor for complications after gastrectomy.
| Characteristic | OR | 95%CI | P value |
| Age ≥ 75 yr | 1.330 | 0.476-3.715 | 0.586 |
| Sex | 0.642 | 0.342-1.202 | 0.166 |
| BMI of < 18.5 kg/m2 | 0.277 | 0.034-2.232 | 0.228 |
| Comorbidities | |||
| Diabetes mellitus | 3.259 | 1.128-9.414 | 0.029 |
| Hypertension | 0.728 | 0.382-1.389 | 0.336 |
| Preoperative serum albumin of < 35 g/L | 1.048 | 1.018-1.109 | 0.015 |
| Preoperative hemoglobin of < 120 g/L | 1.487 | 0.817-2.705 | 0.194 |
| Preoperative CRP of ≥ 10 g/L | 1.336 | 0.512-3.486 | 0.553 |
| CA 19-9 of ≥ 37 ng/mL | 1.328 | 0.647-2.723 | 0.439 |
| CEA of ≥ 5 ng/mL | 1.177 | 0.483-2.865 | 0.720 |
| Lymphocyte count of ≥ 3 × 109/L | 0.314 | 0.038-2.560 | 0.279 |
| CRP on POD 3 in mg/L | 4.653 | 2.435-8.889 | < 0.001 |
| △ALB of ≥ 14.0% | 16.837 | 6.403-44.275 | < 0.001 |
| ASA of ≥ 3 | 1.007 | 0.559-1.815 | 0.981 |
| Clinical stage of ≥ II | 1.798 | 1.255-2.686 | 0.009 |
| Mode of surgical approach | 1.206 | 0.588-2.475 | 0.610 |
| Type of resection | 1.291 | 1.006-1.896 | 0.013 |
| Degree of lymph node dissection ≥ 2 | 2.263 | 0.948-5.399 | 0.066 |
| Operation time of ≥ 250 min | 1.621 | 0.896-2.931 | 0.110 |
| Blood loss of ≥ 200 mL | 1.417 | 0.758-2.651 | 0.275 |
| Characteristic | OR | 95%CI | P value |
| Age ≥ 75 yr | 1.053 | 0.248-4.470 | 0.945 |
| Sex | 0.572 | 0.237-1.380 | 0.214 |
| BMI of < 18.5 kg/m2 | 0.147 | 0.013-1.666 | 0.122 |
| Comorbidities | |||
| Diabetes mellitus | 1.234 | 0.322-4.726 | 0.759 |
| Hypertension | 0.989 | 0.397-2.463 | 0.981 |
| Preoperative serum albumin of < 35 g/L | 0.914 | 0.897-1.067 | 0.093 |
| Preoperative hemoglobin of < 120 g/L | 1.804 | 0.748-4.353 | 0.189 |
| Preoperative CRP of ≥ 10 g/L | 1.008 | 0.210-4.840 | 0.993 |
| CA 19-9 of ≥ 37 ng/mL | 1.197 | 0.431-3.327 | 0.730 |
| CEA of ≥ 5 ng/mL | 0.794 | 0.233-2.706 | 0.713 |
| Lymphocyte count of ≥ 3 × 109/L | 0.192 | 0.018-2.048 | 0.172 |
| CRP on POD 3 in mg/L | 4.296 | 1.887-9.780 | 0.001 |
| △ALB of ≥ 14.0% | 17.957 | 6.073-53.095 | < 0.001 |
| ASA of ≥ 3 | 0.929 | 0.406-2.127 | 0.861 |
| Clinical stage of ≥ II | 1.198 | 0.355-2.286 | 0.109 |
| Mode of surgical approach | 1.737 | 0.576-5.235 | 0.327 |
| Type of resection | 0.791 | 0.506-1.896 | 0.063 |
| Degree of lymph node dissection ≥ 2 | 3.485 | 1.163-10.446 | 0.026 |
| Operation time of ≥ 250 min | 1.448 | 0.624-3.355 | 0.389 |
| Blood loss of ≥ 200 mL | 1.418 | 0.636-3.160 | 0.393 |
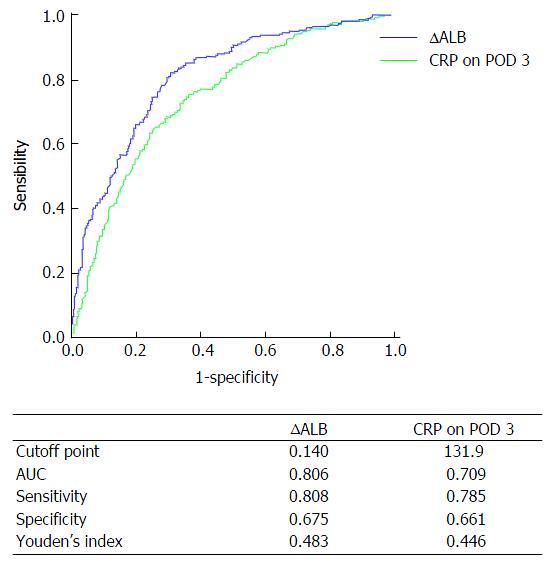
CRP on POD 3 was identified as a practical predictor for complications after gastric cancer surgery in recent studies[7,16]. In this study, the predictive accuracy of ∆ALB and CRP on POD 3 was analyzed by ROC curve. Figure 1 shows the ROC curve parameters. The area under the curve (AUC) of CRP on POD 3 was 0.709, sensitivity was 0.785, specificity was 0.661, Youden’s index was 0.446, and the cutoff point was 131.9; comparatively, the AUC of ∆ALB was 0.806, sensitivity was 0.808, specificity was 0.675, Youden’s index was 0.483, and the cutoff point was 14.0%. Therefore, ∆ALB was a better predictive index than CRP on POD 3 for postoperative complications in patients undergoing gastric cancer surgery.
Based on the cutoff value of ∆ALB, we divided patients into two groups. As shown in Table 4, patients with ∆ALB ≥ 14.0% had more complications after gastrectomy than those with ∆ALB < 14.0% (46.7% vs 5.0%, P < 0.001). Patients with ∆ < 0.001). Patients with ∆ALB ≥ 14.0% were found to have more mild or severe complications than those with ∆ALB < 14.0% (30.3% vs 3.0%, P < 0.001 or 16.4% vs 2.0%, P < 0.001, respectively). In addition, patients who had ∆ALB ≥ 14.0% were suggested to have prolonged postoperative stay (17.2 ± 10.8 vs 14.1 ± 4.2, P = 0.007) and higher CCI (P < 0.001).
| Characteristic | All, n = 223 | ∆ALB < 14.0%, n = 101 | ∆ALB ≥ 14.0%, n = 122 | P value |
| Overall13 | 62 (27.8) | 5 (5.0) | 57 (46.7) | < 0.001 |
| Mild complications13 | 40 (17.9) | 3 (3.0) | 37 (30.3) | < 0.001 |
| Major complications13 | 22 (9.9) | 2 (2.0) | 20 (16.4) | < 0.001 |
| Postoperative stay in d2 | 15.8 ± 8.6 | 14.1 ± 4.2 | 17.2 ± 10.8 | 0.007 |
| CCI all patients4 | 0 (0-20.9) | 0 (0-0) | 8.7 (0-26.2) | < 0.001 |
In this study, serum ALB was mainly considered as an acute phase protein. The ∆ALB was an independent risk factor for prolonged hospital stay and complications after gastrectomy. Patients were distinguished as having low or high risk of complications after gastrectomy by the cutoff value of 14.0% in ∆ALB, which was more accurate than CRP level on POD 3.
Numerous studies have shown that preoperative and postoperative hypoalbuminemia are risk factors for complications after surgery[9-12], while few have specifically focused on the relationship between decreased serum ALB after surgery and clinical outcomes. Hübner et al[21] reported that stress response led to a reduction in postoperative albumin levels, which was consistent with the findings in our study. After major abdominal surgery, the albumin synthesis rate remained the same, whereas the fractional synthesis rate increased, leading to a decrease in plasma ALB[15]. However, sequestration into the interstitial space may contribute most to a postoperative drop in ALB level[14,21]. Capillary leakage is especially common in some malnourished patients with surgical trauma followed by an increased transcapillary escape rate of ≥ 100%[14,22,23]. To summarize, multiple factors affect the reduction in serum ALB after surgery and the stress response plays an important role in the change[23].
Nevertheless, increasing evidence shows that postoperative CRP level, as a risk factor for postoperative inflammation, can be used to predict complications after gastrectomy[7,24]. For instance, Kim et al[16] found that the CRP level on POD 4 was one of the most reliable predictors for complications following gastric cancer resection, when compared to many systematic inflammatory markers (white blood cells, neutrophils, platelet counts and CRP on POD 1). However, in previous studies, certain drawbacks were found by using postoperative CRP as a marker of complications after gastrectomy, which lacks accuracy in certain conditions[16,21,25]. Rettig et al[5] discovered that increased CRP levels after surgery were not early enough to identify patients at high risk for postoperative complications. In our study, ∆ALB was an independent predictive marker for complications after gastrectomy in multivariate analysis. The AUC of ∆ALB was larger than that of CRP on POD 3, suggesting ∆ALB was a better positive predictive marker. According to these findings, ∆ALB had a higher predictive value and tended to be more precise than CRP.
∆ALB could be more precise than CRP in predicting complications after gastrectomy because it is more accurate in reflecting the stress response after surgical trauma. As previously mentioned, the serum ALB level decreases earlier than CRP level after major abdominal surgery. The postoperative reduction in ALB level is associated with systemic inflammatory response syndrome, which leads to increased fractional synthesis and pathological capillary leakage of serum ALB[14]. Besides, serum ALB has a series of significant physiological functions, including free radical scavenging, maintenance of colloid osmotic pressure, change of capillary membrane permeability, and anticoagulant effects[26]. Hypoalbuminemia inhibits the innate immune response by promoting granuloma formation and reducing collagen synthesis. As a result, the systemic immune status is more sensitive to infection and other postoperative complications[27]. In contrast, CRP is involved in innate immunity as an early defense against infection, enhancing phagocytosis by macrophages and assisting complement binding to damaged cells or foreign matter[28]. From the above, the finding that ∆ALB could be more precise than CRP in predicting complications after gastrectomy is understandable, and serum ALB is a better predictor of both systemic inflammation and nutritional status.
However, it remains to be established whether ALB supplementation benefits patients with a large ∆ALB after gastrectomy. Golub et al[29] reported that routine ALB infusion was not beneficial to patients in the surgical intensive care unit. For patients with postoperative hypoalbuminemia, ALB infusion was deemed to be useless after major gastrointestinal surgery[30]. No studies have ever demonstrated the benefit for patients with preoperative hypoalbuminemia. Instead, exogenous ALB administration might increase risks of edema, extravasation of albumin, or other postoperative complications[31]. More intensive perioperative care might relieve an early ∆ALB following gastric cancer resection and improve patient outcomes by reducing postoperative generalized inflammation[9].
There were several limitations to the current study. First, it was a retrospective observational analysis, so the possibility of residual confounding factors could not be entirely excluded. Second, it was a single-center study and the outcome might have been influenced by the small number of patients enrolled and perioperative management strategies in our hospital. To verify the conclusions, multicenter prospective studies involving a large volume of data are needed. Third, whether the findings in our study could be applied to other operations, such as esophagectomy or liver resection, is not known for sure.
In conclusion, this study confirmed that a postoperative ∆ALB can predict short-term complications following gastric cancer resection. The ∆ALB could be more accurate than postoperative CRP in predicting complications after gastrectomy. Surgeons are warned of potential postoperative complications in patients whose serum ALB levels are reduced by > 14.0%.
The authors gratefully acknowledge all of the investigators for their contributions to the trial, and Dr. Xiao-Fei Shen from Nanjing University and Dr. Hua-Tong Liu from Australian National University, who provided medical writing assistance.
To find an accurate and simple predictor for postoperative short-term complications after gastrectomy. A reduction of serum albumin (ALB) level is observed in many patients after gastrectomy, but it remains uncertain whether it could be used as a predictor for short-term outcomes following gastric cancer resection.
Many preoperative or postoperative indexes, such as C-reactive protein (CRP; a proinflammatory cytokine increasing in parallel with surgical stress after operation), white blood cell count and ALB, have been identified as risk factors for complications after gastrectomy. There are many studies revealing the association between serum albumin and postoperative outcomes. However, these studies mainly focused on the impact of serum albumin on nutritional status of patients.
The authors investigated whether the postoperative decrease of serum ALB can predict short-term complications following gastric cancer resection. The decrease of serum ALB could be more accurate than postoperative CRP in predicting complications after gastrectomy. This study involves the first evaluation of the relationship between decrease of ALB and postoperative complications in gastric cancer resection.
Surgeons are warned of potential postoperative complications in patients whose serum ALB levels are reduced by more than 14.0%.
The authors investigated serum ALB changes after gastrectomy and found a correlation between the ALB change with short-term complication rates. Study results are interesting and have novel findings. They know colorectal surgery correlated with ALB changes, but gastric cancer is a new finding and may add some contribution to the literature.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bilir C, Lee YC S- Editor: Qi Y L- Editor: Filipodia E- Editor: Li D
| 1. | Saito T, Kurokawa Y, Miyazaki Y, Makino T, Takahashi T, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. Which is a more reliable indicator of survival after gastric cancer surgery: Postoperative complication occurrence or C-reactive protein elevation? J Surg Oncol. 2015;112:894-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995-999. [PubMed] [Cited in This Article: ] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11444] [Cited by in F6Publishing: 12601] [Article Influence: 1575.1] [Reference Citation Analysis (2)] |
| 4. | Ishino Y, Saigusa S, Ohi M, Yasuda H, Tanaka K, Toiyama Y, Mohri Y, Kusunoki M. Preoperative C-reactive protein and operative blood loss predict poor prognosis in patients with gastric cancer after laparoscopy-assisted gastrectomy. Asian J Endosc Surg. 2014;7:287-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Rettig TC, Verwijmeren L, Dijkstra IM, Boerma D, van de Garde EM, Noordzij PG. Postoperative Interleukin-6 Level and Early Detection of Complications After Elective Major Abdominal Surgery. Ann Surg. 2016;263:1207-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Yamashita K, Ushiku H, Katada N, Hosoda K, Moriya H, Mieno H, Kikuchi S, Hoshi K, Watanabe M. Reduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long-term survival with advanced gastric cancer with positive cytology test. Eur J Surg Oncol. 2015;41:1324-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Warschkow R, Tarantino I, Ukegjini K, Beutner U, Müller SA, Schmied BM, Steffen T. Diagnostic study and meta-analysis of C-reactive protein as a predictor of postoperative inflammatory complications after gastroesophageal cancer surgery. Langenbecks Arch Surg. 2012;397:727-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Jiang N, Deng JY, Ding XW, Ke B, Liu N, Zhang RP, Liang H. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20:10537-10544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 91] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 9. | Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. 2010;252:665-666. [Cited in This Article: ] |
| 10. | Ryan AM, Hearty A, Prichard RS, Cunningham A, Rowley SP, Reynolds JV. Association of hypoalbuminemia on the first postoperative day and complications following esophagectomy. J Gastrointest Surg. 2007;11:1355-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Sang BH, Bang JY, Song JG, Hwang GS. Hypoalbuminemia Within Two Postoperative Days Is an Independent Risk Factor for Acute Kidney Injury Following Living Donor Liver Transplantation: A Propensity Score Analysis of 998 Consecutive Patients. Crit Care Med. 2015;43:2552-2561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Kang SC, Kim HI, Kim MG. Low Serum Albumin Level, Male Sex, and Total Gastrectomy Are Risk Factors of Severe Postoperative Complications in Elderly Gastric Cancer Patients. J Gastric Cancer. 2016;16:43-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Toiyama Y, Yasuda H, Ohi M, Yoshiyama S, Araki T, Tanaka K, Inoue Y, Mohri Y, Kusunoki M. Clinical impact of preoperative albumin to globulin ratio in gastric cancer patients with curative intent. Am J Surg. 2017;213:120-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, Calman KC. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1:781-784. [PubMed] [Cited in This Article: ] |
| 15. | Norberg Å, Rooyackers O, Segersvärd R, Wernerman J. Albumin Kinetics in Patients Undergoing Major Abdominal Surgery. PLoS One. 2015;10:e0136371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Kim EY, Yim HW, Park CH, Song KY. C-reactive protein can be an early predictor of postoperative complications after gastrectomy for gastric cancer. Surg Endosc. 2017;31:445-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Spolverato G, Kim Y, Ejaz A, Frank SM, Pawlik TM. Effect of Relative Decrease in Blood Hemoglobin Concentrations on Postoperative Morbidity in Patients Who Undergo Major Gastrointestinal Surgery. Jama Surg. 2015;150:949-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications. Ann Surg. 2004;240:205-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18532] [Cited by in F6Publishing: 22205] [Article Influence: 1110.3] [Reference Citation Analysis (0)] |
| 19. | Slankamenac K, Nederlof N, Pessaux P, De JJ, Wijnhoven BP, Breitenstein S, Oberkofler CE, Graf R, Puhan MA, Clavien PA. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg. 2014;260:762-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 20. | Soubrane O, De RO, Kim KH, Samstein B, Mamode N, Boillot O, Troisi RI, Scatton O, Cauchy F, Lee SG. Laparoscopic Living Donor Left Lateral Sectionectomy: A New Standard Practice for Donor Hepatectomy. Ann Surg. 2015;262:757-763. [Cited in This Article: ] |
| 21. | Hübner M, Mantziari S, Demartines N, Pralong F, Coti-Bertrand P, Schäfer M. Postoperative Albumin Drop Is a Marker for Surgical Stress and a Predictor for Clinical Outcome: A Pilot Study. Gastroenterol Res Pract. 2016;2016:8743187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 353] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 23. | Smeets HJ, Kievit J, Dulfer FT, Hermans J, Moolenaar AJ. Analysis of post-operative hypalbuminaemia: a clinical study. Int Surg. 1994;79:152-157. [PubMed] [Cited in This Article: ] |
| 24. | Shishido Y, Fujitani K, Yamamoto K, Hirao M, Tsujinaka T, Sekimoto M. C-reactive protein on postoperative day 3 as a predictor of infectious complications following gastric cancer resection. Gastric Cancer. 2016;19:293-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Easton R, Balogh ZJ. Peri-operative changes in serum immune markers after trauma: a systematic review. Injury. 2014;45:934-941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | 260Margarson MP, Soni N. Serum albumin: touchstone or totem? Anaesthesia. 1998;53:789-803. [PubMed] [Cited in This Article: ] |
| 27. | Otranto M, Souza-Netto I, Aguila MB, Monte-Alto-Costa A. Male and female rats with severe protein restriction present delayed wound healing. Appl Physiol Nutr Metab. 2009;34:1023-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Dutta S, Fullarton GM, Forshaw MJ, Horgan PG, Mcmillan DC. Persistent Elevation of C-Reactive Protein Following Esophagogastric Cancer Resection as a Predictor of Postoperative Surgical Site Infectious Complications. World J Surg. 2011;35:1017-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Golub R, Sorrento JJ Jr, Cantu RJ, Nierman DM, Moideen A, Stein HD. Efficacy of albumin supplementation in the surgical intensive care unit: a prospective, randomized study. Critical Care Med. 1994;22:613-619. [PubMed] [Cited in This Article: ] |
| 30. | Cai SR, Luo NX, Yuan XY, He YL, Wu H, Wang Z. Is albumin administration beneficial in early stage of postoperative hypoalbuminemia after gastrointestinal surgery: a prospective randomized control trial. Zhonghua Wai Ke Za Zhi. 2009;47:744-747. [PubMed] [Cited in This Article: ] |
| 31. | Yuan XY, Zhang CH, He YL, Yuan YX, Cai SR, Luo NX, Zhan WH, Cui J. Is albumin administration beneficial in early stage of postoperative hypoalbuminemia following gastrointestinal surgery?: a prospective randomized controlled trial. Am J Surg. 2008;196:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |