Published online May 21, 2017. doi: 10.3748/wjg.v23.i19.3468
Peer-review started: January 20, 2017
First decision: February 9, 2017
Revised: April 25, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: May 21, 2017
To investigate factors causing diabetes recurrence after sleeve gastrectomy (SG) and duodenal-jejunal bypass (DJB).
SG and DJB were performed on rats with diabetes induced by high-fat diet (HFD) and streptozotocin (STZ). HFD was used to induce diabetes recurrence at 4 wk postoperatively. Body weight, oral glucose tolerance test, homeostatic model assessment of insulin resistance (HOMA-IR), insulin signaling [IR, insulin receptor substrate (IRS)1, IRS2, phosphatidylinositol 3-kinase and AKT in liver and skeletal muscle], oral glucose stimulated insulin secretion, beta-cell morphology (mass, apoptosis and insulin secretion), glucagon-like peptide (GLP)-1, PYY and ghrelin were compared among SG rats with common low-fat diet (SG-LFD), SG with HFD (SG-HFD), DJB rats with LFD (DJB-LFD), DJB with HFD (DJB-HFD) and sham-operation with LFD (Sham) at targeted postoperative times.
SG and DJB resulted in significant improvement in glucose tolerance, lower HOMA-IR, up-regulated hepatic and muscular insulin signaling, higher levels of oral glucose-stimulated insulin secretion, bigger beta-cell mass, higher immunofluorescence intensity of insulin, fewer transferase-mediated dUTP-biotin 3’ nick end-labeling (TUNEL)-positive beta cells and higher postprandial GLP-1 and PYY levels than in the Sham group. The improvement in glucose tolerance was reversed at 12 wk postoperatively. Compared with the SG-LFD and DJB-LFD groups, the SG-HFD and DJB-HFD groups showed higher HOMA-IR, down-regulated hepatic and muscular insulin signaling, and more TUNEL-positive beta cells. No significant difference was detected between HFD and LFD groups for body weight, glucose-stimulated insulin secretion, beta-cell mass, immunofluorescence intensity of insulin, and postprandial GLP-1 and PYY levels. Fasting serum ghrelin decreased in SG groups, and there was no difference between HFD-SG and LFD-SG groups.
HFD reverses the improvement in glucose homeostasis after SG and DJB. Diabetes recurrence may correlate with re-impaired insulin sensitivity, but not with alterations of beta-cell function and body weight.
Core tip: To investigate factors causing diabetes recurrence after sleeve gastrectomy (SG) and duodenal-jejunal bypass (DJB), we performed SG and DJB on diabetic rats and high-fat diet was used to induce diabetes recurrence at 4 wk postoperatively. The result showed that diabetes recurrence may correlate with re-impaired insulin sensitivity, but not with alterations of beta-cell function and body weight.
- Citation: Liu T, Zhong MW, Liu Y, Sun D, Wei M, Huang X, Cheng YG, Wu QZ, Wu D, Zhang XQ, Wang KX, Hu SY, Liu SZ. Diabetes recurrence after metabolic surgeries correlates with re-impaired insulin sensitivity rather than beta-cell function. World J Gastroenterol 2017; 23(19): 3468-3479
- URL: https://www.wjgnet.com/1007-9327/full/v23/i19/3468.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i19.3468
The accelerating twin pandemics of obesity and type 2 diabetes mellitus (T2DM) are recognized as two of the greatest global public health threats of our time. Randomized clinical trials have indicated that bariatric surgery achieves rapid and better glycemic control than medical therapy alone in severely obese patients with T2DM[1-4]. Accordingly, bariatric surgery is recommended in the treatment algorithm of T2DM and endorsed by 45 worldwide medical and scientific associations[5]. Although the evidence from clinical and basic research is sufficient to support Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) as anti-diabetes interventions for obese patients, most studies have been restricted to short- and mid-term follow-up, and studies reporting long-term (≥ 5 years) diabetes remission rates have been limited, although they are now emerging[6,7].
Recurrence of diabetes after initial remission has been observed and needs further investigation. In an earlier meta-analysis, Buchwald et al[8] found that the percentage of patients free of diabetes after gastric bypass decreased from 81.6% in the first 2 years to 70.9% after > 2 years. This suggests the potential for relapse of T2DM in some patients whose diabetes resolves after surgery. Several sporadic but convincing studies have documented a 12.1%-53% rate of recurrence of diabetes, with reference to RYGB, SG and biliopancreatic diversion (BPD) as selected procedures[9-13]. Associated risk factors reported include longer duration of T2DM, preoperative use of insulin, old age, poor compliance with doctor’s orders and postoperative high caloric intake[9,14]. Weight regain, lower preoperative body mass index and less excess weight loss (EWL) are also regarded as risk factors, although this is controversial[9,11].
Currently, the physiological and molecular mechanisms underlying diabetes control after bariatric surgery remain incompletely understood, even with regard to diabetes relapse. Against this background, the main goal of our study was to create an animal model of diabetes recurrence after metabolic surgery, and to evaluate alterations of insulin sensitivity, beta-cell function and related indexes in the process of diabetes remission and recurrence. As a secondary aim, we evaluated the association between weight changes, diabetes recurrence and high-fat diet (HFD), which was applied postoperatively to induce diabetes recurrence. In the present study, both SG and duodenal-jejunal bypass (DJB) were performed on rats with diabetes induced by HFD and streptozotocin (STZ). The antidiabetic effects of the two procedures were compared as a third aim. Our study could lead to a deeper understanding of diabetes remission after metabolic surgery and promote better strategies to enhance durable remission.
All experiments were approved by the Animal Care and Utilization Committee of Shandong University, Jinan, China. Animals were housed under conventional conditions and had free access to tap water and food at the Laboratory Animal Center of Shandong University. Male Wistar rats (age, 8 wk; weight, 160-180 g) were fed an HFD (40% of calories as fat) rodent chow for a period of 8 wk, and then injected with STZ intraperitoneally (35 mg/kg). One week later, the rats were fasted overnight and received a 3-h oral glucose tolerance test (OGTT). Rats with a peak blood glucose of ≥ 11.1 mmol/L and ≤ 16.0 mmol/L were considered diabetic and selected for further studies.
Fifty-two diabetic rats were randomly assigned to SG-LFD (n = 11), SG-HFD (n = 10), SG-LFD (n = 11), DJB-HFD (n = 10) and Sham-operated (n = 10) groups. After surgery, all rats in the five groups were given a common low-fat diet (LFD, 15% of calories as fat) rodent chow for 4 wk. An HFD was then provided for the HFD groups for 8 wk. All Sham-operated rats were given LFD after surgery until the end of the study at 12 wk postoperatively. Body weight was monitored weekly during the study.
Before operations, all rats were fed with 10% Ensure (Abbott Laboratories, United States) for 2 d, then fasted overnight, and anesthetized with 10% chloral hydrate (3 mL/kg, Qilu Hospital, China) for surgery. Access to water was allowed at 2 h after surgery. Subsequently, the rats were fed with 10% Ensure for 3 d, followed by LFD or HFD rodent chow according to the protocol until end of the study.
SG: SG involved (1) a 4-cm midline epigastric incision; (2) ligation of all vessels around the greater curvature using 7-0 silk suture (Ningbo Medical Needle, Ningbo, China); (3) resection of the fundus and most of the stomach; and (4) closure of the remnant stomach using 5-0 silk suture (Ningbo Medical Needle).
DJB: DJB involved (1) a 4-cm midline abdominal incision; (2) transection of the duodenum at 0.5 cm from the pylorus and closure of the distal limb using a 7-0 silk suture; (3) transection of the jejunum at 10 cm from the ligament of Treitz; (4) end-to-end anastomosis of the distal jejunal limb to the duodenal stump; and (5) end-to-side anastomosis of the proximal jejunal limb to the small intestine 10 cm distally.
Sham operations: For rats in the Sham group, laparotomy was performed to expose the stomach, esophagus, and small intestine. The operating time was prolonged to generate a comparable degree of anesthetic stress as in the SG and DJB groups. No other procedures were carried out.
OGTT was performed at baseline and 2, 4 and 12 wk postoperatively, and areas under the curves for OGTT (AUCOGTT) were calculated to evaluate the effect of diabetes control in each group. For OGTT, rats were fasted overnight and administered 1 g/kg glucose by oral gavage. Blood samples were obtained from the tail vein at baseline and 10, 30, 60, 120 and 180 min after administration, and glucose was measured using a glucometer (Roche One Touch® Ultra; Lifescan, Johnson & Johnson, Milpitas, CA, United States).
Homeostasis model assessment of insulin resistance (HOMA-IR) was adopted as a surrogate of insulin sensitivity and calculated at baseline, and 4 and 12 wk postoperatively, which was calculated according to the formula: HOMA-IR = fasting insulin (mIU/L) × fasting glucose (mmol/L)/22.5.
All rats were sacrificed at 12 wk postoperatively. Liver and skeletal muscle were sampled and immediately frozen in liquid nitrogen and stored at -80 °C until analysis. Alterations of the insulin signaling pathway were determined by Western blotting, as indicated by protein expression of insulin receptor, insulin receptor substrate (IRS)1, IRS2, phosphatidylinositol 3-kinase (PI3K) and Akt. For Western blotting, samples were mechanically dissociated and lysed in radioimmunoprecipitation assay 37 (RIPA) buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L Na2-EDTA, 1% NP-40, 0.25% Na-deoxycholate) containing protease and phosphatase inhibitor cocktails (Roche, United States). After brief sonication and heating, the supernatants were subjected to SDS-PAGE and transferred to PVDF membranes. Blots were incubated overnight at 4 °C with primary antibodies (anti-insulin receptor antibody, anti-IRS1 antibody, anti-IRS2 antibody, anti-PI3K P85 α antibody, anti-pan-AKT antibody, anti-β-actin antibody; all Abcam Cambridge, MA, United States) and were then incubated with secondary antibodies (Abcam). Blots were visualized with an enhanced chemiluminescence reagent (Millipore, Billerica, MA, United States) and quantified with Image Lab (Bio-Rad, Hercules, CA, United States).
Glucose-stimulated insulin secretion was measured in the serum samples as a surrogate index of beta cell function of insulin secretion at baseline, and postoperative weeks 4 and 12. Rats were deprived of food overnight and then administered 1 g/kg glucose by oral gavage. Blood was collected from the retrobulbar venous plexus at baseline and 15, 30, 60 and 120 min after gavage into tubes containing EDTA and dipeptidyl peptidase IV inhibitor. After centrifugation at 3000 rpm at 4 °C for 15 min, the separated serum was immediately removed to Eppendorf tubes and stored at -80 °C until analyzed. Insulin was measured with rat ELISA kits (Millipore).
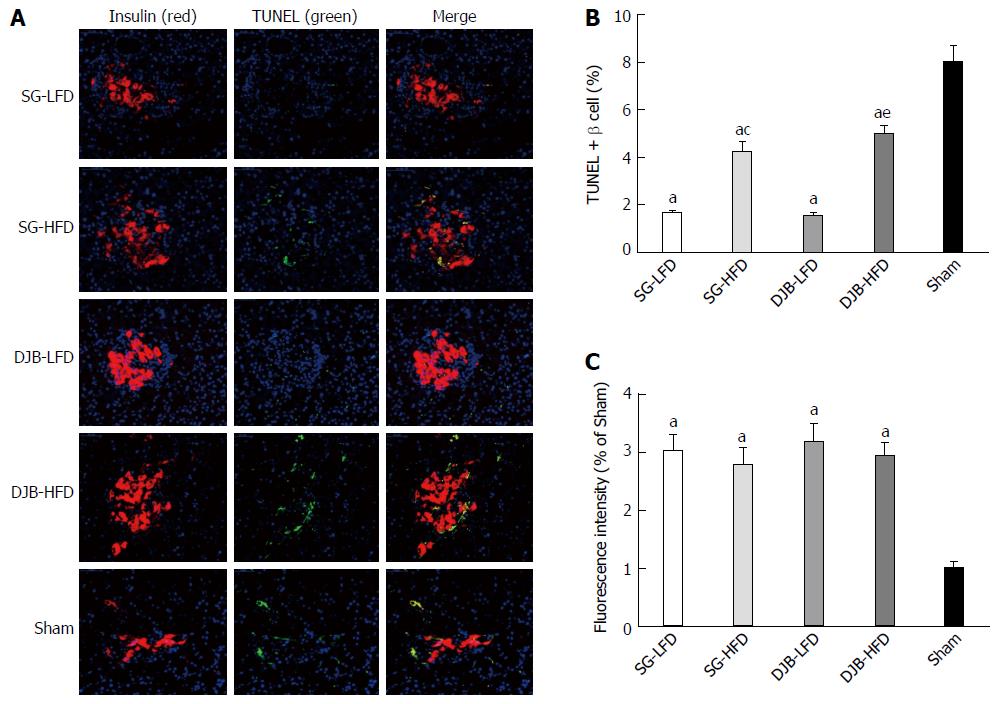
Beta-cell mass, insulin secretion and terminal deoxynucleotidyl transferase-mediated dUTP-biotin 3’ nick end-labeling (TUNEL) assay were performed to evaluate changes in the morphology of beta cells. The rats were killed and pancreases were harvested, flattened and immersed in 4% paraformaldehyde. Tissues were embedded in paraffin after 24 h. Sections of 5 μm were rehydrated, and antigen retrieval was performed in citrate buffer (pH 6) in a pressure cooker. Beta cells were stained with guinea pig anti-insulin antibody (Abcam; 1/200). Apoptosis was assessed using the TUNEL assay (Roche). Nuclei were stained with DAPI (1 μg/mL). Affinity-purified secondary antibodies were from Abcam. Immunofluorescence images were captured on an Olympus FlouView FV1000 confocal microscope at 400 × magnification. Images of sections were analyzed using ImageJ software. The percentage of TUNEL-positive beta cells and the insulin fluorescence intensity of each islet were calculated.
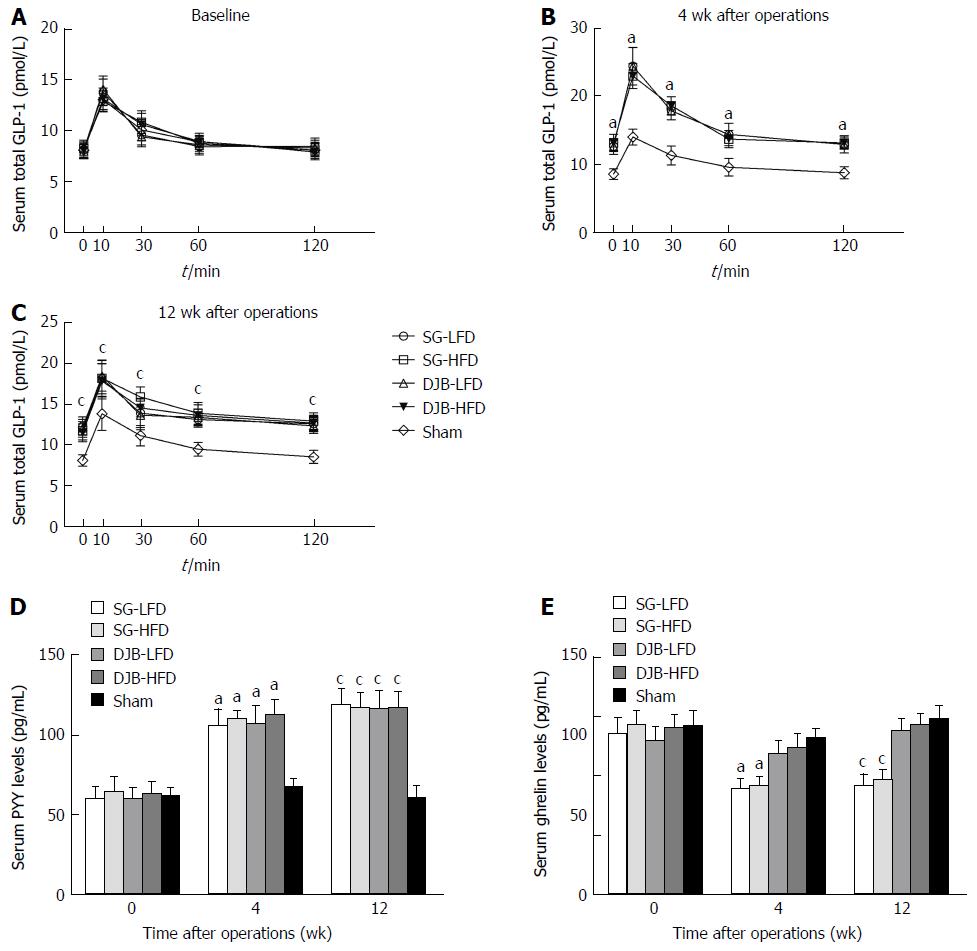
Serum total GLP-1 and PYY levels after glucose gavage and fasting serum ghrelin were measured with the serum collected at 4 and 12 wk postoperatively. GLP-1 was measured with multi-species GLP-1 total ELISA kits (Millipore). PYY was measured with Rat Leptin ELISA (Millipore). Ghrelin was measured with Rat/Mouse Ghrelin (total) ELISA (Millipore).
Data are expressed as mean ± SD. Data that were not normally distributed or did not satisfy homogeneity of variance were logarithmically transformed before analysis. AUCOGTT was calculated by trapezoidal integration. All statistical analyses were performed with SPSS version 19.0. Body weight, AUCOGTT, HOMA-IR and ghrelin data at each time point, band intensity of Western blotting, percentage of TUNEL-positive beta cells, and insulin fluorescence intensity of each islet were compared by one-way analysis of variance (ANOVA). Postprandial insulin, GLP-1 and PYY data were compared by two-factor repeated measures (RM) ANOVA. Post hoc comparisons adjusted by Bonferroni’s correction, were performed when necessary. Differences were considered significant at P < 0.05.
There was no significant difference among the groups in preoperative body weight and oral glucose tolerance. Table 1 shows the number of rats surviving in each group and their body weight. The body weight increased over time in all rats. Body weight in the four surgery groups was lower than the Sham group (4 wk postoperatively: 368.4 g ± 13.2 g; 12 wk postoperatively: 450.7 g ± 15.8 g) at 4 and 12 wk postoperatively (4 wk postoperatively: SG-LFD 343.0 g ± 15.3 g, P < 0.05 vs Sham; SG-HFD 345.6 g ± 20.0 g, P < 0.05 vs Sham; DJB-LFD 346.2 g ± 17.2 g, P < 0.05 vs Sham; DJB-HFD 349.6 g ± 18.5 g, P < 0.05 vs Sham; 12 wk postoperatively: SG-LFD 418.1 g ± 19.4 g, P < 0.05 vs Sham; SG-HFD 422.7 g ± 21.9 g, P < 0.05 vs Sham; DJB-LFD 419.1 g ± 18.4 g, P < 0.05 vs Sham; DJB-HFD 420.9 g ± 21.2 g, P < 0.05 vs Sham; Table 1). No difference in body weight was detected among rats fed with the same diet (all P > 0.05). Although body weight in the HFD groups was higher than in the LFD groups, no difference was detected between the HFD and LFD groups at postoperative weeks 4 and 12 (all P > 0.05).
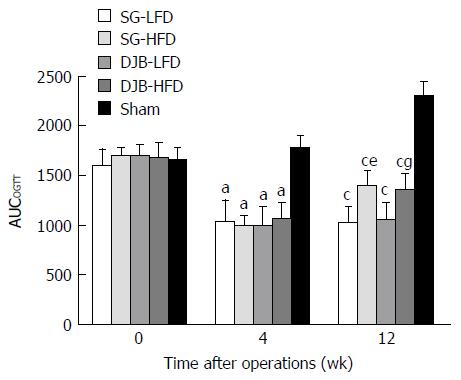
All SG and DJB groups exhibited a significant improvement in glucose tolerance after surgery, as shown by lower AUCOGTT values than in the Sham group (4 wk postoperatively: 1766.4 ± 139.49; 12 wk postoperatively: 2304.62 ± 143.85) at 4 and 12 wk postoperatively (4 wk postoperatively: DJB-LFD 991.94 ± 198.9, P < 0.05 vs Sham; DJB-HFD 1052.88 ± 170.74, P < 0.05 vs Sham; SG-LFD 1031.16 ± 223.73, P < 0.05 vs Sham; SG-HFD 992.64 ± 105.68, P < 0.05 vs Sham; 12 wk postoperatively: DJB-LFD 1049.17 ± 181.03, P < 0.05 vs Sham; DJB-HFD 1344.82 ± 178.73, P < 0.05 vs Sham; SG-LFD 1016.5 ± 170.1, P < 0.05 vs Sham; SG-HFD 1387.53 ± 171.73, P < 0.05 vs Sham; Figure 1). As expected, the improvement in glucose tolerance was reversed after HFD was provided for 8 wk. AUCOGTT in the HFD groups at 12 wk postoperatively was significantly higher than at 4 wk postoperatively (DJB-HFD at 12 wk postoperatively, P < 0.05 vs DJB-HFD at 4 wk postoperatively; SG-HFD at 12 wk postoperatively, P < 0.05 vs SG-HFD at 4 wk postoperatively). Although the improved glucose tolerance was reversed, the HFD groups still had better glucose tolerance than the Sham group had, as shown by lower AUCOGTT at postoperative week 12. SG and DJB groups fed with the same diet showed similar AUCOGTT and no difference was detected at 4 and 12 wk postoperatively (4 wk postoperatively: DJB-LFD, P > 0.05 vs SG-LFD; DJB-HFD, P > 0.05 vs SG-HFD; 12 wk postoperatively: DJB-LFD, P > 0.05 vs SG-LFD; DJB-HFD, P > 0.05 vs SG-HFD).
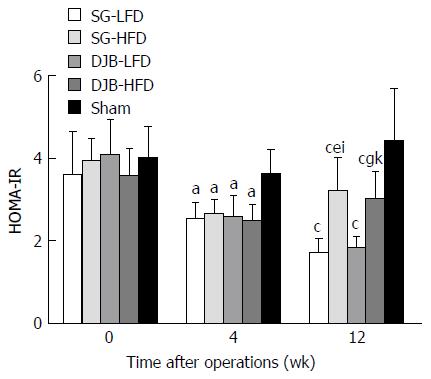
SG and DJB groups exhibited significantly lower HOMA-IR values than the Sham group (4 wk postoperatively: 3.60 ± 0.60; 12 wk postoperatively: 4.40 ± 1.27) at postoperative weeks 4 and 12 (4 wk postoperatively: DJB-LFD 2.56 ± 0.53, P < 0.05 vs Sham; DJB-HFD 2.47 ± 0.42, P < 0.05 vs Sham; SG-LFD 2.51 ± 0.41, P < 0.05 vs Sham; SG-HFD 2.64 ± 0.36, P < 0.05 vs Sham; 12 wk postoperatively: DJB-LFD 1.81 ± 0.28, P < 0.05 vs Sham; DJB-HFD 3.0 ± 0.69, P < 0.05 vs Sham; SG-LFD 1.70 ± 0.34, P < 0.05 vs Sham; SG-HFD 3.19 ± 0.82, P < 0.05 vs Sham; Figure 2). LFD groups resulted in better insulin sensitivity at postoperative week 12 than at week 4, as demonstrated by lower HOMA-IR at postoperative week 12 (DJB-LFD at 12 wk postoperatively, P < 0.05 vs DJB-HFD at 4 wk postoperatively; SG-LFD at 12 wk postoperatively, P < 0.05 vs SG-HFD at 4 wk postoperatively). However, the HFD groups resulted in re-impaired insulin sensitivity at postoperative week 12, as shown by higher HOMA-IR values than at postoperative week 4, but the difference did not reach significance (4 wk postoperatively: DJB-LFD, P > 0.05 vs SG-LFD; DJB-HFD, P > 0.05 vs SG-HFD; 12 wk postoperatively: DJB-LFD, P > 0.05 vs SG-LFD; DJB-HFD, P > 0.05 vs SG-HFD). HOMA-IR in the HFD groups at postoperative week 12 was higher than in the LFD groups (DJB-HFD at 12 wk postoperatively, P < 0.05 vs DJB-LFD at 12 wk postoperatively; SG-HFD at 12 wk postoperatively, P < 0.05 vs SG-LFD at 12 wk postoperatively).
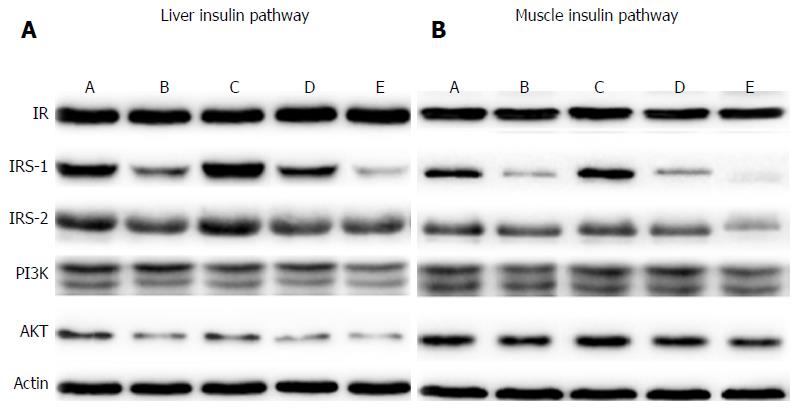
Expression of IRS-1, IRS-2, PI3K and AKT increased in all DJB and SG groups (Figure 3), indicating that the insulin signaling pathway was up-regulated in the liver and skeletal muscle. However, expression of insulin receptor showed no difference among the groups. The HFD groups showed lower expression of IRS-1, IRS-2, PI3K and AKT than the LFD groups, indicating the re-impairment of hepatic and muscular insulin signaling.
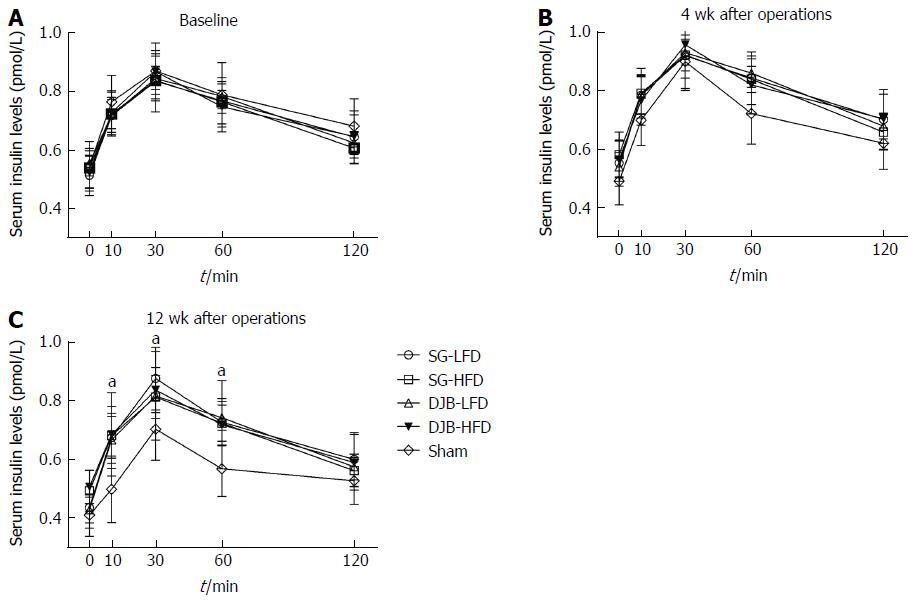
At postoperative week 4, no significant difference in glucose-stimulated insulin secretion was observed among DJB, SG and Sham groups (P > 0.05, two-factor RM ANOVA; Figure 4B), suggesting that the beta-cell function of insulin secretion was not enhanced within a short time after surgery. At postoperative week 12, insulin levels in response to oral glucose gavage increased in HFD and LFD rats, demonstrated by higher insulin curves and peak insulin levels than in the Sham group (P < 0.05 vs Sham group, Figure 4C). However, the peak insulin levels showed no difference between baseline and postoperative week 12 in SG and DJB groups (DJB-LFD at 12 wk postoperatively 0.82 pmol/L ± 0.15 pmol/L, P > 0.05 vs DJB-LFD at baseline 0.85 pmol/L ± 0.08 pmol/L; DJB-HFD at 12 wk postoperatively 0.84 pmol/L ± 0.08 pmol/L, P > 0.05 vs DJB-HFD at baseline 0.87 pmol/L ± 0.06 pmol/L; SG-LFD at 12 wk postoperatively 0.88 pmol/L ± 0.11 pmol/L, P > 0.05 vs SG-LFD at baseline 0.84 pmol/L ± 0.10 pmol/L; SG-HFD at 12 wk postoperatively 0.81 pmol/L ± 0.07 pmol/L, P > 0.05 vs SG-HFD at baseline 0.84 pmol/L ± 0.05 pmol/L; Figure 4), indicating no increase in the beta-cell function of insulin secretion.
At postoperative week 12, all DJB and SG groups exhibited larger and more regulatory beta cell mass (Figure 5A) and fewer TUNEL-positive beta cells (Figure 5B) than in the Sham group. The immunofluorescence intensity of insulin in the DJB and SG groups was about threefold higher than in the Sham group (Figure 5C). These results indicated that DJB and SG resulted in less apoptosis of beta cells and better function of insulin secretion. The HFD groups exhibited more TUNEL-positive beta cells in than LFD groups did (Figure 5A and B), however, no significant difference in the immunofluorescence intensity of insulin was detected between the HFD and LFD groups (both P < 0.05).
Oral glucose gavage resulted in higher GLP-1 levels in all DJB and SG groups than in the Sham group at postoperative weeks 4 and 12 (P < 0.05 vs Sham group, two-factor RM ANOVA) and there was no significant difference between the HFD and LFD groups, and between the SG and DJB groups (Figure 6). Compared with the Sham group (4 wk postoperatively: 66.99 ± 4.27; 12 wk postoperatively: 57.13 ± 5.58), PYY levels in DJB-LFD (4 wk postoperatively: 59.09 ± 3.27, P < 0.05 vs Sham group; 12 wk postoperatively: 106.78 ± 6.08, P < 0.05 vs Sham group), DJB-HFD (4 wk postoperatively: 112.09 ± 5.77, P < 0.05 vs Sham group; 12 wk postoperatively: 91.27 ± 5.58, P < 0.05 vs Sham group), SG-LFD (4 wk postoperatively: 105.10 ± 4.67, P < 0.05 vs Sham group; 12 wk postoperatively: 120.80 ± 5.50, P < 0.05 vs Sham group), SG-HFD (4 wk postoperatively: 108.98 ± 6.31, P < 0.05 vs Sham group; 12 wk postoperatively: 95.11 ± 6.01, P < 0.05 vs Sham group) groups were much higher. SG groups showed lower fasting serum ghrelin values than the DJB and Sham groups.
RYGB and SG are currently the most frequently performed bariatric procedures worldwide and are included in diabetes treatment algorithms[5,15]. Although growing evidence indicates that RYGB and SG improve T2DM with a BMI ≥ 30 kg/m2, even with a lower BMI of 25-27.5 kg/m2) in Asian patients[16,17], diabetes recurrence appears after several years of remission. Few studies have specifically considered diabetes recurrence, compared with the large number that has reported rapid and dramatic remission after bariatric surgery. Identification of the predictors and causes of diabetes recurrence is beneficial to the postoperative glycemic control and reduction of revisional bariatric surgery. In one of our previous studies, we initially reported an animal model of diabetes recurrence after DJB in Goto-Kakizaki (GK) rats and HFD/STZ-induced diabetes in rats[18]. In our study, SG and DJB were both performed on rats with HFD/STZ-induced diabetes. HFD was supplied after initial diabetes remission at 4 wk postoperatively and diabetes recurred at 12 wk postoperatively, which confirms that HFD can induce diabetes recurrence after gastrointestinal metabolic surgery. This remission-recurrence model helps us to understand the mechanisms of diabetes recurrence.
Moreover, it is more beneficial for studying surgery-induced diabetes remission, compared with animal models with long-term diabetes remission. IR is one of the two remarkable pathological characteristics of T2DM, which is usually caused by HFD. Previous studies have discovered that improvement in insulin sensitivity contributes the most to diabetes remission after bariatric surgery, especially in the early stage[19-21]. The present study confirmed this viewpoint with lower HOMA-IR, at 4 and 12 wk postoperatively, and up-regulation of hepatic and muscular insulin signaling provided further evidence for improvement in tissue insulin sensitivity. More importantly, with this remission-recurrence bariatric animal model, we found that the rapidly improved insulin sensitivity was re-impaired after 8 wk HFD gavage, which was shown by higher HOMA-IR in the HFD groups at 12 wk postoperatively compared with before surgery and in the LFD controls. Down-regulated expression of insulin signaling of liver and skeletal muscle in the HFD groups confirmed the re-impairment of tissue insulin sensitivity. HFD was obviously detrimental to durable improvement in our study. Accompanying alterations of gut microbiota, especially adverse higher abundance of Bacteroidetes and Escherichia coli, are considered responsible for diabetes recurrence, possibly by influencing serum lipopolysaccharide and associated low-grade chronic inflammation[22]. A low-calorie diet is included in postoperative instructions from bariatric surgeons, and non-adherence is an important factor associated with durable remission[9-11].
Beta-cell dysfunction, including deficiency of insulin secretion and morphological disorders, is another important pathological characteristic of T2DM. Reports on alterations of beta-cell function after RYGB are controversial in patients or rodents with diabetes[23-28]. In the present study, glucose-stimulated insulin secretion was higher in DJB and SG rats than in Sham rats at 12 wk postoperatively, and no difference was observed between postoperative and preoperative data. These results were identical to studies with non-obese diabetic GK rats[19,23,24], which indicated that DJB and SG procedures preserved but did not increase insulin secretion, at least during 12 wk observation. Meirelles et al[25] reported deceased insulin secretion after RYGB in obese diabetic Zucker rats, and Cummings et al[26] reported a threefold increase in serum insulin after ileal transposition in UCD-T2DM rats. We ascribed these completely distinct alterations of insulin secretion after surgery to different diabetic animal models.
Similar to studies in animals, alterations of insulin secretion after bariatric surgery in patients were also inconsistent[21]. Generally speaking, oral glucose-stimulated insulin secretion, which combines intrinsic and extrinsic regulation of insulin secretion, altered rapidly and significantly after RYGB, with an earlier and exaggerated postprandial rise in insulin concentration that reached a higher peak level than that achieved preoperatively[29-33]. However, intravenous glucose-stimulated insulin secretion, which addressed only the intrinsic regulation, showed that beta-cell function improved minimally and remained significantly impaired after RYGB[34].
We performed pathological examination of the pancreas, which supported the protective effect of DJB and SG on insulin secretion, with larger beta-cell mass, stronger insulin staining, and less apoptosis. Few studies have reported beta-cell morphology, and in all of these, including the present study, only larger beta-cell mass and/or stronger insulin staining were detected in RYGB, SG, DJB or IT animals compared with their diabetic control. It is regrettable that no comparison was performed between pre- and postoperative rats[35-37]. Therefore, there has been no evidence supporting islets hyperplasia or beta-cell turnover after bariatric surgeries until now. DJB and SG preserved insulin secretion and protected beta cells from apoptosis.
Furthermore, we showed that glucose-stimulated insulin secretion was not decreased in DJB and SG rats with recurrent diabetes, and the beta-cell mass and insulin staining showed no difference between the persistent remission and recurrence groups. Consequently, HFD-induced diabetes recurrence after DJB and SG was independent of alterations of beta-cell function during the short observation time. As reported, preoperative old age, higher BMI, lower C-peptide level and long duration of diabetes, which presented poor beta-cell function, predicted the failure of glycemic control[14,38]. Given that more apoptotic beta cells were detected in the groups with recurrent diabetes, re-impaired beta-cell function appeared over the time.
It is controversial whether weight loss is essential to diabetes control after bariatric surgery. In the current study, all non-Sham-operated rats experienced a significant increase in body weight when killed, which was consistent with studies using HFD-STZ and GK rats. This indicated that remission of diabetes was independent of weight changes after surgery. In obese patients accepting bariatric surgery, weight loss undoubtedly benefited diabetes improvement, because the improvement in peripheral insulin sensitivity was significantly related to weight loss[39,40]. EWL is reported to be the only predictor of diabetes remission that is influenced by bariatric procedures[41]. Contradictory voices claim that diabetes resolves independently of weight loss because remission of diabetes occurs before significant weight loss appears, and baseline BMI is unrelated to diabetes remission[42]. Based on the findings that body weight increased when the rats were killed, and no difference in body weight was detected between rats with persistent remission and recurrence of diabetes, we conclude that diabetes recurrence is independent of weight regain. On the contrary, DiGiorgi et al[9] reported that patients with recurrent or worsening diabetes regained a greater percentage of their lost weight, and weight regain was a significant predictor of T2DM recurrence[11]. A possible explanation for the contrary results is that weight regain is a weak predictor of T2DM recurrence[11], and recurrence is more likely to result from a postoperative unhealthy high-calorie diet, which more easily re-impairs insulin sensitivity.
SG and DJB were performed in the present study, and the effect of the two procedures on diabetes showed no significant difference. SG is currently the most performed bariatric procedure and DJB is also performed on diabetic patients, achieving satisfactory disease control[43]. SG and DJB are combined and performed successfully on morbidly obese patients[44]. Better glycemic control in T2DM patients is achieved than with SG alone[45]. As expected and observed in other studies, secretion of GLP-1 and PYY increases after SG and DJB, and ghrelin decreases after SG. However, no alterations of these hormones were observed when diabetes recurred. These results demonstrate that GLP-1, PYY and ghrelin are not correlated with diabetes recurrence.
This study had some limitations. The observation time was only 12 wk. Longer observation and HFD gavage might yield more information about beta-cell apoptosis. Furthermore, lipid profiles (triglycerides, cholesterol and free fatty acids) and inflammatory factors were not examined, and therefore, we cannot further discuss the effect of HFD on the recurrence of diabetes.
In conclusion, this study demonstrated that HFD induced diabetes recurrence after initial remission with SG and DJB surgery. The re-impairment of hepatic and muscular insulin sensitivity was likely responsible for the recurrence, and alterations of beta-cell function, body weight, and gastrointestinal hormones (GLP-1, PYY and ghrelin) seemed not to correlate with recurrence.
Gastrointestinal metabolic surgeries promote dramatic and durable improvement of type 2 diabetes. However, diabetes recurrence happened in part of patients with initial remission after surgeries.
Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are currently the most frequently performed bariatric procedures worldwide and included in diabetes treatment algorithms. Diabetes recurrence appears after years of remission and frustrated both patients and surgeons, but few studies specifically consider diabetes recurrence. This “remission-recurrence” model helped to understand mechanisms of diabetes recurrence.
In this study, the authors created a rat model of diabetes recurrence after SG and duodenal-jejunal bypass (DJB) by feeding the rats a high-fat diet postoperatively. The rats with diabetes recurrence showed re-impairment of hepatic and muscular insulin sensitivity, but no alterations of beta-cell function, body weight, and gastrointestinal hormones (GLP-1, PYY and ghrelin).
The authors established a model using high-fat diet to induce diabetes recurrence after bariatric surgery and to find the mechanism of bariatric surgery to improve glucose metabolism. And they had found in this study that it should focus on the tissue insulin sensitivity but not beta cell apoptosis, and it seem not to depend on the change of gastrointestinal hormones like GLP-1, PYY and ghrelin.
DJB is an experimental procedure that was initially designedto investigate the weight-independent anti-diabetic effects of Roux-en-Y gastric bypass surgery, which is the gold standard in patients with diabetes. SG is a popular bariatric procedures performed worldwide, which has a similar effect to RYGB, and less complications than RYGB.
The study is very interesting. In this study, the authors investigated factors causing diabetes recurrence after SG and DJB. HFD could reverse the improvement in glucose homeostasis induced by SG and DJB surgeries.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kositamongkol P, Pratschke S, Yamaoka Y S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 852] [Cited by in F6Publishing: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 2. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002-2013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1202] [Cited by in F6Publishing: 1125] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 3. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1340] [Cited by in F6Publishing: 1214] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 4. | Kashyap SR, Bhatt DL, Wolski K, Watanabe RM, Abdul-Ghani M, Abood B, Pothier CE, Brethauer S, Nissen S, Gupta M. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36:2175-2182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 5. | Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: a Joint Statement by International Diabetes Organizations. Obes Surg. 2017;27:2-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Juodeikis Ž, Brimas G. Long-term results after sleeve gastrectomy: A systematic review. Surg Obes Relat Dis. 2017;13:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Gadiot RP, Biter LU, van Mil S, Zengerink HF, Apers J, Mannaerts GH. Long-Term Results of Laparoscopic Sleeve Gastrectomy for Morbid Obesity: 5 to 8-Year Results. Obes Surg. 2017;27:59-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248-256.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1816] [Cited by in F6Publishing: 1664] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 9. | DiGiorgi M, Rosen DJ, Choi JJ, Milone L, Schrope B, Olivero-Rivera L, Restuccia N, Yuen S, Fisk M, Inabnet WB. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010;6:249-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Jiménez A, Casamitjana R, Flores L, Viaplana J, Corcelles R, Lacy A, Vidal J. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256:1023-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 11. | Chikunguwo SM, Wolfe LG, Dodson P, Meador JG, Baugh N, Clore JN, Kellum JM, Maher JW. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:254-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, Kashyap SR, Kirwan JP, Rogula T, Kroh M. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258:628-636; discussion 636-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 364] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 13. | Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg. 2012;147:847-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Lee WJ, Chong K, Chen SC, Zachariah J, Ser KH, Lee YC, Chen JC. Preoperative Prediction of Type 2 Diabetes Remission After Gastric Bypass Surgery: a Comparison of DiaRem Scores and ABCD Scores. Obes Surg. 2016;26:2418-2424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25:1822-1832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1090] [Cited by in F6Publishing: 1053] [Article Influence: 117.0] [Reference Citation Analysis (1)] |
| 16. | Wang G, Zhu L, Li W, Yang X, Li P, Zhu S. Can low BMI Chinese patients with type 2 diabetes benefit from laparoscopic Roux-en-Y gastric bypass surgery? Surg Obes Relat Dis. 2016;12:1890-1895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Han X, Yu H, Di J, Zhang P, Jia W. Effect of Roux-en-Y Gastric Bypass on Remission of T2D: Medium-Term Follow-up in Chinese Patients with Different BMI Obesity Class. Obes Surg. 2017;27:134-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Liu SZ, Sun D, Zhang GY, Wang L, Liu T, Sun Y, Li MX, Hu SY. A high-fat diet reverses improvement in glucose tolerance induced by duodenal-jejunal bypass in type 2 diabetic rats. Chin Med J (Engl). 2012;125:912-919. [PubMed] [Cited in This Article: ] |
| 19. | Liu S, Zhang G, Wang L, Sun D, Chen W, Yan Z, Sun Y, Hu S. The entire small intestine mediates the changes in glucose homeostasis after intestinal surgery in Goto-Kakizaki rats. Ann Surg. 2012;256:1049-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care. 2009;32:514-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Dirksen C, Jørgensen NB, Bojsen-Møller KN, Jacobsen SH, Hansen DL, Worm D, Holst JJ, Madsbad S. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55:1890-1901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Zhong MW, Liu SZ, Zhang GY, Zhang X, Liu T, Hu SY. Alterations in gut microbiota during remission and recurrence of diabetes after duodenal-jejunal bypass in rats. World J Gastroenterol. 2016;22:6706-6715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Gavin TP, Sloan RC, Lukosius EZ, Reed MA, Pender JR, Boghossian V, Carter JJ, McKernie RD, Parikh K, Price JW. Duodenal-jejunal bypass surgery does not increase skeletal muscle insulin signal transduction or glucose disposal in Goto-Kakizaki type 2 diabetic rats. Obes Surg. 2011;21:231-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Speck M, Cho YM, Asadi A, Rubino F, Kieffer TJ. Duodenal-jejunal bypass protects GK rats from {beta}-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am J Physiol Endocrinol Metab. 2011;300:E923-E932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Meirelles K, Ahmed T, Culnan DM, Lynch CJ, Lang CH, Cooney RN. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249:277-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Cummings BP, Strader AD, Stanhope KL, Graham JL, Lee J, Raybould HE, Baskin DG, Havel PJ. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138:2437-2446, 2446.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 591] [Cited by in F6Publishing: 479] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 28. | Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care. 2006;29:1554-1559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, Kirwan JP, Schauer PR. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond). 2010;34:462-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 30. | Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring). 2008;16:298-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 31. | Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227-2235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 32. | Reed MA, Pories WJ, Chapman W, Pender J, Bowden R, Barakat H, Gavin TP, Green T, Tapscott E, Zheng D. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab. 2011;96:2525-2531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Umeda LM, Silva EA, Carneiro G, Arasaki CH, Geloneze B, Zanella MT. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg. 2011;21:896-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Dutia R, Brakoniecki K, Bunker P, Paultre F, Homel P, Carpentier AC, McGinty J, Laferrère B. Limited recovery of β-cell function after gastric bypass despite clinical diabetes remission. Diabetes. 2014;63:1214-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Patriti A, Aisa MC, Annetti C, Sidoni A, Galli F, Ferri I, Gullà N, Donini A. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery. 2007;142:74-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Seyfried F, Miras AD, Rotzinger L, Nordbeck A, Corteville C, Li JV, Schlegel N, Hankir M, Fenske W, Otto C. Gastric Bypass-Related Effects on Glucose Control, β Cell Function and Morphology in the Obese Zucker Rat. Obes Surg. 2016;26:1228-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Zhou X, Qian B, Ji N, Lui C, Liu Z, Li B, Zhou H, Yan C. Pancreatic hyperplasia after gastric bypass surgery in a GK rat model of non-obese type 2 diabetes. J Endocrinol. 2016;228:13-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Lee MH, Lee WJ, Chong K, Chen JC, Ser KH, Lee YC, Chen SC. Predictors of long-term diabetes remission after metabolic surgery. J Gastrointest Surg. 2015;19:1015-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S, Baldi S, Nannipieri M, Rebelos E, Anselmino M. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54:2093-2102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 40. | Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 41. | Hamza N, Abbas MH, Darwish A, Shafeek Z, New J, Ammori BJ. Predictors of remission of type 2 diabetes mellitus after laparoscopic gastric banding and bypass. Surg Obes Relat Dis. 2011;7:691-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Panunzi S, De Gaetano A, Carnicelli A, Mingrone G. Predictors of remission of diabetes mellitus in severely obese individuals undergoing bariatric surgery: do BMI or procedure choice matter? A meta-analysis. Ann Surg. 2015;261:459-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 43. | Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 44. | Kasama K, Tagaya N, Kanehira E, Oshiro T, Seki Y, Kinouchi M, Umezawa A, Negishi Y, Kurokawa Y. Laparoscopic sleeve gastrectomy with duodenojejunal bypass: technique and preliminary results. Obes Surg. 2009;19:1341-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Zachariah PJ, Chen CY, Lee WJ, Chen SC, Ser KH, Chen JC, Lee YC. Compared to Sleeve Gastrectomy, Duodenal-Jejunal Bypass with Sleeve Gastrectomy Gives Better Glycemic Control in T2DM Patients, with a Lower β-Cell Response and Similar Appetite Sensations: Mixed-Meal Study. Obes Surg. 2016;26:2862-2872. [PubMed] [Cited in This Article: ] |