Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5259
Peer-review started: October 28, 2014
First decision: December 26, 2014
Revised: January 18, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 7, 2015
AIM: To evaluate the feasibility of low contrast medium and radiation dose for hepatic computed tomography (CT) perfusion of rabbit VX2 tumor.
METHODS: Eleven rabbits with hepatic VX2 tumor underwent perfusion CT scanning with a 24-h interval between a conventional tube potential (120 kVp) protocol with 350 mgI/mL contrast medium and filtered back projection, and a low tube potential (80 kVp) protocol with 270 mgI/mL contrast medium with iterative reconstruction. Correlation and agreement among perfusion parameters acquired by the conventional and low dose protocols were assessed for the viable tumor component as well as whole tumor. Image noise and tumor-to-liver contrast to noise ratio during arterial and portal venous phases were evaluated.
RESULTS: A 38% reduction in contrast medium dose (360.1 ± 13.3 mgI/kg vs 583.5 ± 21.5 mgI/kg, P < 0.001) and a 73% decrease in radiation dose (1898.5 mGy • cm vs 6951.8 mGy • cm) were observed. Interestingly, there was a strong positive correlation in hepatic arterial perfusion (r = 0.907, P < 0.001; r = 0.879, P < 0.001), hepatic portal perfusion (r = 0.819, P = 0.002; r = 0.831, P = 0.002), and hepatic blood flow (r = 0.945, P < 0.001; r = 0.930, P < 0.001) as well as a moderate correlation in hepatic perfusion index (r = 0.736, P = 0.01; r = 0.636, P = 0.035) between the low dose protocol with iterative reconstruction and the conventional protocol for the viable tumor component and the whole tumor. These two imaging protocols provided a moderate but acceptable agreement for perfusion parameters and similar tumor-to-liver CNR during arterial and portal venous phases (5.63 ± 2.38 vs 6.16 ± 2.60, P = 0.814; 4.60 ± 1.27 vs 5.11 ± 1.74, P = 0.587).
CONCLUSION: Compared with the conventional protocol, low contrast medium and radiation dose with iterative reconstruction has no significant influence on hepatic perfusion parameters for rabbits VX2 tumor.
Core tip: We performed hepatic computed tomography (CT) perfusion of liver VX2 tumor in rabbits by using both conventional tube potential (120 kVp) protocol with 350 mgI/mL concentration contrast medium and low tube potential (80kVp) protocol with 270 mgI/mL concentration contrast medium at a 24-h interval. This study demonstrated that there were a 38% reduction in contrast medium consumption and a 73% reduction in radiation dose for low dose protocol without significant influence on the hepatic perfusion parameters. It is promising that hepatic CT perfusion with low contrast medium dose and tube potential protocol may be clinically used to decrease radiation dose and the risk of contrast-induced nephropathy.
- Citation: Zhang CY, Cui YF, Guo C, Cai J, Weng YF, Wang LJ, Wang DB. Low contrast medium and radiation dose for hepatic computed tomography perfusion of rabbit VX2 tumor. World J Gastroenterol 2015; 21(17): 5259-5270
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5259.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5259
Angiogenesis is essential for tumor growth and metastasis, and has significant implications in the diagnosis and treatment of solid tumors. Functional computed tomography (CT) perfusion of the liver was first introduced in 1991, and has been reported to provide quantitative assessment of tumor angiogenesis with high spatial resolution[1]. This multi-detector row technique has the potential to identify the dynamic features of contrast agents passing through the tumor neovessels with high temporal resolution[2]. However, application of functional CT perfusion is limited in routine clinical practice due to concerns regarding the relatively high radiation dose and contrast-induced nephropathy[3,4].
To date, many techniques have been used for radiation dose reduction, mainly including automatic exposure control (AEC), tube current adjustment, and tube potential optimization. Although AEC was shown to achieve acceptable image quality with effectively reduced radiation dose, it may be less effective in small patients[5]. Low tube potential is closer to the iodine K-edge of 33 keV, which increases the contrast of iodine. This technique enhances the iodinated contrast medium and therefore reduces the administered contrast medium dose. However, low tube potential may increase image noise during abdominal CT scanning[6].
A new method for noise reduction has been recently developed for clinical use. With this new algorithm called iterative reconstruction, image data are corrected with a system of models to improve image noise. Multiple studies have assessed the iterative reconstruction with the aim to reduce the radiation dose and improve image quality[7,8]: this technique achieved diagnostic image quality with a CT dose index reduction of 32%-65%. A previous report found that combination of low tube potential and 40% hybrid iterative algorithm yielded a dramatic decrease in both radiation dose and contrast medium administration for hepatic CT scanning without compromising the image quality[9]. To our knowledge, no study has reported the use of low tube potential in combination with iterative reconstruction and low concentration contrast medium for hepatic CT perfusion. We hypothesized that CT perfusion could be performed to assess the angiogenesis of hepatic VX2 tumor in rabbits by using a combination of 80 kVp tube potential with 270 mgI/mL contrast medium and iterative reconstruction.
Therefore, this study aimed to evaluate the feasibility of low radiation dose and low concentration contrast medium in quantitative assessment of liver VX2 tumor in rabbits with implementation of iterative reconstruction on a 256-slice CT.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Approval No, XHEC-F-2013-016). All surgery was performed under ketamine hydrochloride and xylazine anesthesia, and animal suffering was minimized.
Twelve adult New Zealand white rabbits (Xinhua Hospital Laboratory Animal Center) weighing between 2.88 and 3.15 kg were implanted with a hepatic VX2 tumor using methodology similar to Kim et al[10]. Three weeks after implantation, the tumor size was expected to be approximately 3 cm. Non-contrast enhanced CT scanning was performed for the 12 experimental rabbits to evaluate the tumor size. No tumor was observed in the liver of one experimental rabbit. The tumor sizes for the remaining eleven rabbits were 3.3 ± 0.43 cm, ranging from 2.8 to 3.8 cm. One daughter nodule (0.51 cm in size) adjacent to the main tumor was detected in the liver of one rabbit. The eleven main tumors were evaluated in the perfusion CT study.
Before perfusion CT scan, each rabbit was anesthetized by intramuscular injection of 25 mg/kg body weight ketamine hydrochloride (Ketamine, Fujian, China) and 0.1 mL/kg 2% xylazine (Lu mian ning, Jilin, China). A 24-gauge cannula was inserted into the auricular vein. The rabbit was fixed on a board in supine position and properly covered with a bandage on the belly to minimize the respiratory motion.
Eleven rabbits were examined with a 256-slice CT scanner (Brilliance iCT; Philips Healthcare, Cleveland, Ohio). Repeated perfusion CT scanning of the upper abdomen was performed by using two different protocols: (1) conventional tube potential (120 kVp, 100 mAs) and concentration contrast medium (5 mL omnipaque at 350 mgI/mL); and (2) low tube potential (80 kVp, 80 mAs) and low concentration contrast medium (4 mL, visipaque at 270 mgI/mL). We used 80 kVp and 80 mAs setting as low radiation dose scanning protocol, based on Verdun et al[11] recommendation regarding low radiation dose CT scan protocol for pediatric patients with body weight from 2.5 to 5 kg. The dedicated perfusion scanning protocol with 8 cm wide coverage in one gantry rotation was used for whole liver perfusion CT. A 24-h interval was allowed between the use of low and conventional dose protocols for each rabbit. Iohexol (Omnipaque 350 mgI/mL, GE healthcare, Shanghai, China) and Iodixanol (visipaque, 270 mgI/mL, GE healthcare, Cork, Ireland) for conventional and low dose protocols, respectively, were administered through the auricular vein with a power injector (Mallinckrodt, Liebel-Flarseim Company, United States) at the rate of 1.5 mL/s. The perfusion CT scanning of the entire liver began at 2 s before bolus injection of contrast medium and continued for 90 s (gantry cycle time: 1.5 s, gantry cycle: 60 times). The scanning parameters were as follows: hepatic perfusion mode with field of view (FOV) 15 cm; slice thickness, 1.5 mm; no gap; matrix size, 512 × 512.
Conventional 120 kVp (protocol A) image data were reconstructed using the filtered back projection (FBP) algorithm. Images obtained from 80 kVp imaging were reconstructed by using the hybrid iterative reconstruction algorithm (protocol B) and FBP (protocol C). The level of hybrid iterative reconstruction was selected as 40% that theoretically provides 40% noise reduction for abdominal CT image data.
The perfusion images were transferred to the dedicated post-processing workstation (Extended Brilliance Workspace, V4.5.3, Philips Healthcare) for functional analysis using the maximum slope method. The region of interest (ROI) was placed on the abdominal aorta at the level of hepatic hilum, the main portal vein and the cortex of right kidney, to generate time-density curve (TDC). The rabbit’s relative small spleen cannot be easily identified; therefore, we selected the right renal cortex to differentiate the arterial and portal venous phases[12]. A radiologist (YFC) with 4 years of abdominal CT interpretation experience manually drew the ROI, outlining the contour of the whole tumor as well as the portion of viable tumor with the most marked enhancement. All measurements were performed three times. Three ROIs each with 50 mm2 area were drawn on the hepatic lobe without tumor, avoiding the large vessels. Each ROI on the 120 kVp images was matched with that on 80 kVp images as much as possible. Subsequently, hepatic blood flow (HBF), hepatic arterial perfusion (HAP), hepatic portal perfusion (HPP), and hepatic perfusion index (HPI) for the whole tumor, viable tumor and normal liver parenchyma were automatically calculated with protocols A, B and C.
Three ROIs each with 50 mm2 area were drawn on the hepatic parenchyma without tumor, avoiding large vessels at the level of maximum tumor diameter. The normal liver attenuation (ROIliver) was defined as the mean CT value of the three ROIs. The mean tumor attenuation (ROItumor) was assessed by manually placing circle or ovoid ROIs as much as possible to encompass the enhanced portion of the tumor in three consecutive scanning slices. The lesion-to-liver contrast-to-noise ratio (CNR) was calculated using the following equation: CNR = (ROItumor-ROIliver)/SDnoise, where ROIliver is the mean attenuation of normal hepatic parenchyma; ROItumor is the mean attenuation of viable tumor component; and SDnoise is measured as the standard deviation of the pixel values from a circular or ovoid ROI drawn in the subcutaneous fat of the abdominal wall at the level of maximum tumor diameter[13]. All measurements were performed in both the hepatic arterial and portal venous phases; namely, CNR in the arterial phase (CNRHAP) and portal venous phase (CNRPVP) were both obtained. The timings of arterial and portal venous phases were defined as 11 s and 25 s, respectively, after enhancement in the aorta reached 100 HU[10].
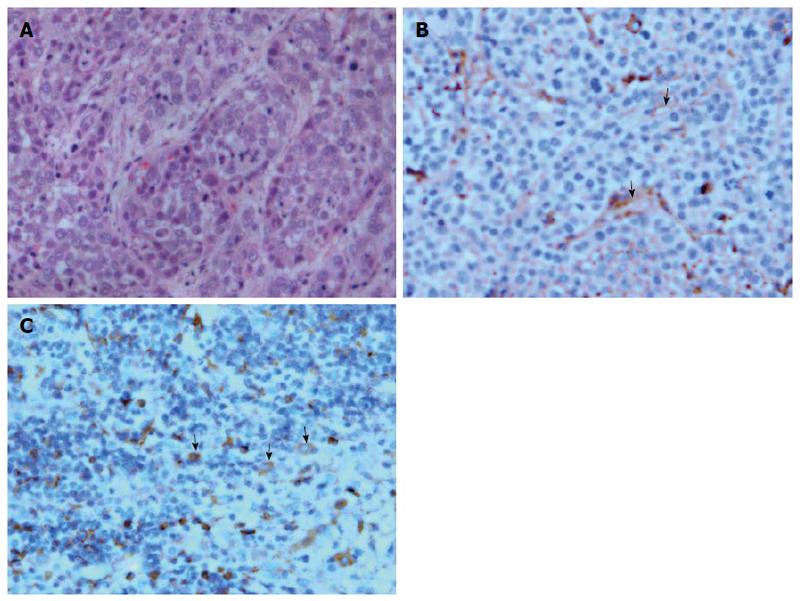
After perfusion CT with low and conventional dose protocols, rabbits were sacrificed by injection of xyazine overdose (Lu mian ning, Jilin, China). Then, liver tissues were sliced into 1 mm thick sections in the orientation matching the CT scanning. The specimens were fixed with formalin for 24 h, embedded in paraffin and stained with hematoxylin and eosin, anti-CD31 and anti-vascular endothelial growth factor (VEGF) antibodies. The histological characterization of tumor was assessed by a pathologist (XY W).
All numeric values are expressed as mean ± standard deviation (SD). To compare CT perfusion parameters in whole tumor, viable tumor, and normal liver parenchyma for protocols A, B and C, one-way analysis of variance (ANOVA) was used. P < 0.05 was considered statistically significant. If a statistically significant difference was obtained, the Dunnett test was performed with protocol A serving as a control. Correlations among perfusion parameters for the whole tumor, viable tumor, and normal liver parenchyma measured in protocols A and B were assessed by Pearson correlation analysis. A correlation coefficient (r) between 0.5 and 0.8 indicated a moderate positive relationship; r ranging from 0.81 to 1.0 indicated a strong positive relationship[14]. Bland-Altman plots were employed to evaluate the agreement of perfusion parameters between protocols A and B. Dunnett test was used to compare the noise, CNR, and CT values for ROI in the three sets of perfusion CT, with protocol A used as a control. All statistical analyses were performed with SPSS version 15.0 for Microsoft Windows (SPSS, Inc., Chicago, IL) and the Medcalc software (Ostend, Belgium).
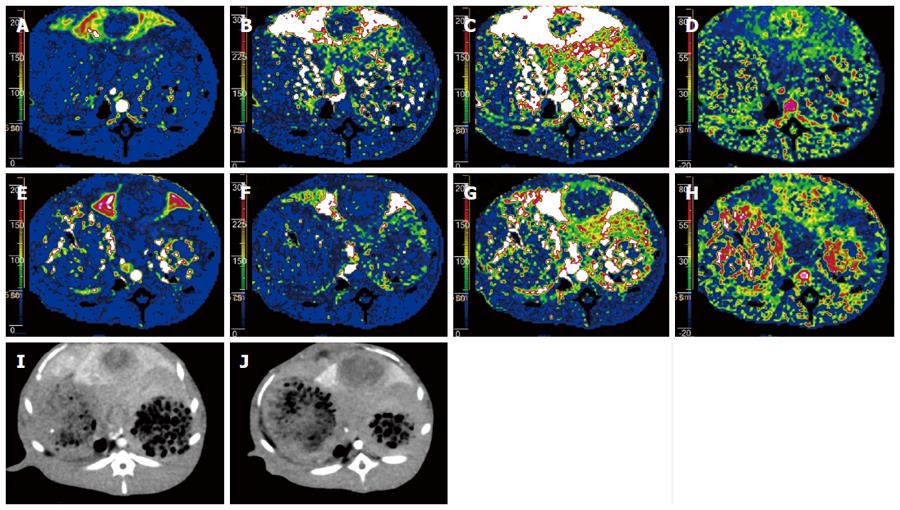
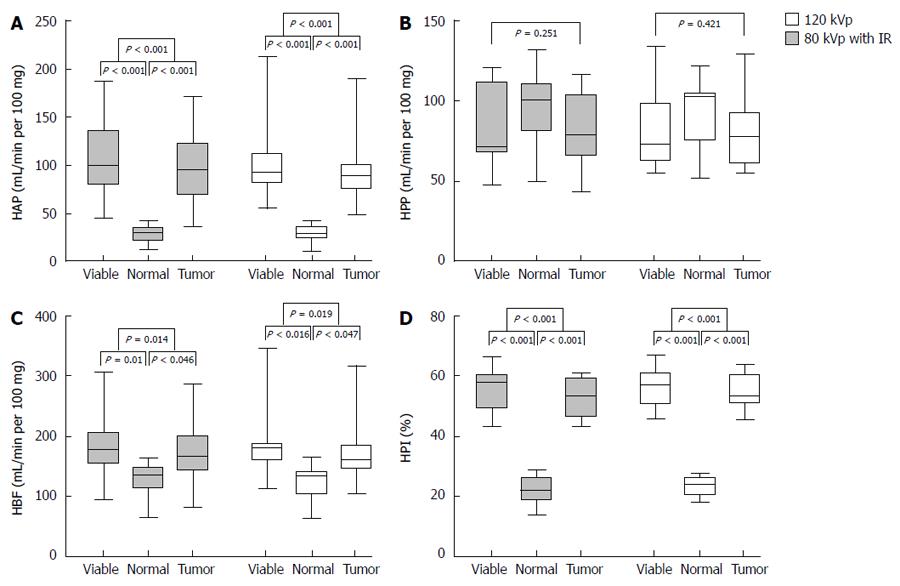
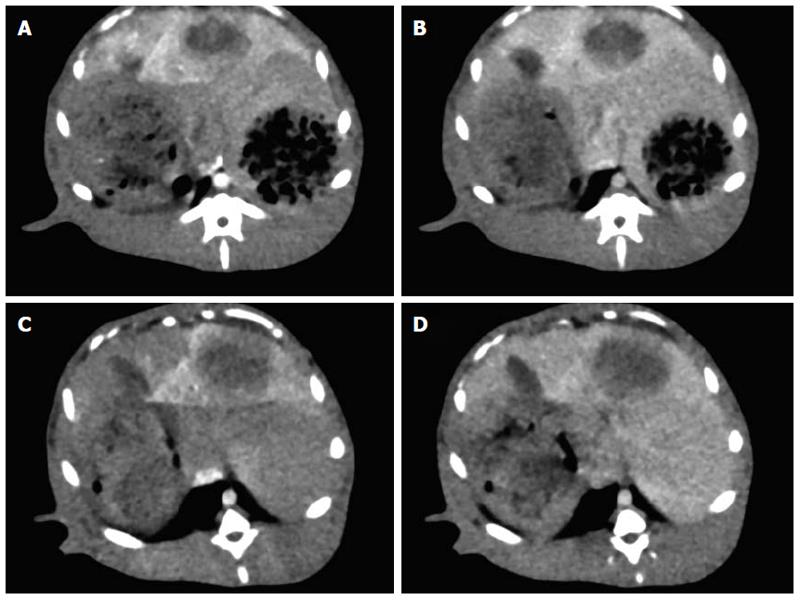
Perfusion CT was successfully performed in all 11 rabbits (Figure 1). Figure 2 shows that perfusion parameters for both viable tumor and whole tumor were significantly higher compared with those obtained for the normal liver parenchyma with protocols A and B, respectively (P < 0.05). However, no significant differences in HPP among viable tumor, whole tumor, and normal liver parenchyma were obtained using protocols A and B (P = 0.421 and 0.251, respectively). Therefore, a Dunnett test was not performed for this group.
Similarly, analysis of variance showed that there were no significant differences among protocols A, B and C with regard to HAP, HPP, HBF and HPI for normal liver, viable tumor component and whole tumor (P > 0.05; Table 1).
| HAP (mL/min per 100 mg) | HPP (mL/min per 100 mg) | HBF (mL/min per 100 mg) | HPI (%) | |
| Normal liver 120 kVp | 31.27 ± 9.22 | 99.44 ± 22.99 | 130.71 ± 31.08 | 23.60 ± 3.99 |
| 80 kVp IR | 28.38 ± 9.45 | 97.5 ± 22.11 | 125.93 ± 29.19 | 22.24 ± 4.55 |
| 80 kVp | 28.5 ± 9.56 | 97.56 ± 22.02 | 125.96 ± 29.35 | 22.20 ± 4.34 |
| P value | 0.721 | 0.974 | 0.912 | 0.699 |
| Viable tumor | ||||
| 120 kVp | 112.68 ± 50.22 | 82.67 ± 25.21 | 195.36 ± 72.38 | 56.65 ± 6.42 |
| 80kVp IR | 108.94 ± 43.35 | 83.83 ± 25.04 | 192.77 ± 63.56 | 55.66 ± 6.90 |
| 80 kVp | 106.76 ± 42.99 | 83.91 ± 25.36 | 192.49 ± 64.08 | 55.46 ± 7.23 |
| P value | 0.954 | 0.992 | 0.994 | 0.909 |
| Whole tumor | ||||
| 120 kVp | 102.06 ± 45.53 | 81.65 ± 25.55 | 183.71 ± 68.66 | 54.60 ± 6.01 |
| 80 kVp IR | 96.71 ± 38.69 | 81.99 ± 22.79 | 178.70 ± 57.81 | 53.10 ± 6.71 |
| 80 kVp | 99.48 ± 40.32 | 83.37 ± 23.11 | 182.85 ± 59.63 | 53.22 ± 7.03 |
| P value | 0.956 | 0.984 | 0.980 | 0.842 |
HAP (r = 0.577, P = 0.031), HPP (r = 0.527, P = 0.048), HBF (r = 0.583, P = 0.03), and HPI (r = 0.531, P = 0.046) for normal liver parenchyma at 80 kVp with the iterative reconstruction protocol showed a moderate positive correlation with values measured at 120 kVp.
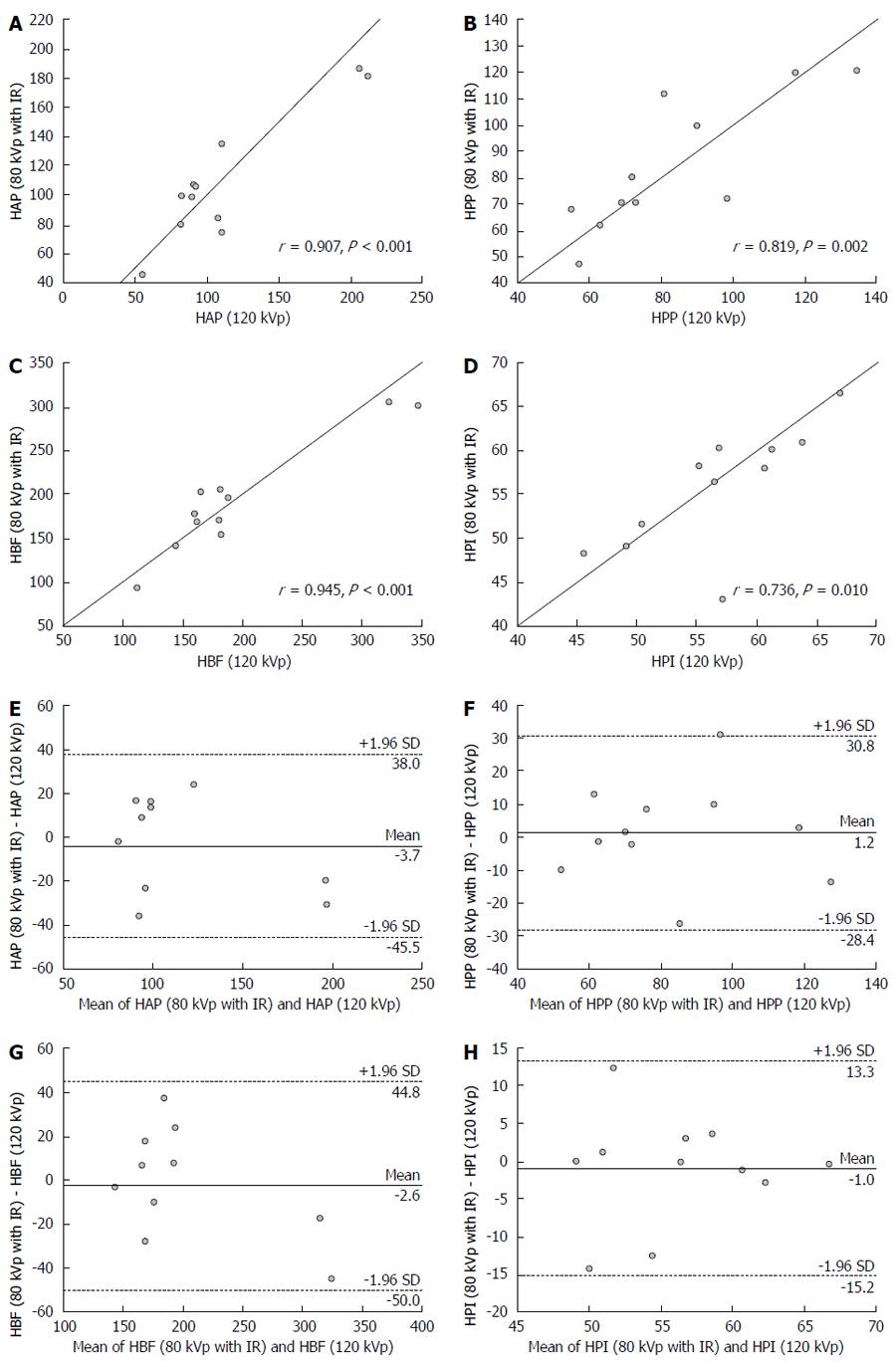
As shown in Figure 3, strong positive correlations were obtained in HAP (r = 0.907; P < 0.001), HPP (r = 0.819; P = 0.002) and HBF (r = 0.945; P < 0.001) values for viable tumor between protocols A and B. HPI (r = 0.736; P = 0.01) for viable tumor determined with protocol A showed a moderate positive correlation with the value measured with protocol B. Bland-Altman plots for viable tumor yielded mean differences of -3.7 for HAP (95% limits of agreement, -45.5 to 38 mL/min per 100 mg), 1.2 for HPP (95% limits of agreement, -28.4 to 30.8 mL/min per 100 mg), -2.6 for HBF (95% limits of agreement, -50.0 to 44.8 mL/min per 100 mg), and 1.0 for HPI (95% limits of agreement, -15.2% to 13.3%), which did not differ significantly from zero. In clinical interpretation, the 95% limits of agreement may lead to misinterpretation of tumor angiogenesis and liver perfusion in few cases, and are therefore considered to be a moderately acceptable agreement. For example, all points were located within 95% limits of agreement values (Figure 3), and HPI at 80 kVp with iterative reconstruction differed from HPI at 120 kVp by 15.2% below and 13.3% above; with mean value of these two HPIs being 56.16%, the misinterpretation of tumor perfusion may occur in few atypical cases.
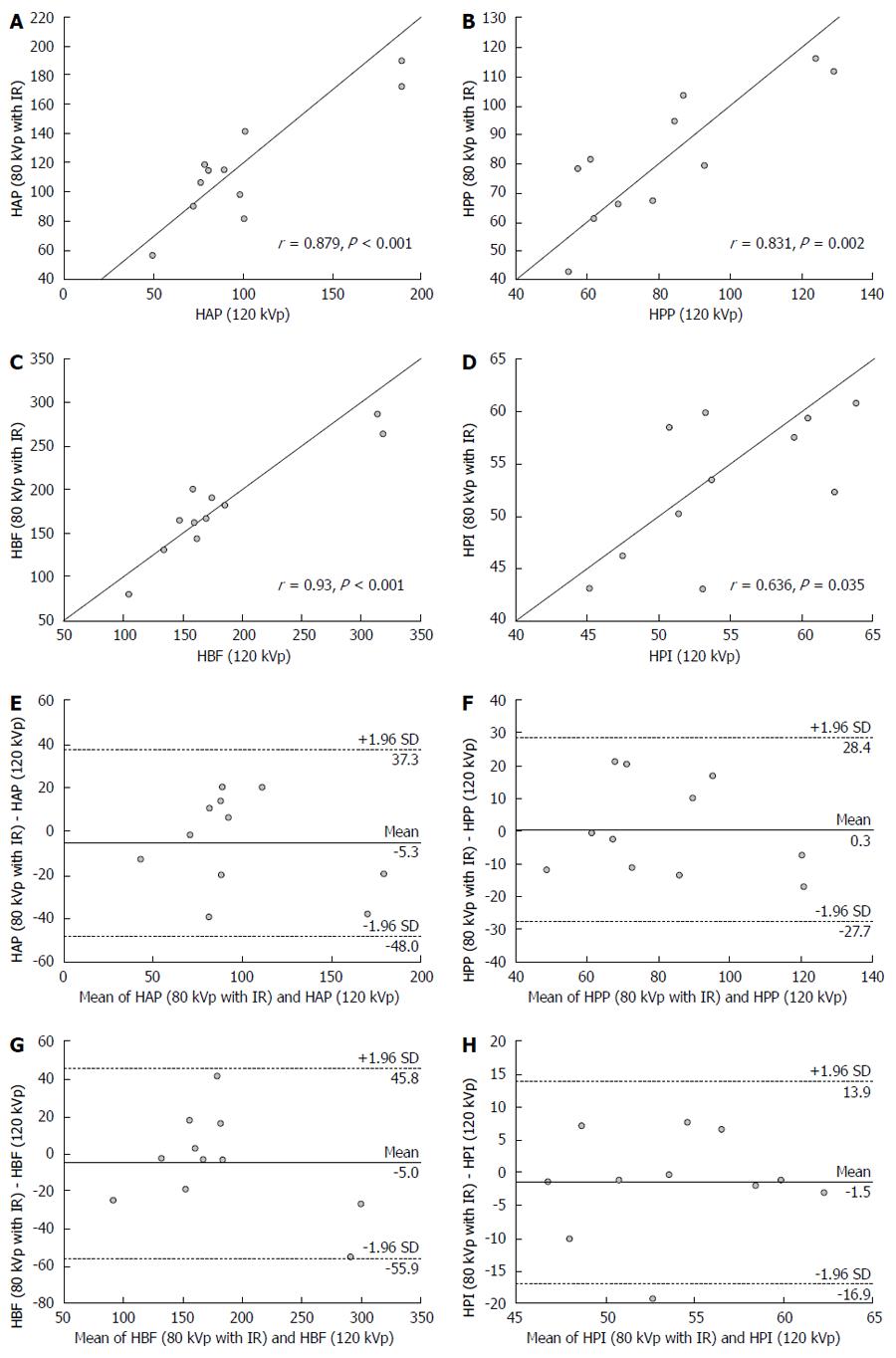
Similarly, correlation and agreement data among perfusion parameters for whole tumor between protocols A and B are shown in Figure 4. HAP, HPP and HBF determined with protocol A showed a strong positive correlation with values measured at 80 kVp. There was a moderate positive correlation for HPI of whole tumor between protocols A and B. Bland-Altman plots suggested a moderate acceptable agreement for these perfusion parameters between protocols A and B.
As shown in Table 2, the CT values obtained for normal liver parenchyma in arterial and portal venous phases with protocols B and C were significantly different from those obtained with protocol A (P < 0.05). However, there was no significant difference among protocols A, B and C for the same parameters of viable tumor in arterial and portal venous phases, respectively (P > 0.05). Image noises in arterial and portal venous phases with protocol C were 70% and 63% higher than values obtained with protocol A (13.85 ± 1.05 HU vs 8.16 ± 0.95 HU, P < 0.001; 13.47 ± 1.27 HU vs 8.27 ± 1.17 HU, P < 0.001, respectively). In comparison with image noises determined by protocol C, 80 kVp with iterative reconstruction reduced 33% and 29% of image noises in arterial and portal venous phases (9.31 ± 0.94 HU vs 13.85 ± 1.05 HU; 9.50 ± 0.93 HU vs 13.47 ± 1.27 HU, respectively). However, there was a significant difference in image noises during arterial and portal venous phases between protocols B and A (9.31 ± 0.94 HU vs 8.16 ± 0.95 HU, P = 0.019; 9.50 ± 0.93 HU vs 8.27 ± 1.17 HU, P = 0.031). There was no significant difference between protocols B and A in tumor-to-liver CNR values during both arterial and portal venous phases (P = 0.814, P = 0.587), while CNR values obtained with protocol C significantly differed from those measured with protocol A (P = 0.048, P = 0.004).
| P value | |||||
| 80 kVp with IR | 80 kVp | 120 kVp | 80 IR vs 120 | 80 vs 120 | |
| Normal liver | |||||
| Arterial phase (HU) | 78.88 ± 7.1 | 78.61 ± 7.27 | 71.57 ± 6.93 | 0.041 | 0.049 |
| Portal venous phase (HU) | 129.00 ± 5.70 | 129.5 ± 5.67 | 120.34 ± 5.26 | 0.002 | 0.001 |
| Viable tumor | |||||
| Arterial phase (HU) | 131.68 ± 24.24 | 131.74 ± 24.29 | 122.15 ± 23.25 | 0.552 | 0.549 |
| Portal venous phase (HU) | 86.05 ± 12.82 | 87.77 ± 13.39 | 79.43 ± 10.59 | 0.357 | 0.211 |
| Noise | |||||
| NoiseHAP (HU) | 9.31 ± 0.94 | 13.85 ± 1.05 | 8.16 ± 0.95 | 0.019 | < 0.001 |
| NoisePVP (HU) | 9.50 ± 0.93 | 13.47 ± 1.27 | 8.27 ± 1.17 | 0.031 | < 0.001 |
| CNR | |||||
| CNRHAP | 5.63 ± 2.38 | 3.88 ± 1.82 | 6.16 ± 2.60 | 0.814 | 0.048 |
| CNRPVP | 4.60 ± 1.27 | 3.16 ± 1.04 | 5.11 ± 1.74 | 0.587 | 0.004 |
The mean values of contrast medium dose delivered for the 80 kVp and 120kVp protocols were 360.1 ± 13.3 and 583.5 ± 21.5 mgI/kg, respectively (P < 0.001). Low tube potential allowed a 38% decrease in contrast medium dose in comparison with conventional tube potential (Table 3).
| 80 kVp | 120 kVp | P value | |
| Body weight (kg) | 3.0 ± 0.11 | 3.0 ± 0.11 | |
| Concentration of contrast medium (mgI/mL) | 270 | 350 | |
| Volume of contrast medium (mL) | 4 | 5 | |
| Contrast medium dose (mgI/kg) | 360.1 ± 13.3 | 583.5 ± 21.5 | < 0.001 |
Figure 5 illustrates the histopathological features of hepatic VX2 tumor in rabbits. The epithelial cells in tumor microvessels were stained brown with anti-CD31 and protoplasm of tumor cells was stained brown with anti-VEGF. In addition, microvessels were heterogeneously distributed within the tumor and showed a predominantly peripheral distribution. These findings were correlated with perfusion CT images.
Radiation doses were automatically measured by the CT scanner during scanning. The radiation dose for the 80 kVp group was 73% lower than that used in the 120 kVp group (CTDIvol: 237.31 mGy, DLP: 1898.5 mGy • cm vs CTDIvol 868.98 mGy, DLP 6951.8 mGy • cm).
Our data revealed that perfusion parameters (HAP, HBF and HPI) of viable tumor component and whole tumor obtained by a 256-slice CT scanner using 80 kVp and conventional 120 kVp protocols were significantly higher than those of normal liver parenchyma, indicating a successful detection of all liver tumors in experimental rabbits. Slightly lower mean HPP values were measured for viable tumor and whole tumor in comparison with tumor free liver parenchyma. Consistent with previous experimental and clinical studies, HAP and HPI in both hepatocellular carcinoma and hepatic metastases were significantly elevated[2,15-18]. As a result of increased formation of new blood vessels from the hepatic artery during angiogenesis, which was also observed in our immunohistochemical analysis of tumor specimen, hepatic arterial flow in the region of viable tumor was increased. Moreover, owing to obstruction, stenosis and compression of the hepatic sinusoidal capillaries, the increased intrahepatic resistance contributed to decreased portal venous flow. This indicated that the blood supply of rabbit liver VX2 tumor mainly derived from arterial rather than portal venous circulation, which resulted in increased HPI. Consequently, compared to 120 kVp with conventional concentration contrast agent protocol, we believed that 80 kVp with low concentration contrast agent protocol has similar capability in providing valuable information for rabbit liver VX2 tumor identification.
In the past few years, with the development of imaging technique, perfusion CT has played a vital role in quantitatively assessing the perfusion of abdominal organs and lesions. However, the relatively high radiation dose limits the wide application of this functional image modality[19]. The low-kVp technique allows a sharp decrease in radiation dose (proportionally to the square of tube voltage) and increase in the attenuation of iodinated contrast agent[20-23]. Previous studies have reported that the delivered dose of the contrast agent is closely related to contrast-induced nephropathy and affects the mortality rate in patients with acute myocardial infarction[24-27]. In our study, the use of 80 kVp with 40% iterative reconstruction protocol offers a 73% reduction of the radiation dose with a 38% decrease in contrast agent concentration in comparison with the conventional 120 kVp protocol. Thus, the low tube potential protocol may reduce the risk of contrast-induced nephropathy. Another important finding was the positive correlation among perfusion parameters between the 80 kVp and 120 kVp protocols. Despite the low concentration of contrast medium administrated in the 80kVp protocol, the latter achieved highly positive correlations for HAP, HPP, and HBF, and moderate positive correlation for HPI in viable tumor and whole tumor compared with the conventional 120 kVp protocol. It has been suggested that correlation coefficient may not reflect the level of agreement and cannot be used to explain the possibility of interchangeability between two methods of measurements[28]. In order to avoid systematic bias, Bland-Altman analysis of perfusion parameters was performed for the whole tumor and viable tumor component between the two image settings, and a moderate acceptable agreement was obtained. Therefore, perfusion CT with low radiation dose and low concentration of contrast agent may be more helpful in evaluating tumor angiogenesis and blood perfusion in comparison with the conventional protocol.
The iterative reconstruction algorithm has been routinely applied in the field of nuclear imaging and was introduced in the past six years to reduce the noise and artifacts of image for MDCT with the advance in computer technology[29]. We found that 80 kVp with 40% iterative reconstruction decreases image noise by 33% in hepatic arterial phase and 29% in hepatic portal venous phase. Iterative reconstruction algorithm allows similar tumor-to-liver CNR (P > 0.05) in hepatic arterial and portal venous phases, compared with 120 kVp with the FBP algorithm. However, Marin et al[13] reported that low tube voltage and high tube current CT technique increases the tumor-to-liver CNR and improves the conspicuity of malignant hypervascular liver tumors during late hepatic arterial phase (12). This discrepancy can mainly be explained by the 38% lower dose of contrast media used in our study. At the same tube potential setting and lower contrast agent delivered, less lesion enhancement was obtained. These findings indicate that the iterative reconstruction algorithm together with 80 kVp tube potential and low contrast medium dose dramatically reduced image noise with similar conspicuity of tumor, compared with FBP algorithm used in combination with 120 kVp and conventional contrast medium dose (Figure 6).
This study has several limitations. First, a relatively small number of rabbits was involved, which might limit the statistical significance. Second, we only performed one level of hybrid iterative reconstruction. Although an increased level of iterative reconstruction might result in a more pronounced decrease of image noise, Leipsic et al[30] reported that a high level of iterative reconstruction causes loss of image nature. Third, imaging was set for the low dose radiation protocol based on body weight recommendation; however, body size such as body mass index and cross sectional diameter are key points to assessing image noise and CNR as well[31]. Combination of body size and body weight may offer more accurate measurements of perfusion parameters in further studies. Fourth, rabbit liver VX2 is a hypervascular tumor model with only one input blood supply, and the use of low dose protocol in evaluating hepatic metastatic tumors might be limited. Fifth, our low concentration contrast medium and radiation dose protocol was implemented using a rabbit model, and clinical application with patients’ larger body weight and body size could pose a challenge. The low dose radiation and contrast medium protocol needs to be modified for patient suitability in further clinical research. In addition, we performed maximum slope perfusion CT, which was reported to possibly underestimate hepatic perfusion, especially for portal venous perfusion[32]. Finally, we present the correlation and agreement of HAP, HPP, HBF and HPI and did not evaluate other useful parameters such as hepatic permeability of capillary vessel surface (PS), hepatic blood volume, mean transit time, because these measurements were not achievable in the maximum slope perfusion CT protocol.
In conclusion, perfusion CT with low tube potential and low concentration contrast agent can dramatically decrease radiation dose and image noise with similar conspicuity of tumor compared to conventional tube potential with conventional concentration contrast medium and does not significantly influence perfusion parameters for liver VX2 tumor in rabbits. Therefore, the proposed protocol has a potential for clinical use in evaluating hepatic tumor angiogenesis and the response of anti-angiogenesis therapy.
Computed tomography (CT) perfusion plays an important role in quantitatively assessing the perfusion of abdominal organs and lesions. However, concerns regarding the relatively high radiation dose and contrast-induced nephropathy limited its wide use. There were rarely reported data on simultaneously implementing low radiation dose and low contrast medium concentration protocol for hepatic CT perfusion.
The technique of low tube potential combined with iterative reconstruction has emerged as a robust tool to dramatically decrease the radiation dose without compromising image quality and increase the conspicuity of hypervascular tumor during contrast enhanced CT scans. Therefore, the author aimed to evaluate whether hepatic CT perfusion with low tube potential and low contrast medium concentration protocol could provide similar perfusion parameters of rabbit VX2 tumor in comparison with conventional protocol.
This study reported for the first time that CT perfusion with low dose protocol achieved a 38% reduction in contrast medium consumption and a 73% reduction in radiation dose without significant influence on the hepatic perfusion parameters in rabbit VX2 tumor.
It is promising that hepatic CT perfusion with the combination of low tube potential with iterative reconstruction and low concentration contrast medium may be clinically used to decrease radiation dose and the risk of contrast-induced nephropathy without significant influence on perfusion parameters.
Since low tube potential is closer to the iodine K-edge of 33 keV, this technique increases the contrast of iodine and may allow for a reduction in the administered contrast medium dose.
Authors evaluate the feasibility of low radiation dose and low concentration contrast medium with iterative reconstruction on a 256-slice CT for hepatic VX2 tumor in rabbits, and conclude that perfusion CT with low tube potential and low concentration contrast agent can dramatically decrease radiation dose and image noise with similar conspicuity of tumor compared to conventional tube potential with conventional concentration contrast medium and does not significantly influence perfusion parameters for liver VX2 tumor in rabbits. This is an important study and if it could be repeated in human subjects would improve the safety of CT scanning for patients and allow the technique to be used in patients who would otherwise be contraindicated because of potential toxicity.
P- Reviewer: Bramhall S, Hashimoto N S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Miles KA, Hayball M, Dixon AK. Colour perfusion imaging: a new application of computed tomography. Lancet. 1991;337:643-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 144] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005;234:661-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | From AM, Bartholmai BJ, Williams AW, Cha SS, McDonald FS. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc. 2008;83:1095-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Negi N, Yoshikawa T, Ohno Y, Somiya Y, Sekitani T, Sugihara N, Koyama H, Kanda T, Kanata N, Murakami T. Hepatic CT perfusion measurements: a feasibility study for radiation dose reduction using new image reconstruction method. Eur J Radiol. 2012;81:3048-3054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Kalra MK, Maher MM, Toth TL, Schmidt B, Westerman BL, Morgan HT, Saini S. Techniques and applications of automatic tube current modulation for CT. Radiology. 2004;233:649-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 552] [Cited by in F6Publishing: 506] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 6. | Yeh BM, Shepherd JA, Wang ZJ, Teh HS, Hartman RP, Prevrhal S. Dual-energy and low-kVp CT in the abdomen. AJR Am J Roentgenol. 2009;193:47-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Hara AK, Paden RG, Silva AC, Kujak JL, Lawder HJ, Pavlicek W. Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR Am J Roentgenol. 2009;193:764-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 578] [Cited by in F6Publishing: 512] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 8. | Silva AC, Lawder HJ, Hara A, Kujak J, Pavlicek W. Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR Am J Roentgenol. 2010;194:191-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 473] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 9. | Nakaura T, Nakamura S, Maruyama N, Funama Y, Awai K, Harada K, Uemura S, Yamashita Y. Low contrast agent and radiation dose protocol for hepatic dynamic CT of thin adults at 256-detector row CT: effect of low tube voltage and hybrid iterative reconstruction algorithm on image quality. Radiology. 2012;264:445-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Kim KW, Lee JM, Kim JH, Klotz E, Kim HC, Han JK, Choi BI. CT color mapping of the arterial enhancement fraction of VX2 carcinoma implanted in rabbit liver: comparison with perfusion CT. AJR Am J Roentgenol. 2011;196:102-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Verdun FR, Lepori D, Monnin P, Valley JF, Schnyder P, Gudinchet F. Management of patient dose and image noise in routine pediatric CT abdominal examinations. Eur Radiol. 2004;14:835-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Miles KA, Hayball MP, Dixon AK. Functional images of hepatic perfusion obtained with dynamic CT. Radiology. 1993;188:405-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Marin D, Nelson RC, Samei E, Paulson EK, Ho LM, Boll DT, DeLong DM, Yoshizumi TT, Schindera ST. Hypervascular liver tumors: low tube voltage, high tube current multidetector CT during late hepatic arterial phase for detection--initial clinical experience. Radiology. 2009;251:771-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 14. | Zou KH, Tuncali K, Silverman SG. Correlation and simple linear regression. Radiology. 2003;227:617-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 437] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 15. | Stewart EE, Chen X, Hadway J, Lee TY. Correlation between hepatic tumor blood flow and glucose utilization in a rabbit liver tumor model. Radiology. 2006;239:740-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Yang HF, Du Y, Ni JX, Zhou XP, Li JD, Zhang Q, Xu XX, Li Y. Perfusion computed tomography evaluation of angiogenesis in liver cancer. Eur Radiol. 2010;20:1424-1430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Choi SH, Chung JW, Kim HC, Baek JH, Park CM, Jun S, Kim MU, Lee ES, Cho HR, Jae HJ. The role of perfusion CT as a follow-up modality after transcatheter arterial chemoembolization: an experimental study in a rabbit model. Invest Radiol. 2010;45:427-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Cuenod C, Leconte I, Siauve N, Resten A, Dromain C, Poulet B, Frouin F, Clément O, Frija G. Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology. 2001;218:556-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Goh V, Dattani M, Farwell J, Shekhdar J, Tam E, Patel S, Juttla J, Simcock I, Stirling J, Mandeville H. Radiation dose from volumetric helical perfusion CT of the thorax, abdomen or pelvis. Eur Radiol. 2011;21:974-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Funama Y, Awai K, Nakayama Y, Kakei K, Nagasue N, Shimamura M, Sato N, Sultana S, Morishita S, Yamashita Y. Radiation dose reduction without degradation of low-contrast detectability at abdominal multisection CT with a low-tube voltage technique: phantom study. Radiology. 2005;237:905-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Leschka S, Stolzmann P, Schmid FT, Scheffel H, Stinn B, Marincek B, Alkadhi H, Wildermuth S. Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. Eur Radiol. 2008;18:1809-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Nakayama Y, Awai K, Funama Y, Liu D, Nakaura T, Tamura Y, Yamashita Y. Lower tube voltage reduces contrast material and radiation doses on 16-MDCT aortography. AJR Am J Roentgenol. 2006;187:W490-W497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Itatani R, Oda S, Utsunomiya D, Funama Y, Honda K, Katahira K, Morishita S, Yamamura S, Namimoto T, Yamashita Y. Reduction in radiation and contrast medium dose via optimization of low-kilovoltage CT protocols using a hybrid iterative reconstruction algorithm at 256-slice body CT: phantom study and clinical correlation. Clin Radiol. 2013;68:e128-e135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, De Metrio M, Galli S, Fabbiocchi F, Montorsi P. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773-2782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 394] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 25. | Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, Leon MB. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542-1548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 535] [Cited by in F6Publishing: 501] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 26. | Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1317] [Cited by in F6Publishing: 1243] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 27. | Barrett BJ, Katzberg RW, Thomsen HS, Chen N, Sahani D, Soulez G, Heiken JP, Lepanto L, Ni ZH, Ni ZH. Contrast-induced nephropathy in patients with chronic kidney disease undergoing computed tomography: a double-blind comparison of iodixanol and iopamidol. Invest Radiol. 2006;41:815-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Bland JM, Altman DG. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol. 2003;22:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 967] [Cited by in F6Publishing: 956] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 29. | Xu J, Mahesh M, Tsui BM. Is iterative reconstruction ready for MDCT? J Am Coll Radiol. 2009;6:274-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Leipsic J, Labounty TM, Heilbron B, Min JK, Mancini GB, Lin FY, Taylor C, Dunning A, Earls JP. Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol. 2010;195:649-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 31. | Menke J. Comparison of different body size parameters for individual dose adaptation in body CT of adults. Radiology. 2005;236:565-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Kanda T, Yoshikawa T, Ohno Y, Kanata N, Koyama H, Takenaka D, Sugimura K. CT hepatic perfusion measurement: comparison of three analytic methods. Eur J Radiol. 2012;81:2075-2079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |