Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7457
Peer-review started: September 21, 2014
First decision: October 14, 2014
Revised: November 27, 2014
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: June 28, 2015
AIM: To detect the mechanism by which colon tumor escapes the growth constraints imposed on normal cells by cell crowding and dense pericellular matrices.
METHODS: An immunohistochemical study of integrin αvβ6 and matrix metalloproteinase-9 (MMP-9) was performed on tissue microarrays of 200 spots, including 100 cases of colon tumors.
RESULTS: High immunoreactivity for αvβ6 (73.7%; 28/38) and MMP-9 (76.5%; 52/68) was observed in invasive tumor portions. Furthermore, the effects of integrin αvβ6 on tumor invasive growth in nude mice were detected. Tumor invasive growth and high expression of both αvβ6 and MMP-9 were only seen in tumors resulting from WiDr cells expressing αvβ6 in the tumorigenicity assay. Flow cytometry was applied to analyze αvβ6 expression in colon cancer WiDr and SW480 cells. The effects of cell density on αvβ6 expression and MMP-9 secretion were also detected by Biotrak MMP-9 activity assay and gelatin zymography assay. High cell density evidently enhanced αvβ6 expression and promoted MMP-9 secretion compared with low density.
CONCLUSION: Integrin αvβ6 sustains and promotes tumor invasive growth in tumor progression via a self-perpetuating mechanism. Integrin ανβ6-mediated MMP-9 secretion facilitates pericellular matrix degradation at high cell density, which provides the basis of invasive growth.
Core tip: This study is designed to identify the mechanisms by which integrin αvβ6 sustains and promotes tumor invasive growth in colon cancer progression. Our results suggested that integrin αvβ6 sustains and promotes tumor invasive growth in tumor progression via a self-perpetuating mechanism. Integrin ανβ6-mediated matrix metalloproteinase-9 secretion facilitates pericellular matrix degradation at high cell density, which provides the basis of invasive growth.
- Citation: Yang GY, Guo S, Dong CY, Wang XQ, Hu BY, Liu YF, Chen YW, Niu J, Dong JH. Integrin αvβ6 sustains and promotes tumor invasive growth in colon cancer progression. World J Gastroenterol 2015; 21(24): 7457-7467
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7457
Invasive growth is one of the main features that distinguish malignant tumor cells from normal cells. The mechanisms by which tumor cells escape the growth constraints imposed on normal cells by cell crowding and dense pericellular circumstance are controversial. The reason why colon cancer cells sustain invasive growth via a self-perpetuating manner in tumor progression is also unclear. There is a general consensus, nevertheless, that this demonstrates a cell-surface problem, and the cell adhesion molecules integrin αvβ6 and matrix metalloproteinase-9 (MMP-9) are likely to be involved in tumor progression[1,2].
Within the integrin αv subfamily, integrin αvβ6 is expressed only on abnormal epithelial cells. It is highly expressed during morphogenesis and tumorigenesis[2-4], and de novo expression has been observed at the margins of advanced colon tumors. One potential mechanism for the growth-promoting effect of integrin αvβ6 may be via enhanced MMP-9 activity. The invasive growth of colon cancer cells is also likely to reflect the ability of tumor cells to digest their surrounding matrix scaffold through the secretion of MMP-9 because integrin αvβ6 expression in colon cancer cells has been shown by our group to induce MMP-9 secretion[5], and the inhibition of MMP-9 activity abolishes the integrin αvβ6-mediated growth effect[6].
As an adhesion protein involved in both the nuclear Wnt/beta-catenin pathway and the mesenchymal transition of colorectal cancer cells, nuclear beta-catenin expression increases from the central area towards the invasive margin. It has been reported that the expression of integrin αvβ6, which is also an adhesion protein, is induced during the epithelial-transition of aggressive colon carcinoma[3,7-9]. MMP-9 overexpression related to tumor invasive growth in gastric carcinoma has also been reported. The induction of MMP-9 mRNA in endothelial cells has been reported to be dependent on direct cell adhesion with cancer cells[10]. The maximal expression of MMPs has also been displayed at the invasive margin of colon tumor cell islands. This finding is consistent with the observation that integrin αvβ6 preferentially localizes at the leading edge of epithelial ovarian cancer with a malignant potential of invasiveness and metastasis. The consequence of integrin ανβ6-mediated MMP-9 secretion may provide the basis for a self-perpetuating system of tumor invasive growth that operates through integrin ανβ6. However, the effects of both integrin αvβ6 and MMP-9 on invasive growth in colon cancer progression remain controversial.
This study was designed to identify the mechanisms by which integrin αvβ6 sustains and promotes tumor invasive growth in colon cancer progression.
The human colon cancer cell lines WiDr and SW480 and the normal human keratinocyte cell line HaCaT were obtained from the ATCC (Rockville, MD, United States). SW480 cells, which lack constitutive integrin αvβ6 expression, were stably transfected with pcDNA1neo constructs that contained either the β6 gene construct or the expression plasmid only (SW480 β6 or SW480 mock) as previously described[11].
For flow cytometry analysis, low-density cultures were established by seeding 5 to 7.5 × 105 cells in 2.5 mL of standard medium into 6 cm-diameter tissue culture dishes or 25 cm2 tissue culture flasks. High density cultures were established using identical cell numbers and medium volume seeded into 24-well tissue culture plates (Falcon Becton Dickinson, Oxnard, CA). At 72 h, the low- and high-density cultures were approximately 40% and 100% confluent, respectively. Then, cells were harvested by trypsin/EDTA at 72 h for the assessment of integrin ανβ6 expression. The ratio of cell number to surface area was in the range of 1.5 to 2 × 105 cells/cm2 and 3.5 to 4 × 105 cells/cm2 for the low- and high-density cultures, respectively.
The anti-integrin αvβ6 mAb 2G2 was obtained from Biogen, and the specificity of the antibody has previously been reported[12].
FITC-conjugated goat-anti-mouse IgG was obtained from Jackson (ImmunoResearch Laboratories, Inc., United States); anti-MMP9 mAbs-whole molecule (ab38898, Abcam, United Kingdom) was purchased from Jingmei Biotech (Shenzhen, China). Briefly, the conditioned medium (CM) for MMP-9 estimation was prepared by the removal of fetal bovine serum-containing medium and three washes of the adherent cells with phosphate-buffered saline prior to the addition of chemically defined serum-free medium.
From January 2007 to December 2007, 100 patients who underwent curative resection by the same surgical team for pathologically confirmed colon carcinoma at the Department of Pathology of Qilu Hospital (Shandong University, China) had their formalin-fixed, paraffin-embedded tissue blocks that contained colon carcinoma specimens selected. The specimens were constructed into tissue microarray (TMA; 200 spots each) slides under the Human Investigative Committee protocol of Shandong University by Chaoying Biochip Company, Ltd. (Xi’an, China). The maximum tumor diameter in each colon tumor specimen we selected was not less than 1.5 cm to suit the requirement of the TMA. After screening hematoxylin and eosin-stained (HE) slides for optimal tumor content, two cores, one obtained from tumor edge portions that contained the invasive margin of the lesion (generally located in the superficial part of the tumor) and the other from the central portion of the tumor (that contained none or little of the invasive margin, generally located in the deep part of the tumor), were obtained from each sample using punch cores; these punch cores measured 1.0 mm in the greatest dimension and were spaced 0.8 mm apart. The invasive front of the tumor has ample blood supply, and because the most biologically relevant portion of tumors with histologic heterogeneity is the area with the deepest invasion, tumor “budding” in this area is also enriched[13,14]. The invasive fronts were defined as expanding or infiltrating in HE slides according to the morphological guidelines previously defined by Jass and colleagues.
Immunohistochemistry (IHC) for integrin ανβ6 was performed using the avidin-biotin complex (ABC) method. Overnight incubation occurred at 4 °C with primary antibody against integrin ανβ6 (2G2, 1.67 μg/mL, Biogen, Idec., United States). The antibody is specific for integrin αvβ6 and does not recognize the αv or other αv integrins. Negative controls were treated identically but with the primary antibody omitted.
Immunohistochemical staining for MMP-9 was performed using a monoclonal antibody, clone ab38898, against MMP-9 (5 μg/mL; Abcam, United Kingdom). The antibody recognizes murine and human MMP-9 but does not cross-react with the other MMP family members (MMP-1, MMP-2, or MMP-3). TMA sections, 5 μm thick, were thaw-mounted onto Fisherbrand Super Frost/Plus slides. After air drying at 37 °C for 12 h and incubation for 20 min at 60 °C, the sections were deparaffinized and rehydrated. No antigen exposure procedure of any type was necessary. Staining was performed after incubation with the antibody at 4 °C for 12 h using labeled avidin-biotin. Negative controls underwent a similar staining procedure with the exclusion of the primary antibody application.
The sections were assessed for both integrin αvβ6 and MMP-9 immunoreactivity microscopically via positive DAB staining by three trained observers (Yang GY, Wang YQ and Guo S). The integrin αvβ6 is a trans-membrane protein with immunohistochemical staining located in both the membrane and cytoplasm, whereas the immunohistochemical staining for MMP-9 was predominately located in the cytoplasm. The percentage of positive cells and staining intensity were determined by three observers with 100% agreement.
The cells were incubated with anti-β6 mAb 10D5 (10 μg/mL), E7P6 (10 μg/mL) or an isotype-matched control IgG (10 μg/mL) for 30 min. After washing, cells were stained with goat anti-mouse IgG conjugated with phycoerythrin/FITC for 30 min prior to flow cytometry analysis.
For the tumorigenicity assay, BALB/C female nude mice (6 wk of age purchased from the Animal Resource Center, Shandong University, China) were maintained under pathogen-free conditions and fed standard mouse chow and water ad lib. All mice within each group were inoculated with a single cell line. The cells used were WiDr wild-type and WiDr antisense β6 transfectants. The mice received subcutaneous flank injections of approximately 2 × 106 viable tumor cells (cell counts and viability were assessed by counting cells stained with 0.4% trypan blue in a hemocytometer) suspended 0.1 mL of DMEM culture medium. Tumor sizes (breadth and length as measured with calipers) were recorded weekly. Six weeks following the last injection, the mice were sacrificed and visible subcutaneous tumors were excised. The formalin-fixed paraffin-embedded sections were analyzed for HE and IHC staining to inspect routinely for invasive growth.
MMP-9 levels in TCM obtained from low-and high-density cultures were assayed using a commercially available kit, the MMP-9 activity assay system.
Following electrophoresis, the gels were washed twice in 2.5% Triton X-100 for 30 min at RT to remove the SDS. The gels were subsequently incubated at 37 °C overnight in substrate buffer that contained 50 mmol/L Tris HCl and 5 mmol/L CaCl2 (pH 8.0). The gels were stained with 0.15% Coomassie blue R250 (Bio-Rad, Hercules CA, United States) in 50% methanol and 10% glacial acetic acid for 20 min at room temperature.
The continuous variables are expressed as the mean ± SD and were compared between groups using Student’s t tests. The categorical variables were compared using χ2 tests. Survival curves were drawn by the Kaplan-Meier method, and their comparisons were analyzed by the log-rank test. All statistical analyses were conducted using SPSS 20.0 statistical software (SPSS, Inc., Chicago, IL). Statistical significance was defined as a P value < 0.05.
To examine the role of integrin ανβ6 in the invasive growth of colon carcinoma cells, the expression and distribution of integrin ανβ6 and MMP-9 in invasive tumors were evaluated by IHC in both the edge portion that contained the invasive margin of the lesion (generally located in the superficial part of the tumor) and in the non-invasive central area of the tumor (that contained little or none of the invasive margin, generally located in the deep part of the tumor). Two hundred spot tissue microarrays (TMAs) including 100 cases of malignant colon tumors were used, and the clinicopathological characteristics are shown in Table 1. Table 2 summarizes the findings of both ανβ6 and MMP-9 expression on the TMAs.
| Characteristic | Number of patients | Percentage |
| Sex | ||
| Male | 54 | 54 |
| Female | 46 | 46 |
| Age (yr) | ||
| < 60 | 61 | 61 |
| ≥ 60 | 39 | 39 |
| Tumor location | ||
| Right | 39 | 39 |
| Transverse | 10 | 10 |
| Left colon | 51 | 51 |
| Depth1 | ||
| < 5 mm | 72 | 72 |
| ≥ 5 mm | 28 | 28 |
| Maximum diameter of tumor (cm) | ||
| ≥ 1.0 and < 5.0 | 67 | 67 |
| ≥ 5.0 | 33 | 33 |
| Tumor differentiation | ||
| Well | 19 | 19 |
| Moderate | 53 | 53 |
| Poor | 21 | 21 |
| Unknown | 7 | 7 |
| Lymph node metastasis | ||
| 0 | 23 | 23 |
| 1-4 | 32 | 32 |
| ≥ 5 | 45 | 45 |
| Venous vessel invasion | ||
| Absent | 42 | 42 |
| Present | 58 | 58 |
| Tumor margin | ||
| Expanding | 81 | 81 |
| Infiltrating | 19 | 19 |
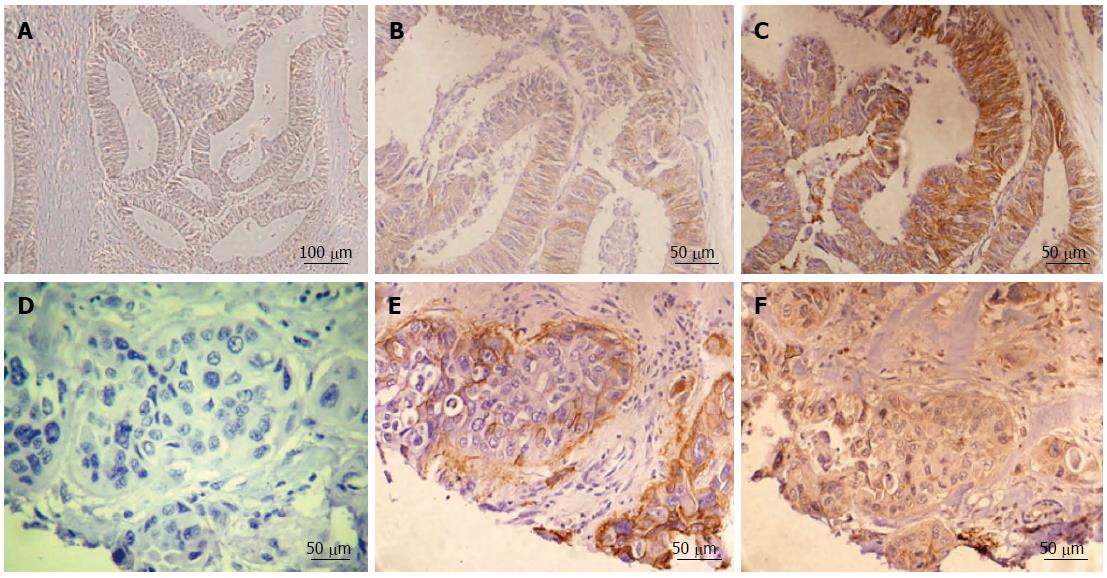
Negative integrin αvβ6 expression is shown in Figure 1A. A sample with low integrin αvβ6 expression is indicated in the deep part of the tumor (Figure 1B), whereas high expression of integrin αvβ6 was detected in the tumor edge cells (Figure 1C). In paired serial sections, Figure 1D is a negative control. At the stage of tumor progression, tumor budding is exhibited as an enrichment in the invasive front[15,16].
Intense up-regulation of αvβ6 expression, and particularly, preferential localization at the edges of both aggressive tumor islands and tumor budding are shown in Figure 1E, which is consistent with our recent report. Similarly, strong MMP-9 expression was also identified in paired serial sections as shown in Figure 1F. High integrin αvβ6 expression was detected in tumor invasive edge portions in 73.7% (28 cases of high expression/38 cases of positive expression) of cases; strong MMP-9 staining intensity was also identified in 76.5% (52 cases of high expression/68 cases of positive expression) of cases in the same tumor edge portions. These data indicate that both integrin αvβ6 and MMP-9 are strongly associated with invasive tumor growth.
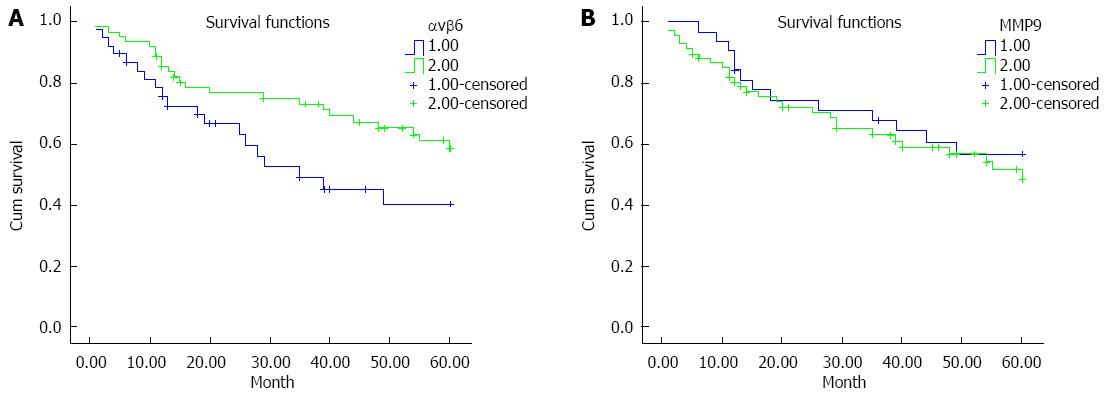
To determine whether there is an association between either αvβ6 or MMP-9 expression in primary colon cancer and patient survival, a five-year follow-up of prognosis was performed. Corresponding Kaplan-Meier plot is shown in Figure 2. There was a significant difference between the integrin αvβ6 positive patients and the integrin αvβ6 negative patients (P = 0.048; P < 0.05). The survival estimates also exhibited a striking difference in the median survival: the integrin αvβ6 positive patients averaged 45.9 mo, whereas the integrin αvβ6 negative patients averaged only 36.1 mo and exhibited an 18.3% lower 5-year survival as shown in Table 3. However, the log-rank test indicated that there was no significant difference in the median survival or 5-year survival between the MMP-9 positive patients and the MMP-9 negative patients. From a biological perspective, we conclude that elevated expression of integrin αvβ6 in colon cancer has been associated with a poor prognosis.
| Groups | Negative/positive | n | Median survival (mo) and 95%CI | 5-yr survival rate (%) and 95%CI |
| αvβ6 | Negative | 62 | 45.948 (40.575-51.320) | 0.585 (0.452-0.718) |
| Positive | 38 | 36.114 (28.501-43.728) | 0.402 (0.220-0.584)1 | |
| MMP-9 | Negative | 32 | 44.071 (36.812-51.330) | 0.570 (0.392-0.748) |
| Positive | 68 | 41.686 (36.040-47.331) | 0.485 (0.344-0.626)2 |
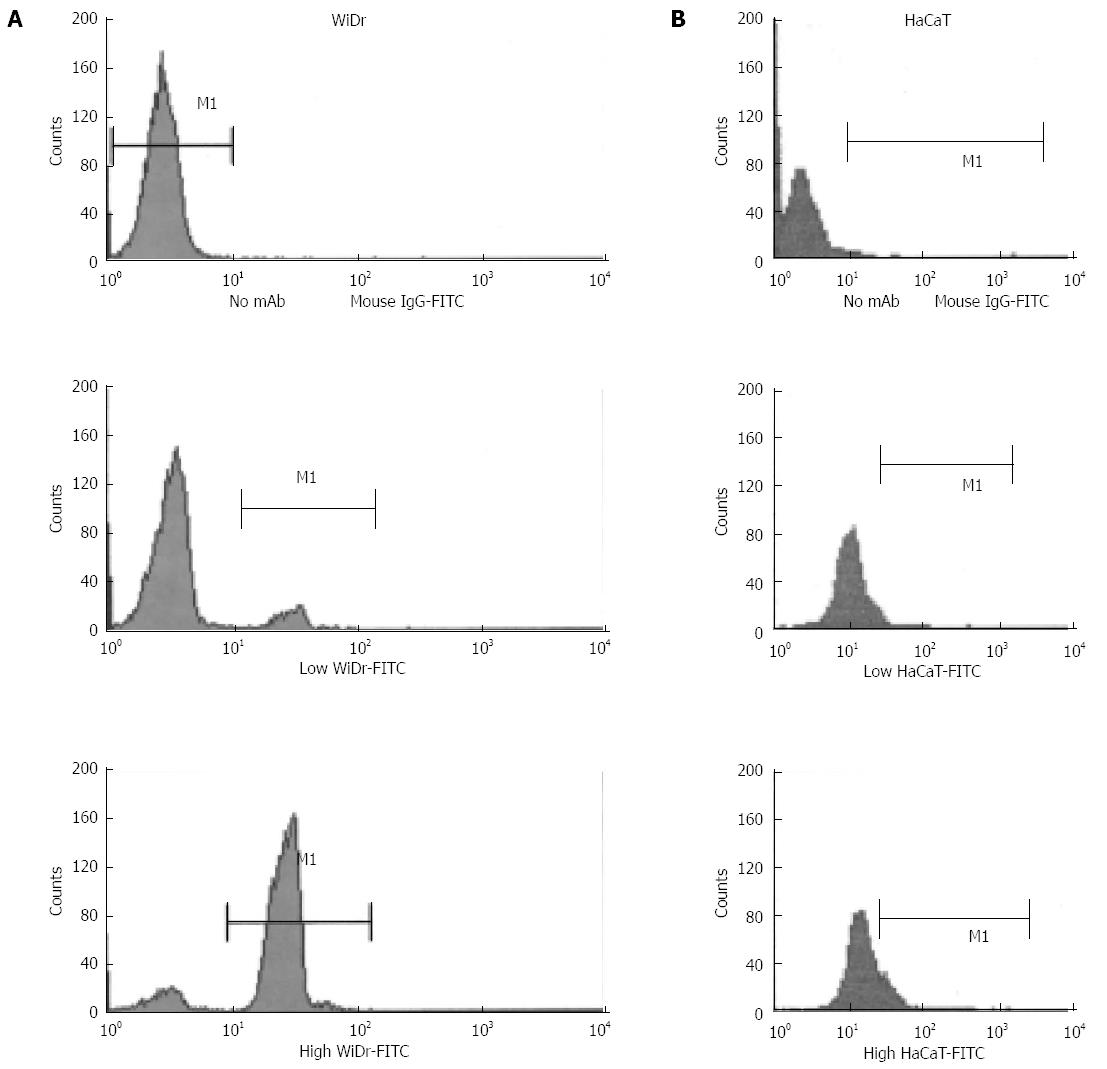
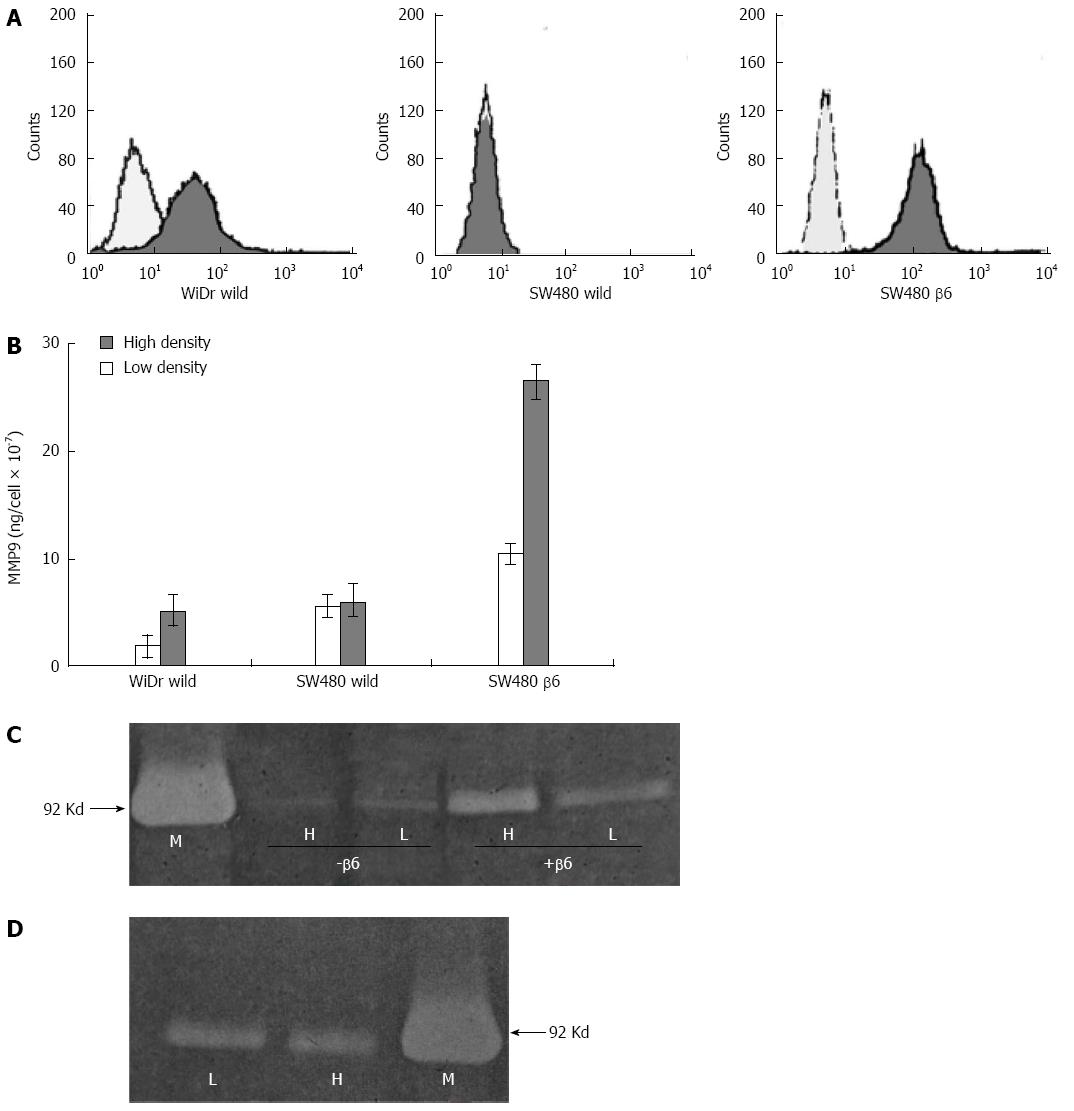
To detect the mechanism by which colon carcinoma cells maintain invasive growth via escaping the growth constraints, the surface expression of integrin ανβ6 was evaluated by flow cytometry in the human colon cancer cell line WiDr and the normal human cell line cultured at low or high cell densities. As shown in Figure 3A, the integrin ανβ6 surface expression was higher in the cells with high density compared with low-density cultures, and no cell density-dependent increase in integrin ανβ6 expression was displayed in HaCaT cells (Figure 3B).
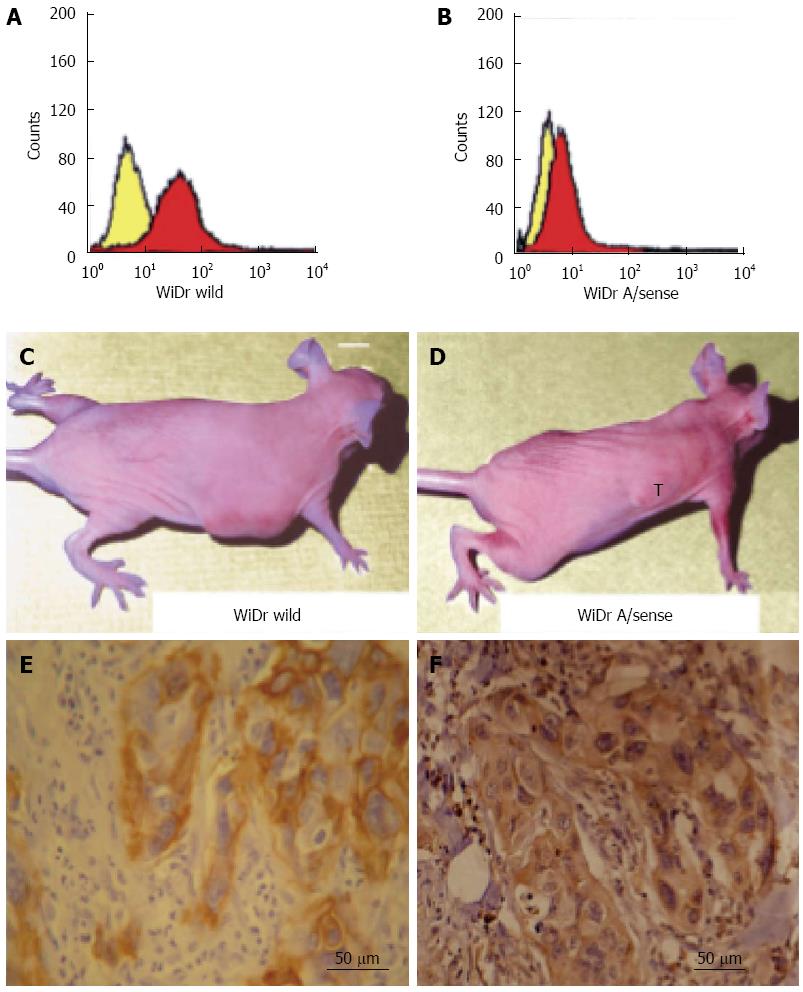
To evaluate the effect of integrin ανβ6 on the invasive growth of colon cancer tumors in vivo, we injected 2 × 106 wild-type WiDr cells that expressed integrin ανβ6 (Figure 4A) and antisense β6 WiDr cells that expressed less integrin ανβ6 (Figure 4B) into the right flank of 20 athymic mice per cell line. Tumor growth was subsequently followed for 6 wk. The tumors formed by the wild-type WiDr cells (Figure 4C) grew to a substantially larger size (breadth and length) compared with the tumors formed by the antisense β6 WiDr cells. Of the 20 mice inoculated with antisense β6 WiDr cells, the tumors completely disappeared in 65% (13/20) of the animals. In the remaining 7 mice, the largest tumor size was only 6 mm2 (Figure 4D) compared with tumor sizes of at least 16-25 mm2 in the other 20 mice inoculated with wild-type WiDr cells that expressed normal levels of integrin ανβ6. These results indicate that a fundamental difference of tumor growth exists between wild-type WiDr and antisense β6 WiDr cells.
A significant difference in infiltrating behavior between the two tumor types was noted from routine histochemistry and IHC. Histologically, we demonstrated that the tumors formed by the antisense β6 WiDr cells grew adjacent to, but did not infiltrate, the underlying muscle, whereas the tumors formed by the wild-type WiDr cells extensively infiltrated the underlying muscle. The results of IHC for both integrin αvβ6 and MMP-9 indicated that integrin αvβ6 was expressed highly in tumors resulting from wild-type WiDr cells as shown in Figure 4E, and MMP-9 also stained strongly in the serial sections as shown in Figure 4F. However, in tumors resulting from antisense β6 WiDr cells, integrin αvβ6 was barely detectable and MMP-9 was weakly expressed (data not shown). The data indicate that integrin αvβ6 expression significantly enhances invasive tumor growth in vivo.
To examine the effects of cell density on integrin ανβ6 and MMP-9 as colon carcinoma cells maintain invasive growth when cells are crowded and dense, the surface expression of integrin ανβ6 in human colon cancer cell lines was evaluated by flow cytometry as shown in Figure 5A. Wild-type WiDr and SW480 cells and β6 SW480 cells were cultured at low and high cell densities under serum-free conditions in tumor-conditioned medium (TCM) and analyzed for the presence of MMP-9 using the Biotrak MMP-9 activity assay system. As shown in Figure 5B, both the wild-type WiDr and β6 SW480 cells secreted approximately 2-3 times more MMP-9 per cell at high cell density compared with low-density conditions. However, no increase in MMP-9 was identified in the high-density cultures of the wild-type SW480 cells relative to the low-density cultures.
To examine the effect of cell density on MMP-9 secretion in the absence of integrin αvβ6, TCM from SW480 cells was analyzed for the presence of the enzyme using gelatin zymography. As shown in Figure 5C, a marked increase in the amount of MMP-9 in TCM from high-density compared with low-density cultures was identified only for the β6 SW480 cells, which express integrin αvβ6. However, no increase in MMP-9 was identified for the high-density cultures of the wild-type SW480 cells, which lack integrin αvβ6. These data indicate that the promotion of MMP-9 secretion as colon cancer cells reach confluence occurs in an αvβ6-dependent manner.
Integrin αvβ6 is undetectable on HaCaT cells in situ, but it significantly increases in wound healing and culture-established keratinocytes[1]. The HaCaT cells were cultured under identical conditions to the colon cancer cell lines. However, no difference in integrin αvβ6 expression was identified in the HaCaT cells cultured at low vs high cell densities (Figure 3B). MMP-9 is the predominant type of collagenase in dermal keratinocytes, and in contrast to the colon cancer cell lines, MMP-9 secretion was not enhanced for the HaCaT cells cultured at high density (Figure 5D).
Invasive growth is the main characteristic feature that distinguishes malignant tumor cells from normal cells, and this relies on the capability of tumor cells to digest surrounding matrix through the secretion of MMP-9. For example, MMP activators have been implicated in colon cancer invasive growth[17]. The increased expression of integrin αvβ6 has also been demonstrated to promote cell growth[17-19] and to induce MMP-9 secretion both in vitro and in vivo[5,6,20]. Furthermore, the inhibition of MMP-9 activity abolishes the integrin αvβ6-mediated growth effect. Nevertheless, the effect of integrin αvβ6 on invasive growth remains unknown. According to Weiss et al[21], the term ‘‘crowdedness’’ by its mere literal sense signals that the size and conformation of a test particle dictates if it feels an environment is crowded. Here, cell crowding and density are references to a specific environment in which the cell concentration or density is more crowded than the normal status. The expression of certain genes that regulate cell growth in colon cancer may be cell density-dependent. Takeha also reported that increased MMP-9 expression in primary cancer cells is associated with infiltrating growth in human colorectal cancers, essentially distributed along the invasive margin. We have demonstrated that increased high integrin αvβ6 immunoreactivity is coincident with high MMP-9 staining in invasive tumor portions that contain the invasive margin of the lesion compared with the non-invasive tumor central area (that contains little or none of the invasive margin). Furthermore, the culmination of our observations is detected in the invasive front (at the stage of tumor development when tumor cells migrate into and invade the surrounding tissue either as single cells or in collective clusters, thereby forming an invasive front), especially in the crowded cell zone, of colon cancer tumors. In this study, we demonstrate that the colon cancer cell lines WiDr wild-type and SW480 β6 transfectants compared with HaCaT normal human keratinocyte cells at high cell density exhibit evidently enhanced integrin αvβ6 expression. The present study suggests that integrin αvβ6 is preferentially expressed over normal human cells during invasive growth when colon cancer cells become crowded and dense in progression. These data also indicate that invasive tumor growth is strongly associated with the induced expression of integrin αvβ6 at a high cell density.
The maximal expression of MMP-9 has been identified at the invasive margin of tumor cell islands in colon cancer[12,14,20]. We have also reported that integrin ανβ6 induces MMP-9 secretion[12,17]. In contrast, no density-dependent secretion of MMP-9 was identified for colon cancer cells that lacked integrin ανβ6. We also recently reported that the down-regulation of constitutive integrin αvβ6 expression dramatically reduced MMP-9 levels in the cultures of human colon cancer cell lines[22]. Therefore, the present study suggests that integrin ανβ6-mediated MMP-9 secretion at high cell density provides the basis for a self-perpetuating system of invasive tumor growth for integrin ανβ6-expressing cells that operates through integrin ανβ6.
In vivo, a variety of factors and events affect tumor cell invasive growth. To associate cell density-dependent increase in integrin ανβ6 expression and MMP-9 secretion with invasive growth in vivo, we performed tumorigenesis assays. At least in theory, some requirements, such as the need for angiogenesis, degradation of ECM, and local invasive growth, can be recapitulated in this system. Our experimental data demonstrated that tumor invasive growth was enhanced for the wild-type WiDr cells that expressed normal integrin ανβ6 compared with the antisense β6 WiDr cells. More importantly, the tumors formed from wild-type WiDr cells infiltrated deep into the underlying muscle, whereas the tumors from WiDr antisense β6 cells grew adjacent to, but did not invade, the underlying muscle; the increased expression of integrin αvβ6 and MMP-9 was only identified in the tumors resulting from wild-type WiDr cells. Furthermore, according to the five-year follow-up data for 100 cases of patients with colon cancer, the log-rank test indicated that there was no significant survival difference between the MMP-9 positive patients and the MMP-9 negative patients (P = 0.48). There was, however, a significant difference between the integrin αvβ6 negative patients and the integrin αvβ6 positive patients (P = 0.048). Biologically, we observed that the elevated expression of integrin αvβ6 in colon cancer was associated with a poor prognosis. This finding provided strong evidence for integrin ανβ6 as a potential independent factor of invasive growth.
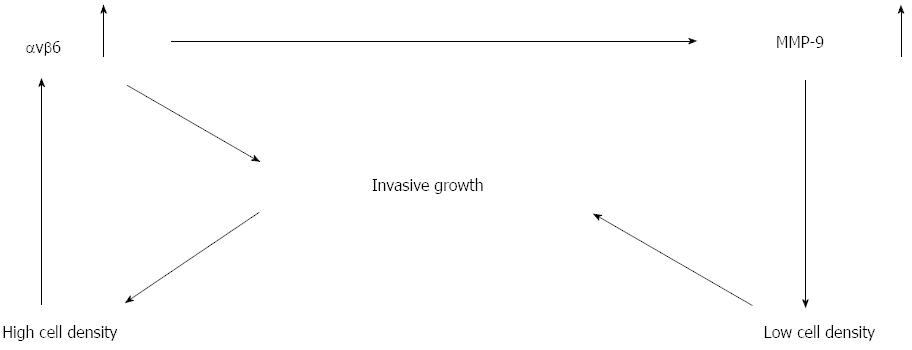
We suggest that the expression of integrin αvβ6 in colon cancer cells as they become crowded and dense during progression may be critical to the invasive growth that is characteristic of this type of tumor. We propose a self-perpetuating model that explains tumor cell invasive growth as shown in Figure 6. The stimulatory effect of cell density on integrin ανβ6 expression is enhanced in colon cancer cells that express integrin ανβ6. As a consequence of this integrin ανβ6-mediated MMP-9 secretion, peri-cellular matrix degradation is facilitated, which helps to overcome cell crowding. This effect reduces the matrix density and facilitates invasive growth because a high collagen density exerts an inhibitory effect on colon cancer invasive growth in vitro, and the cells are converted from low to high density again. The repeating cycle from low to high cell densities resembles a closed infinity symbol. Our study indicated that suppression of integrin ανβ6 expression inhibits both tumor invasive growth and high secretion of MMP-9 in tumor xenografts in nude mice.
Integrin ανβ6 is expressed in basal keratinocytes during wound healing and in culture-established keratinocytes[23,24]. Our study indicated that no increase in integrin ανβ6 expression or MMP-9 secretion was identified at a high cell density. Invasive growth contributes to the embrace of novel therapeutic strategies that target specific cancer cell characteristics.
We wish to thank Biogen Idec, Cambridge, MA, United States for invaluable contributions to this manuscript by way of antibodies and techniques.
To detect the mechanism for tumor invasive growth in colon cancer progression, an immunohistochemical study of integrin αvβ6 and matrix metalloproteinase-9 (MMP-9) was performed on tissue microarrays of 200 spots, including 100 cases of colon tumors.
The results showed that high immunoreactivity for integrin αvβ6 (73.7%; 28/38) and MMP-9 (76.5%; 52/68) was observed in invasive tumor portions. Furthermore, the effects of integrin αvβ6 on tumor invasive growth in nude mice were detected. Tumor invasive growth and high expression of both integrin αvβ6 and MMP-9 were only seen in tumors resulting from WiDr cells expressing integrin αvβ6 in the tumorigenicity assay. Flow cytometry was applied to analyze integrin αvβ6 expression in colon cancer cell lines WiDr and SW480. The effects of cell density on integrin αvβ6 expression and MMP-9 secretion were also detected by Biotrak MMP-9 activity assay and gelatin zymography assay. The study indicated that high cell density evidently enhanced integrin αvβ6 expression and promoted MMP-9 secretion compared with low density.
Integrin αvβ6 sustains and promotes tumor invasive growth in tumor progression via a self-perpetuating mechanism. Integrin ανβ6-mediated MMP-9 secretion facilitates pericellular matrix degradation at high cell density, which provides the basis of invasive growth.
This is an interesting manuscript. In this manuscript, the mechanisms by which integrin αvβ6 sustains and promotes tumor invasive growth in colon cancer progression are identified. The study design is good, and the results are interesting.
P- Reviewer: Meropol NJ, Wenzel SE S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Hecht JL, Dolinski BM, Gardner HA, Violette SM, Weinreb PH. Overexpression of the alphavbeta6 integrin in endometrial cancer. Appl Immunohistochem Mol Morphol. 2008;16:543-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Zhuang Z, Zhou R, Xu X, Tian T, Liu Y, Liu Y, Lian P, Wang J, Xu K. Clinical significance of integrin αvβ6 expression effects on gastric carcinoma invasiveness and progression via cancer-associated fibroblasts. Med Oncol. 2013;30:580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Mogi S, Dang D, Van Waes C, Ellis D, Atakilit A, Ramos DM. The expression of integrin alpha(v)beta6 promotes the epithelial cell morphology and suppresses invasive behavior in transformed oral keratinocytes. Anticancer Res. 2005;25:751-755. [PubMed] [Cited in This Article: ] |
| 4. | Moore KM, Thomas GJ, Duffy SW, Warwick J, Gabe R, Chou P, Ellis IO, Green AR, Haider S, Brouilette K. Therapeutic targeting of integrin αvβ6 in breast cancer. J Natl Cancer Inst. 2014;106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Niu J, Gu X, Turton J, Meldrum C, Howard EW, Agrez M. Integrin-mediated signalling of gelatinase B secretion in colon cancer cells. Biochem Biophys Res Commun. 1998;249:287-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Agrez M, Gu X, Turton J, Meldrum C, Niu J, Antalis T, Howard EW. The alpha v beta 6 integrin induces gelatinase B secretion in colon cancer cells. Int J Cancer. 1999;81:90-97. [PubMed] [Cited in This Article: ] |
| 7. | Lee C, Lee C, Lee S, Siu A, Ramos DM. The cytoplasmic extension of the integrin β6 subunit regulates epithelial-to-mesenchymal transition. Anticancer Res. 2014;34:659-664. [PubMed] [Cited in This Article: ] |
| 8. | Ramos DM, Dang D, Sadler S. The role of the integrin alpha v beta6 in regulating the epithelial to mesenchymal transition in oral cancer. Anticancer Res. 2009;29:125-130. [PubMed] [Cited in This Article: ] |
| 9. | Katoh D, Nagaharu K, Shimojo N, Hanamura N, Yamashita M, Kozuka Y, Imanaka-Yoshida K, Yoshida T. Binding of αvβ1 and αvβ6 integrins to tenascin-C induces epithelial-mesenchymal transition-like change of breast cancer cells. Oncogenesis. 2013;2:e65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Hasebe Y, Egawa K, Shibanuma M, Nose K. Induction of matrix metalloproteinase gene expression in an endothelial cell line by direct interaction with malignant cells. Cancer Sci. 2007;98:58-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Agrez M, Chen A, Cone RI, Pytela R, Sheppard D. The alpha v beta 6 integrin promotes proliferation of colon carcinoma cells through a unique region of the beta 6 cytoplasmic domain. J Cell Biol. 1994;127:547-556. [PubMed] [Cited in This Article: ] |
| 12. | Yang GY, Xu KS, Pan ZQ, Zhang ZY, Mi YT, Wang JS, Chen R, Niu J. Integrin alpha v beta 6 mediates the potential for colon cancer cells to colonize in and metastasize to the liver. Cancer Sci. 2008;99:879-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004;240:832-839. [PubMed] [Cited in This Article: ] |
| 14. | Park KJ, Choi HJ, Roh MS, Kwon HC, Kim C. Intensity of tumor budding and its prognostic implications in invasive colon carcinoma. Dis Colon Rectum. 2005;48:1597-1602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Christofori G. New signals from the invasive front. Nature. 2006;441:444-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 734] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 16. | Liang X. EMT: new signals from the invasive front. Oral Oncol. 2011;47:686-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Wang J, Wu J, Hong J, Chen R, Xu K, Niu W, Peng C, Liu E, Wang J, Liu S. PKC promotes the migration of colon cancer cells by regulating the internalization and recycling of integrin αvβ6. Cancer Lett. 2011;311:38-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Eberlein C, Kendrew J, McDaid K, Alfred A, Kang JS, Jacobs VN, Ross SJ, Rooney C, Smith NR, Rinkenberger J. A human monoclonal antibody 264RAD targeting αvβ6 integrin reduces tumour growth and metastasis, and modulates key biomarkers in vivo. Oncogene. 2013;32:4406-4416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Hezel AF, Deshpande V, Zimmerman SM, Contino G, Alagesan B, O’Dell MR, Rivera LB, Harper J, Lonning S, Brekken RA. TGF-β and αvβ6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res. 2012;72:4840-4845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Peng C, Liu X, Liu E, Xu K, Niu W, Chen R, Wang J, Zhang Z, Lin P, Wang J. Norcantharidin induces HT-29 colon cancer cell apoptosis through the alphavbeta6-extracellular signal-related kinase signaling pathway. Cancer Sci. 2009;100:2302-2308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Weiss M, Elsner M, Kartberg F, Nilsson T. Anomalous subdiffusion is a measure for cytoplasmic crowding in living cells. Biophys J. 2004;87:3518-3524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 582] [Cited by in F6Publishing: 449] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 22. | Rimm DL, Camp RL, Charette LA, Olsen DA, Provost E. Amplification of tissue by construction of tissue microarrays. Exp Mol Pathol. 2001;70:255-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Xie Y, Gao K, Häkkinen L, Larjava HS. Mice lacking beta6 integrin in skin show accelerated wound repair in dexamethasone impaired wound healing model. Wound Repair Regen. 2009;17:326-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Ponugoti B, Xu F, Zhang C, Tian C, Pacios S, Graves DT. FOXO1 promotes wound healing through the up-regulation of TGF-β1 and prevention of oxidative stress. J Cell Biol. 2013;203:327-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |