Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6543
Peer-review started: November 30, 2014
First decision: January 8, 2015
Revised: February 4, 2015
Accepted: March 31, 2015
Article in press: March 31, 2015
Published online: June 7, 2015
AIM: To investigate the effect of repeated lower +Gz exposure on liver injury induced by high +Gz exposure in rats.
METHODS: Sixty male Wister rats were randomly divided into a blank control group, a low G preconditioning group (LG) (exposed to +4 Gz/5 min per day for 3 d before +10 Gz/5 min exposure), and a +10 Gz/5 min group (10G) (n = 20 in each group). Blood specimens and liver tissue were harvested at 0 h and 6 h after +10 Gz/5 min exposure. Liver function was analyzed by measuring serum alanine transaminase (ALT) and aspartate aminotransferase (AST) levels, and liver injury was further assessed by histopathological observation. Malondialdehyde (MDA), superoxide dismutase (SOD) and Na+-K+-ATPase were determined in hepatic tissue.
RESULTS: The group LG had lower ALT, AST, and MDA values at 0 h after exposure than those in group 10G. SOD values and Na+-K+-ATPase activity in the LG group were higher than in group 10G 0 h post-exposure. Hepatocyte injury was significantly less in group LG than in group 10G on histopathological evaluation.
CONCLUSION: It is suggested that repeated low +Gz exposure shows a protective effect on liver injury induced by high +Gz exposure in rats.
Core tip: We conducted this experimental study to explore an optimized strategy of reducing liver injury induced by high +Gz exposure, and to observe more specific indices of liver function, such as alanine transaminase, aspartate aminotransferase, malondialdehyde, superoxide dismutase, Na+-K+-ATPase and hepatic pathology. We found that low G preconditioning reduced oxidative stress and significantly improved Na+-K+-ATPase activity, inducing minimal liver injury.
- Citation: Shi B, Feng ZQ, Li WB, Zhang HY. Low G preconditioning reduces liver injury induced by high +Gz exposure in rats. World J Gastroenterol 2015; 21(21): 6543-6549
- URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6543.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6543
Ischemic preconditioning (IPC) refers to a phenomenon in which the tissue can not only increase resistance to further ischemic injury but also reduce the degree of organ dysfunction or subsequent damage following ischemia reperfusion[1,2]. To some extent, ischemia of a brief period initiates an endogenous protection for the following sustained ischemic period[3]. At first, the protective effect of IPC was verified in the heart[4]. Although the potential mechanism is not fully understood, the protective effect of IPC in delaying cell injury of skeletal muscle[5], brain[6], kidney[7], liver[8] and small intestine[9] is generally accepted. More and more studies have demonstrated that many different stimuli can induce preconditioned status of the liver. Ren et al[10] reported that liver IPC played a beneficial role in hepatic graft function and intestinal barrier function, contributing to stabilization of intestinal microbiota in liver transplantation. Currin et al[11] found that IPC markedly reduced hepatocellular injury after aortic clamping and ameliorated the survival rate. Lee et al[12] showed that hepatic IPC directly reduced distant renal ischemia and reperfusion injury in animal experiments. Figueira et al[13] demonstrated that hepatic IPC could not only recover portal vein flow, but also relieve hepatocellular injury.
During a flight, pilots may experience high sustained +Gz acceleration that results in gravity load and hemodynamic changes. Repeated +Gz exposure can cause accumulative stress damage in the body[14], inducing organ dysfunction and triggering pathologic changes. For a long time, many researchers were interested in the problem and tried to figure out some useful safeguard measures[15]. Cao et al[16] found that repeated low +Gz preconditioning could obviously ameliorate memory and balance changes induced by high +Gz exposure in rats. It was also found that lower gravity preconditioning was able to dramatically improve rat learning and memory impairment induced by high gravity exposure[17]. Li et al[18]’s study showed that brain injury induced by high +Gz exposure could be remarkably alleviated by low +Gz exposures in rats. It was also found that the left ventricular contractility and secretions of vascular endotheliocytes in the heart of rats following high +Gz exposure were significantly improved by low G preconditioning[19]. Moreover, it was discovered that low G preconditioning was protective for several enzyme activities in myocardial tissue after high +Gz stress in rats[20].
The liver is the largest internal organ, and an important metabolic organ[21,22]. Without timely and effective preventive measures, the natural protective mechanism of the liver may be overpowered by continuous and repeated exposures to +Gz acceleration. In experimental studies, repeated +Gz exposure can transiently cause liver dysfunction and trigger pathologic changes. We are interested in whether low G preconditioning has a similar protective effect on liver injury and dysfunction induced by high +Gz stress. The aim of this study is to investigate the possible protective effect of repeated low +Gz exposure on liver injury induced by high +Gz exposure in rats.
Sixty male Wister rats (provided by the Experimental Animal Center of the Academy of Military Medical Science, Beijing, China), weighing between 250 and 300 g, were randomly divided into three groups:blank control group (group BC, n = 20), low G preconditioning group (group LG, n = 20) (exposed to +4 Gz/5 min per day for 3 d before +10 Gz/5 min exposure) and +10 Gz/5 min group (group 10G, n = 20). All rats were housed under standard experimental conditions: 12:12-h light-dark cycle, humidity 70%-80%, and room temperature 23-26 °C. Standard laboratory chow and water were provided and rats were allowed to acclimatize for 7 d. The rats were fasting but had access to water for 12 h before the experiment to reduce experimental errors. All experiments were conducted between 8:00 am and 12:00 am. The experimental schemes were approved by the Animal Care and Use Committee of China PLA Air Force General Hospital and carried out according to the Guide for the Care and Use of Laboratory Animals.
The animal centrifuge had an arm length of 2 m and was provided by the Air Force Aeromedicine Institute (Beijing, China), with an onset rate of 0.1-6 Gz/s, and acceleration range of 1-15 G. Each rat was placed inside a 15 cm × 5 cm × 3 cm cylindrical plastic restraint device which was mounted in the centrifuge arm with the head of the rat facing the axis of the centrifuge for +Gz orientation. In the +10 Gz/5min group, the rats were exposed to +10 Gz lasting for 5 min as reported elsewhere[23]. For the low G preconditioning group, the rats were exposed to +4 Gz/5 min every day for 3 d before +10 Gz/5 min exposure. The onset/offset rate of +Gz was set at +1 G/s. The rats in the blank control group were mounted on the arms of centrifuge, but were free from acceleration. After exposure to acceleration for 0 h and 6 h, general anesthesia, routine disinfection, and laparotomy were performed for specimen collection. A blood sample of about 2 mL was drawn from the inferior vena cava to measure serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Liver tissue was quickly removed and weighed. One part of the tissue was fixed in 4% formaldehyde for the histopathological examination, and the other part was immediately frozen in liquid nitrogen and stored under -80 °C for determination of malondialdehyde (MDA), superoxide dismutase (SOD) and Na+-K+-ATPase.
Blood samples were obtained and separated by centrifugation (1500 rpm, 20 min). Activity changes of serum ALT and AST were measured using a serum analyzer (Cobas-Mira Plus; Roche Manheim, Germany).
MDA level and SOD activity were measured spectrophotometrically using the corresponding kits (Nanjing Jiancheng Biotechnology Institute, Nanjing, China), respectively. Liver specimens were homogenized and treated in accordance with the manufacturer’s recommendations. The results were expressed as nmol/mg protein for MDA, and U/mg protein for SOD.
Na+-K+-ATPase activity in liver specimens was measured using an ATPase Assay Kit (Nanjing Jiancheng Biotechnology Institute, Nanjing, China) according to the manufacture’s protocol. Na+-K+-ATPase activity was measured based upon the principle of inorganic phosphate measurement that was decomposed by adenosine triphosphate[24]. Changes of enzymatic activity indirectly indicated membrane damage or not. Na+-K+-ATPase activity was expressed as μmolPi/mg protein/h.
After being fixed in 4% formaldehyde solution, the tissue samples were embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin-eosin (HE). The histological changes after repeated +Gz exposure were graded using Suzuki’s criteria[25]. There were three features to assess the morphometric parameters: sinusoidal congestion, hepatocyte necrosis, and ballooning degeneration, graded from 0 to 4. A specific grading method was introduced: 0, none; 1, minimal congestion and ballooning degeneration as well as single cell necrosis; 2, minor congestion and ballooning degeneration as well as < 30% lobular necrosis; 3, moderate congestion and ballooning degeneration as well as 30%-60% lobular necrosis; 4, severe congestion and ballooning degeneration as well as > 60% lobular necrosis. The pathological changes were observed under microscope by an experienced blinded histologist.
The data was expressed as the mean ± SD. The unpaired t-test was used to compare low G preconditioning group and +10 Gz/5 min group. SPSS 13.0 software (SPSS, Chicago, IL, United States) was involved for data analysis, and P < 0.05 indicated significant difference.
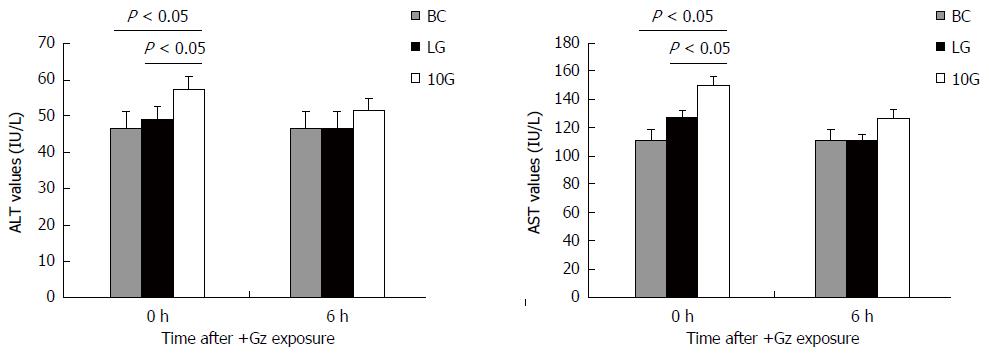
Plasma ALT and AST levels were measured to assess liver damage at 0 and 6 h after exposure. ALT and AST values in the BC group were 46.7 ± 4.6 IU/L and 110.6 ± 7.4 IU/L, respectively. In group LG or group 10G, ALT and AST levels were higher than those in BC group (P < 0.05) at 0 h after exposure, respectively. However, the rats in LG group showed lower ALT and AST levels than those in 10G group at 0 h after exposure (P < 0.05). Group LG displayed normal ALT and AST levels at 6 h after exposure (Figure 1). These results demonstrate that low G preconditioning had a protective effect on liver function in rats after high G stress.
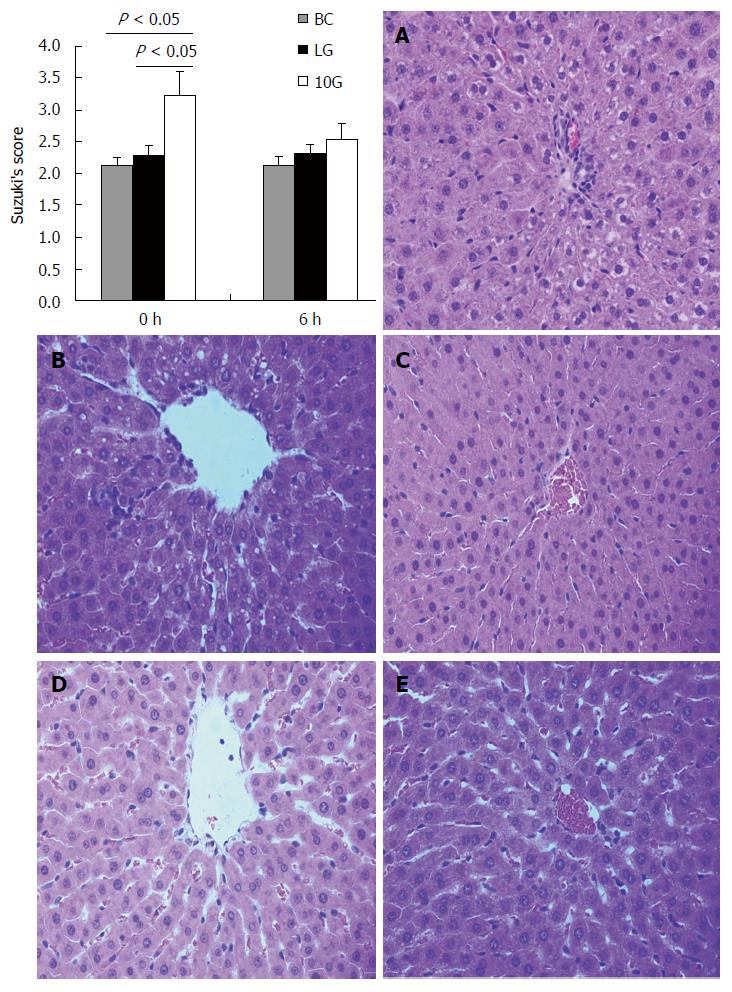
To analyze the extent of hepatocyte injury induced by high +Gz acceleration, liver sections were stained with HE at 0 and 6 h after exposure (Figure 2). No significant injury was found in the blank control group (Figure 2A; Suzuki’s score = 2.12 ± 0.13). At 0 h after exposure, there was a disorderly hepatic sinus cord-like structure associated with hepatocyte edema in the 10GS group (Figure 2B; Suzuki’s score = 3.23 ± 0.37). In sharp contrast, there was regular liver lobule structure in the LG group (Figure 2C; Suzuki’s score = 2.28 ± 0.16). At 6 h after exposure, hepatocyte edema became lighter, and liver lobule structure was arranged in a more orderly manner in the 10GS group (Figure 2E; Suzuki’s score = 2.53 ± 0.25; P < 0.01). There was no significant difference between 0 and 6 h after exposure in the LG group (Figure 2B and D; Suzuki’s score = 2.28 ± 0.16 vs 2.31 ± 0.14; P < 0.01).
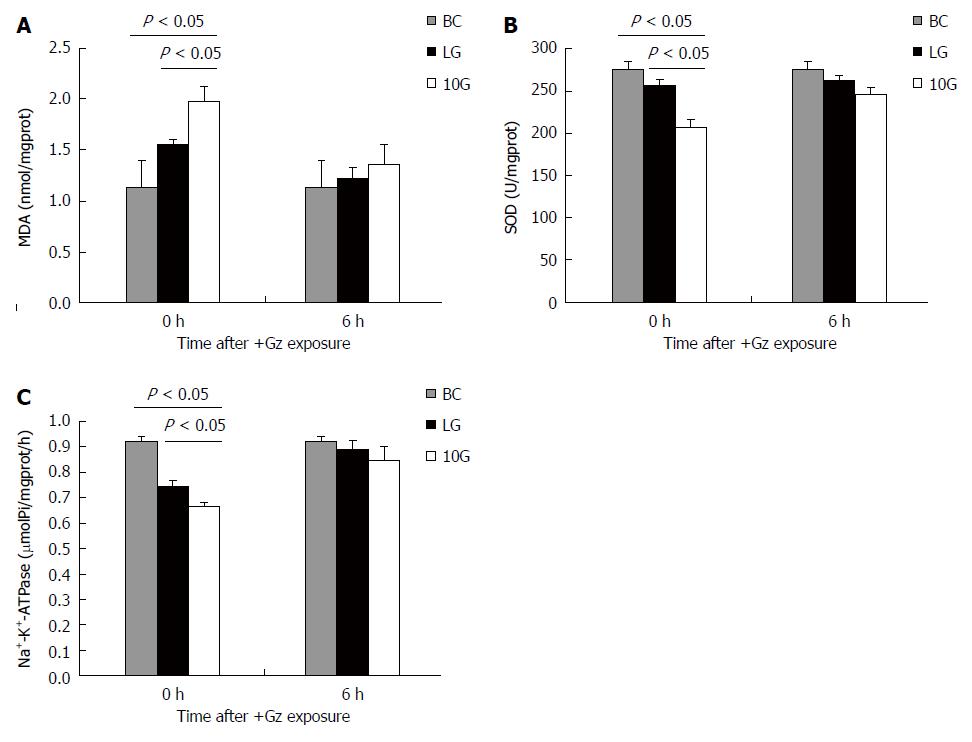
To assess oxidative stress effect on hepatocytes induced by +Gz exposure, MDA and SOD were measured at 0 and 6 h after exposure. The MDA level in the BC group was 1.14 ± 0.25 nmol/mgprot. At 0 h after exposure, levels of MDA activity in the LG and 10G groups were 1.56 ± 0.05, 1.99 ± 0.14 nmol/mgprot, respectively, which were higher than that of the blank control group (P < 0.05). However, MDA level in liver tissue in the LG group was lower than that in the 10G group at 0 h after exposure. There was no significant difference between MDA level in liver tissue of LG and 10G groups at 6 h after exposure (P > 0.05, Figure 3A). Compared with the blank control group, liver tissue SOD level in the LG or 10G groups reduced significantly at 0 h after exposure (255.5 ± 8.15 U/mgprot vs 207.28 ± 8.36 U/mgprot, P < 0.05). Compared to the 10G group, SOD level was higher in the LG group at 0 h after exposure (P < 0.05). There was no significant difference between LG and 10G groups at 6 h after exposure (P > 0.05; Figure 3B). Therefore, low G preconditioning could reduce oxidative stress injury induced by high +Gz exposure in rats.
The Na+-K+-ATPase activity in the liver tissue of the LG and 10G groups was decreased compared to that of the blank control group. The Na+-K+-ATPase activity in the LG group was higher than that in the 10G group at 0 h after exposure (0.742 ± 0.023 μmolPi/mgprot vs 0.666 ± 0.016 μmolPi/mgprot, P < 0.05). The difference between group LG and group 10G at 6 h after exposure was not significant (P > 0.05; Figure 3C).
With the progress of aviation science and technology, maneuverability of the fighter plane has been improved to a large extent. Modern aircrafts are capable of generating positive acceleration of +9 Gz range and +6 Gz/s rapid onset rate which is sustained for 15-45 s, which may exceed the human body’s physical capabilities[26,27]. More attention should be paid to pilots’ health and flight safety.
In clinical practice, IPC is a surgical tactic to increase tissue tolerance and reduce ischemia-reperfusion injury (I/R)[4,28]. Several studies support the safety and efficacy of IPC against liver IR[29,30]. During flight, direct action and stress response caused by repeated +Gz exposure may cause liver I/R injury. Several anti-G measures have been adopted to reduce organism damage and build G tolerance, such as anti-G suit[31], anti-G straining maneuvers and positive pressure breathing[32], tilt-back seat[33], and comprehensive protection measures[34]. It is worth mentioning that centrifuge training is an important way of improving the strength and stamina of pilots[35]. Low G training might be a better way to help build endurance. In the field of gravitation physiology, other studies have shown that low G training could increase reserves of circulatory system and elevate the G tolerance of pilots[36]. In the preliminary experiments, it has been demonstrated that long-term exposure to the +4 G environment shows no harmful effects on liver morphology, which is the basis of selecting +4 G as the preconditioning condition in this study (data is not shown). The liver is a vital organ in the body. Liver injury after high +Gz exposure may be life threatening. Hence, we carried out the study to ascertain whether low G training could reduce liver injury induced by high +Gz exposure.
Both ALT and AST are markers of hepatic damage after +Gz exposure. Our results indicated that the rats in the LG group had lower serum ALT and AST level than those in the 10G group. Thereby, low Gz preconditioning was able to reduce liver injury induced by high +Gz exposure.
MDA is used widely as a sensitive marker of oxidative stress[37]. In our study, hepatic MDA in the LG group was lower compared to the 10G group. Furthermore, SOD activity was significantly decreased after +10 Gz/5 min exposure, indicating that liver tissue was vulnerable to oxidative damage. Our results showed that low G preconditioning may reduce the oxidative stress caused by high +Gz exposure and attenuate subsequent tissue damage.
Na+-K+-ATPase activity decreased significantly in liver tissue after +Gz exposures compared to the blank control group. Nevertheless, Na+-K+-ATPase activity in the LG group was significantly higher than that in the 10G group at 0 h after exposure, further supporting that low G preconditioning improved hepatic energy metabolism. Morphologically, our study of rat liver in the +Gz exposure model demonstrated that low +Gz preconditioning could reduce hepatocyte injury.
In summary, low G preconditioning is protective for liver injury induced by high +Gz exposure in rats, and the precise mechanism includes decrease of oxidative stress, preservation of hepatic energy metabolism and improvement of cellular morphology.
Many studies have shown that ischemic preconditioning can not only increase resistance to further ischemic injury but also reduce the degree of organ dysfunction or subsequent damage following ischemia reperfusion. In basic research, repeated +Gz exposure can transiently impair liver function and trigger pathologic changes. However, no experimental study has been carried out to investigate the possible protective effect of repeated low +Gz exposure on liver injury induced by high +Gz exposure in rats.
Flight safety and the protective effect of low G preconditioning are the key research frontiers.
The authors established animal models to study the possible protective effect of lower +Gz exposures. The experimental results showed that low G preconditioning could decrease liver enzymes and malondialdehyde levels and improve superoxide dismutase and Na+-K+-ATPase activity.
Low G preconditioning shows a protective effect on liver injury induced by high +Gz exposure in rats.
A low G preconditioning group was exposed to +4 Gz/5 min per day for 3 d before +10 Gz/5 min exposure.
This study result may provide an important method to combat liver injury induced by high +Gz exposure.
P- Reviewer: Czubkowski P, Gobejishvili L, Sis B S- Editor: Yu J L- Editor: Logan S E- Editor: Ma S
| 1. | Ishida T, Yarimizu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock. 1997;8:86-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Kharbanda RK, Peters M, Walton B, Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J, MacAllister R. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation. 2001;103:1624-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 235] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | de Groot PC, Thijssen DH, Sanchez M, Ellenkamp R, Hopman MT. Ischemic preconditioning improves maximal performance in humans. Eur J Appl Physiol. 2010;108:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5406] [Cited by in F6Publishing: 5434] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 5. | Gürke L, Marx A, Sutter PM, Frentzel A, Salm T, Harder F, Seelig J, Heberer M. Ischemic preconditioning improves post-ischemic skeletal muscle function. Am Surg. 1996;62:391-394. [PubMed] [Cited in This Article: ] |
| 6. | Sang H, Li J, Liu J, Wang Z, Huo T, Sun J, Xiong L. Preconditioning with +Gz acceleration (head-to-foot inertial load) produces neuroprotection against transient focal cerebral ischemia in rats. Neurosci Lett. 2008;445:78-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Cochrane J, Williams BT, Banerjee A, Harken AH, Burke TJ, Cairns CB, Shapiro JI. Ischemic preconditioning attenuates functional, metabolic, and morphologic injury from ischemic acute renal failure in the rat. Ren Fail. 1999;21:135-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 9. | McCallion K, Wattanasirichaigoon S, Gardiner KR, Fink MP. Ischemic preconditioning ameliorates ischemia- and reperfusion-induced intestinal epithelial hyperpermeability in rats. Shock. 2000;14:429-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Ren Z, Cui G, Lu H, Chen X, Jiang J, Liu H, He Y, Ding S, Hu Z, Wang W. Liver ischemic preconditioning (IPC) improves intestinal microbiota following liver transplantation in rats through 16s rDNA-based analysis of microbial structure shift. PLoS One. 2013;8:e75950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Currin RT, Peng XX, Lemasters JJ. Ischemic preconditioning of rat livers from non-heart-beating donors decreases parenchymal cell killing and increases graft survival after transplantation. HPB Surg. 2012;2012:236406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Lee JA, Choi JW, In JH, Jung HS, Kim YS, Jeon YS, Kang YJ, Kim DW, Lim YG, Park JH. Hepatic ischemic preconditioning provides protection against distant renal ischemia and reperfusion injury in mice. J Korean Med Sci. 2012;27:547-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Figueira ER, Rocha-Filho JA, Nakatani M, Buto MF, Tatebe ER, Andre VO, Cecconello I, D’Albuquerque LA. Hepatic ischemic preconditioning increases portal vein flow in experimental liver ischemia reperfusion injury. Hepatobiliary Pancreat Dis Int. 2014;13:40-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Martin DS, D’Aunno DS, Wood ML, South DA. Repetitive high G exposure is associated with increased occurrence of cardiac valvular regurgitation. Aviat Space Environ Med. 1999;70:1197-1200. [PubMed] [Cited in This Article: ] |
| 15. | Siitonen SL, Kauppinen T, Leino TK, Vanninen E, Kuronen P, Länsimies E. Cerebral blood flow during acceleration in flight measured with SPECT. Aviat Space Environ Med. 2003;74:201-206. [PubMed] [Cited in This Article: ] |
| 16. | Cao XS, Sun XQ, Wei YB, Yao YJ, Feng DY, Yang CB. [Effects of repeated lower g exposures on high-G induced memory and balance changes in rats]. Space Med Med Eng (Beijing). 2004;17:16-19. [PubMed] [Cited in This Article: ] |
| 17. | Cao XS, Wu XY, Sun XQ, Liu TS. [Effects of low gravity preconditioning on rat learning and memory impairment induced by high gravity exposure]. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:212-215. [PubMed] [Cited in This Article: ] |
| 18. | Li JS, Sun XQ, Wu XY, Rao ZR, Liu HL, Xie XP. [Influences of repeated lower +Gz exposures on high +Gz exposure induced brain injury in rats]. Space Med Med Eng (Beijing). 2002;15:339-342. [PubMed] [Cited in This Article: ] |
| 19. | Fu FY, Zhan H, Zhang Z, Li T, Xin YM, Wei SH. [Effects of low-G preconditioning on contractive ability of left ventricle and contents of endothelin and prostacycline in myocardium of rats after repeated +10 Gz stress]. Space Med Med Eng (Beijing). 2003;16:414-417. [PubMed] [Cited in This Article: ] |
| 20. | Zhang Z, Zhan H, Lu JY, Xin YM, Li T, Zhang QJ. [Effects of repeated high +Gz exposure on several enzyme activities in cardiomyocytes in rats and some protective measures]. Space Med Med Eng (Beijing). 2001;14:410-413. [PubMed] [Cited in This Article: ] |
| 21. | Starr S, Hand H. Nursing care of chronic and acute liver failure. Nurs Stand. 2002;16:47-54; quiz 55-56. [PubMed] [Cited in This Article: ] |
| 22. | Zakaria ZA, Rofiee MS, Somchit MN, Zuraini A, Sulaiman MR, Teh LK, Salleh MZ, Long K. Hepatoprotective activity of dried- and fermented-processed virgin coconut oil. Evid Based Complement Alternat Med. 2011;2011:142739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Feng S, Wang Q, Wang H, Peng Y, Wang L, Lu Y, Shi T, Xiong L. Electroacupuncture pretreatment ameliorates hypergravity-induced impairment of learning and memory and apoptosis of hippocampal neurons in rats. Neurosci Lett. 2010;478:150-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Reading HW, Isbir T. The role of cation-activated ATPases in transmitter release from the rat iris. Q J Exp Physiol Cogn Med Sci. 1980;65:105-116. [PubMed] [Cited in This Article: ] |
| 25. | Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 653] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 26. | Jones DR. A review of central nervous system effects of G-induced loss of consciousness on volunteer subjects. Aviat Space Environ Med. 1991;62:624-627. [PubMed] [Cited in This Article: ] |
| 27. | Öztürk C, İlbasmış MS, Akın A. Cardiac responses to long duration and high magnitude +Gz exposure in pilots: an observational study. Anadolu Kardiyol Derg. 2012;12:668-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Yoshizumi T, Yanaga K, Soejima Y, Maeda T, Uchiyama H, Sugimachi K. Amelioration of liver injury by ischaemic preconditioning. Br J Surg. 1998;85:1636-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Sindram D, Rüdiger HA, Upadhya AG, Strasberg SM, Clavien PA. Ischemic preconditioning protects against cold ischemic injury through an oxidative stress dependent mechanism. J Hepatol. 2002;36:78-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Stevenson AT, Lythgoe DT, Darby CL, Devlin JM, Connolly DM, Scott JP. Garment fit and protection from sustained +Gz acceleration with ‘full-coverage’ anti-G trousers. Aviat Space Environ Med. 2013;84:600-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Eiken O, Kölegärd R, Bergsten E, Grönkvist M. G protection: interaction of straining maneuvers and positive pressure breathing. Aviat Space Environ Med. 2007;78:392-398. [PubMed] [Cited in This Article: ] |
| 33. | Burns JW. Prevention of loss of consciousness with positive pressure breathing and supinating seat. Aviat Space Environ Med. 1988;59:20-22. [PubMed] [Cited in This Article: ] |
| 34. | Cohen MM. Combining techniques to enhance protection against high sustained accelerative forces. Aviat Space Environ Med. 1983;54:338-342. [PubMed] [Cited in This Article: ] |
| 35. | Jing X, Wu P, Liu F, Wu B, Miao D. Guided imagery, anxiety, heart rate, and heart rate variability during centrifuge training. Aviat Space Environ Med. 2011;82:92-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Wojtkowiak M, Markiewicz L, Kempa G. Effect of low acceleration simulator training on +Gz acceleration tolerance level. J Gravit Physiol. 1998;5:P31-P32. [PubMed] [Cited in This Article: ] |
| 37. | Takayama F, Egashira T, Kudo Y, Yamanaka Y. Chemiluminescence-HPLC assay of phosphatidylcholine hydroperoxide generated by ischemia-reperfusion in the liver of rats. Biochem Pharmacol. 1992;44:2412-2414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |