Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4517
Peer-review started: November 27, 2014
First decision: December 11, 2014
Revised: December 29, 2014
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: April 21, 2015
AIM: To investigate the molecular mechanism for regulation of cholesterol metabolism by hepatitis C virus (HCV) core protein in HepG2 cells.
METHODS: HCV genotype 1b core protein was cloned and expressed in HepG2 cells. The cholesterol content was determined after transfection. The expression of sterol regulatory element binding protein 2 (SREBP2) and the rate-limiting enzyme in cholesterol synthesis (HMGCR) was measured by quantitative real-time PCR and immunoblotting after transfection. The effects of core protein on the SREBP2 promoter and 3’-untranslated region were analyzed by luciferase assay. We used different target predictive algorithms, microRNA (miRNA) mimics/inhibitors, and site-directed mutation to identify a putative target of a particular miRNA.
RESULTS: HCV core protein expression in HepG2 cells increased the total intracellular cholesterol level (4.05 ± 0.17 vs 6.47 ± 0.68, P = 0.001), and this increase corresponded to an increase in SREBP2 and HMGCR mRNA levels (P = 0.009 and 0.037, respectively) and protein expression. The molecular mechanism study revealed that the HCV core protein increased the expression of SREBP2 by enhancing its promoter activity (P = 0.004). In addition, miR-185-5p expression was tightly regulated by the HCV core protein (P = 0.041). Moreover, overexpression of miR-185-5p repressed the SREBP2 mRNA level (P = 0.022) and protein expression. In contrast, inhibition of miR-185-5p caused upregulation of SREBP2 protein expression. miR-185-5p was involved in the regulation of SREBP2 expression by HCV core protein.
CONCLUSION: HCV core protein disturbs the cholesterol homeostasis in HepG2 cells via the SREBP2 pathway; miR-185-5p is involved in the regulation of SREBP2 by the core protein.
Core tip: In this study, we investigated the regulation relationship between hepatitis C virus (HCV) core protein and sterol regulatory element binding protein 2 (SREBP2). The microRNA miR-185-5p was confirmed in the regulation of SERBP2 expression, and it was the target for HCV core protein. The regulation relationship between HCV core, miR-185-5p and SREBP2 was established at transcriptional, post-transcriptional, and translational levels.
- Citation: Li M, Wang Q, Liu SA, Zhang JQ, Ju W, Quan M, Feng SH, Dong JL, Gao P, Cheng J. MicroRNA-185-5p mediates regulation of SREBP2 expression by hepatitis C virus core protein. World J Gastroenterol 2015; 21(15): 4517-4525
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4517.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4517
Liver steatosis is defined as accumulation of lipid in the liver. In patients with chronic hepatitis C (CHC), it is frequently found as a histologic appearance. Epidemiologic studies revealed that hepatitis C virus (HCV) infection is a primary cause of liver steatosis. It was reported that prevalence of steatosis is much higher (up to 2.5 times) in CHC than in other liver diseases, such as autoimmune liver diseases (17%) and hepatitis B infection (26%)[1]. Steatosis is correlated with the effect of interferon treatment, fibrosis, and the development of hepatocellular carcinoma[2]. So both pathogenesis study and new drug development are important against HCV infection.
HCV belongs to the Flaviviridae family. The HCV genome has a positive single-stranded RNA genome of 9.6 kb[3]. It contains a single open reading frame that encodes a large polyprotein of 3000 amino acids. After translation, this polyprotein is processed to the viral structural (core, E1, E2, and possibly P7) and non-structural (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) proteins[4]. The HCV core protein is thought to be a pathogenic factor for inducing steatosis, hepatocellular carcinoma, and oxidative stress[5]. Several studies have reported that lipid accumulation can be induced by the core protein in transgenic mouse models and cell culture, suggesting that the HCV core protein is sufficient to induce a lipid accumulation in hepatocytes[6].
The sterol response element binding protein (SREBP) family of proteins, including SREBP1a, SREBP1c, and SREBP2, are major regulators of lipid metabolism[7]. SREBP1c is mainly involved in the lipid synthesis pathway by regulating related genes[8], whereas SREBP2 specifically regulates cholesterol homeostasis[9]. In the liver, cholesterol synthesis is controlled by the microsomal HMG-CoA reductase (HMGCR). HMGCR regulates the rate-limiting step in cholesterol biosynthesis by converting HMG-CoA to mevalonate. Results from transgenic mice suggest that overexpression of SREBP2 increases the mRNA level of HMGCR[10]. The influence of the HCV core protein on this transcription factor could contribute to the development of HCV-associated steatosis.
MicroRNAs (miRNAs) are about 22 nucleotide single-stranded small noncoding RNAs. miRNAs can suppress target gene expression by imperfect pairing to miRNA response elements (MREs) within the 3’-untranslated regions (UTRs). miRNA regulation results in the inhibition of target gene expression through mRNA degradation and/or translational inhibition[11].
The pathogenesis of HCV-induced steatosis remains largely unknown. In this study, we investigated the relationship between host genes and the HCV core protein to reveal the molecular pathogenic mechanism of HCV-induced steatosis.
HepG2 cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (Life Technologies of Thermo Fisher Scientific, Waltham, MA, United States) at 37 °C in a humid atmosphere of 5% CO2.
The HCV core fragment of genotype 1b (573 bp) was cloned from the template of a full-length HCV cDNA by PCR. The primers used for PCR amplification were as follows: forward 5’-ctcgagCCATGAGCACGAATCCTAAAC-3’ and reverse 5’-ggatccGGCGGAAACTGGGATGGT C-3’. The amplified product was digested with BamHI and XhoI and inserted into BamHI- and XhoI-digested pcDNA3.1/myc-His(-) vector.
The promoter region of SREBP2 (from nucleotide -933 to +263) was cloned from the genomic DNA of HepG2 cells using primers as follows: forward, 5’-gagctcTGATCCCATGGTATTCCATCG-3’; reverse, 5’-gctagcACCACTCACC GTCGATGTCT-3’. The PCR product was digested with SacI and NheI and inserted into SacI- and NheI-digested pGL4.10 vector (Promega, Madison, WI, United States) after sequencing.
To generate a luciferase reporter to determine the miRNA activity, the 3’-UTR (3596-4306; 776 bp) of SREBP2 was cloned by PCR using the following primers: forward, 5’-gctagcGCAGATGATTGTTAAGCTG-3’; reverse, 5’-ctcgagGGAAGGAACAGGACAATT-3’. The genomic DNA of HepG2 cells was used as the template. After digestion with NheI/XhoI, the PCR product was inserted into the pmirGLO reporter construct (Promega) at the NheI/XhoI restriction site after sequencing. Mutations of the seed region binding sites were generated by Pfu DNA polymerase (M7741; Promega) and DpnI (Promega). Three nucleotides in the 3’-UTR (binding for miR-185-5p) of the SREBP2 gene were mutated (T to G) by PCR. Wild-type and mutant constructs were confirmed by sequencing. The primers used for mutagenesis are shown in Table 1.
| Mutant binding site | 5'-3' | |
| a | Forward | CCTTCCTGAGTTTCGCGCGCCTGAACCCTACTCTC |
| Reverse | GAGAGTAGGGTTCAGGCGCGCGAAACTCAGGAAGG | |
| b | Forward | CTCTCCTGAACCCTACGCGCGCCTTTTTGCTTCCTC |
| Reverse | GAGGAAGCAAAAAGGCGCGCGTAGGGTTCAGGAGAG | |
| c | Forward | CTGTAGCGTCTTGATGCGCGCCCTGGGTCTGCG |
| Reverse | CGCAGACCCAGGGCGCGCATCAAGACGCTACAG | |
| d | Forward | CTCTCTCTCGATTTCTCGCGCGCCCCCTCAGCATC |
| Reverse | GATGCTGAGGGGGCGCGCGAGAAATCGAGAGAGAG | |
Determination of intracellular total cholesterol
After seeding in a 6-well culture plate, HepG2 cells were incubated in DMEM containing 10% fetal bovine serum until reaching 70%-80% confluency. After quantifying the cell number, a Cholesterol/Cholesteryl Ester Quantitation Kit (K603-100; Biovision, Inc., Milpitas, CA, United States) was used to extract and measure the intracellular TC according to the manufacturer’s instructions. The intracellular total cholesterol (TC) quantity was expressed as μg/106 cells.
RNA was isolated from HepG2 cells using a Total RNA Kit (R6834; Omega Bio-Tek, Inc., Norcross, GA, United States), and then converted into cDNA by PrimeScript® RT Reagent Kit (DRR027A; TaKaRa, Shanghai, China). One-fourth of the cDNAs were subjected to qRT-PCR amplification (4367659; Applied Biosystems of Thermo Fisher Scientific). The relative mRNA levels of SREBP2 and HMGCR were measured using the comparative Ct method (∆∆Ct) with β-actin as the internal control. The following primers were used for PCR amplification: SREBP2, sense 5’-CCCTTCAGTGCAACGGTCATTCAC-3’ and antisense 5’-TGCCATTGGCCGTTTGTGTC-3’; HMGCR, sense 5’-CTTGTGTGTCCTT GGTATTAGAGC-3’ and antisense 5’-ATCATCTTGACCCTCTGAGTTACAG-3’; β-actin, sense 5’-CATCCGCAAAGACCTGTACGC-3’ and antisense 5’-AGTACTTGCGCTCAGGAGG AG-3’ (Sangon, Shanghai, China).
Total RNAs including miRNAs were isolated with the mirVana miRNA Isolation Kit (AM1560; Ambion of Thermo Fisher Scientific) according to the manufacturer’s instructions. The high-capacity cDNA reverse transcription kit (4368814; Applied Biosystems) and Bulge-LoopTM miRNA qRT-PCR Primer Set (MQP-0105; Ribobio, Guangzhou, China) were used to convert selected miRNAs to cDNAs. Expressions of selected miRNAs were analyzed by qRT-PCR using Power SYBR® Green PCR Master Mix (4367659; Applied Biosystems). RNU6B was used to normalize the mature miRNAs expression levels (MQP-0105; Ribobio).
Cell extracts were isolated in lysis buffer (150 mmol/L NaCl, pH 7.5, 1 mmol/L EDTA, 50 mmol/L Tris-Cl, 10% glycerol, 1% NP-40, and protease inhibitors) for 30 min on ice. The Pierce BCA assay (23225; Pierce Biotechnology, Inc., Rockford, IL, United States) was used to determine the protein concentration. Samples (100 μg) were separated on 10% SDS-polyacrylamide gels and then transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, United States). The membranes were incubated with anti-β-actin (sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX, United States), anti-SREBP2 (ab30682; Abcam, Cambridge, United Kingdom), anti-HMGCR (AP6577c; Abgent, San Diego, CA, United States), or anti-His (sc-803; Santa Cruz Biotechnoogy, Inc.) antibodies in blocking buffer for 2 h at room temperature. After the membranes were incubated with secondary antibodies, the enhanced chemiluminescence system (32209; Thermo Fisher Scientific) was used to detect the immunoreactive bands.
Transfection assays were performed using jetPRIME™ (Polyplus-transfection Inc., Illkirch, France). Either pGL4.10-SREBP2-P (250 ng) or pmirGLO-SREBP2 3’-UTR (250 ng) and the Renilla luciferase vector (pRL-TK; 25 ng) were transfected into HepG2 cells 24 h after seeding in a 48-well culture plate. For some experiments, HepG2 cells were co-transfected with pcDNA3.1/myc-His(-)-core with or without miR-185-5p mimic/inhibitor, whereas the pcDNA3.1/myc-His(-) empty vector and the indicated miR-185-5p negative control were used to normalize the amount of DNA. The cells were harvested and then lysed using the passive lysis buffer (E1941; Promega) at 24 h or 48 h after transfection. A microplate luminometer was used to measure SREBP2 promoter activity and/or SREBP2 3’-UTR activity in some experiments according to the manufacturer’s instructions for the Dual-Luciferase-Reporter Assay System (E1910; Promega). The miR-185-5p mimics/inhibitors and the corresponding negative controls were transfected according to the manufacturer’s protocols (4464014/4464009; Life Technologies).
Data analysis from three independent experiments was performed by the paired Student’s t test. All data are presented as the means ± SE. P < 0.05 was considered statistically significant. The statistical method evaluate by the author Min Li.
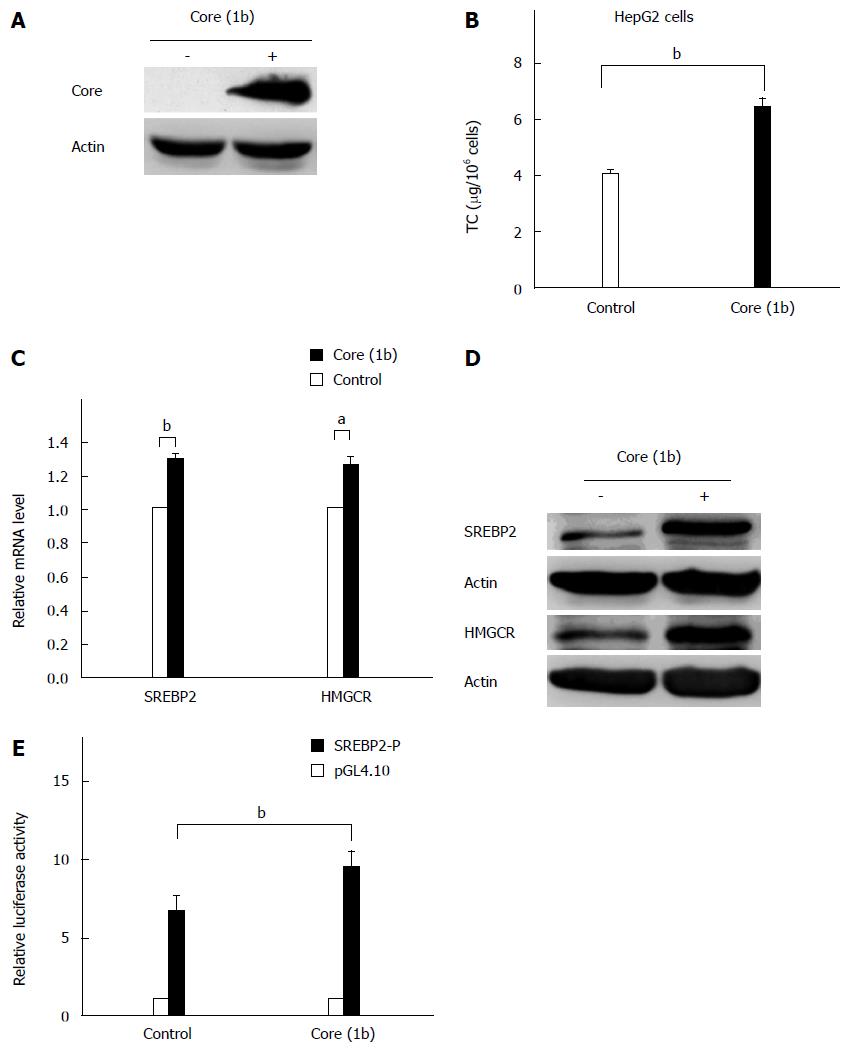
To investigate the effects of HCV core protein on lipid metabolism, we cloned the HCV core gene from CHC patients of genotype 1b. Western blotting was performed to confirm whether HCV core protein was expressed or not. The results indicated that HCV core protein was expressed successfully after transient transfection in HepG2 cells (Figure 1A). In HepG2 cells expressing core protein, the intracellular TC was significantly increased (P = 0.001; Figure 1B).
Further results suggested that the expression of several lipid-associated genes were upregulated by the HCV core protein, including SREBP1c, SREBP2, HMGCR, HMGCS, and Sirt1 (data not shown). As SREBP2 has been reported to play a key role in cholesterol metabolism, and HMGCR acts not only as the rate-limiting enzyme in cholesterol synthesis, but also a predominant target gene of SREBP2, the effects of the HCV core protein on SREBP2 and HMGCR were investigated. The results showed that core protein increased both the mRNA level (P = 0.009 and 0.037, respectively; Figure 1C) and the protein expression (Figure 1D) of SREBP2 and HMGCR. To explore the mechanism by which HCV core protein upregulates SREBP2 and induces steatosis, the impact of core protein on SREBP2 promoter activity was investigated. The results from the luciferase assay indicate that the SREBP2 promoter activities were enhanced in core-expressing cells (P = 0.004; Figure 1E).
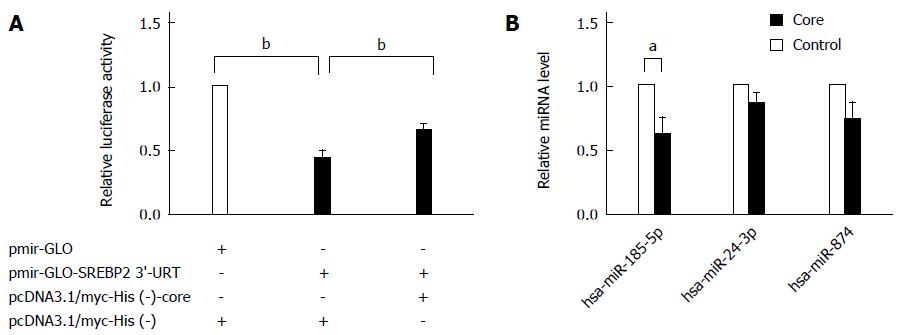
miRNAs have been recently suggested to regulate lipid metabolism genes involved in fatty acid oxidation and cholesterol homeostasis post-transcriptionally[12]. So we proposed that miRNAs may be involved in the regulation of SREBP2 expression by the HCV core protein. To examine whether miRNAs indeed bind to its putative binding sites on the SREBP2 3’-UTR, the pmirGLO-SREBP2 3’-UTR luciferase-reporter construct was transfected into HepG2 cells, and luciferase activity was determined. In pmirGLO-SREBP2 3’-UTR-transfected cells, luciferase activity was repressed significantly compared with the controls, suggesting that certain miRNAs may be involved in the regulation of SREBP2 expression (P < 0.001; Figure 2A). In cells overexpressing core protein, the luciferase activity of SREBP2 3’-UTR was partially restored (P = 0.001). Five miRNAs were predicted to target SREBP2 by a target prediction algorithm (http://www.microrna.org/), including miR-542-3p, 24-3p, 185-5p, 874, and 543. Of these, miR-185-5p (previous ID: miR-185) was also predicted by DIANAmicroT_v3_targets, Targetscan, PITA, and miRDB, to regulate SREBP2 (Table 2). In addition, miR-185-5p is evolutionarily conserved throughout vertebrates[13]. Moreover, miR-185-5p was downregulated by HCV core protein (P = 0.041; Figure 2B), suggesting that misregulation of miR-185-5p by HCV core protein may be an event responsible for SREBP2 overexpression.
| DIANAmicroT_v3_targets | Targetscan | Microrna. org | miRDB | PITA | ||
| Has-miR-98 | 24 | 4644 | 542-3p | 4279 | 423-5p | 1275 |
| 124 | 185 | 1193-5p | 24 | 4306 | 185 | 1303 |
| 185 | 193 | 125a-3p | 185 | 4644 | 298 | 1207-5p |
| 506 | 370 | 193-3p | 543 | 149-3p | 346 | 1207-5p |
| 650 | 379 | 193b | 874 | 185-5p | 448 | 296-3p |
| 940 | 539 | 24-3p | 1915-3p | 608 | 296-5p | |
| 331-3p | 543 | 24ab | 4446-3p | 612 | 664b | |
| 532-3p | 544 | 296-3p | 4628-3p | 637 | 671-5p | |
| let-7a | 873 | 324-5p | 638 | |||
| let-7b | 874 | 539-5p | 650 | |||
| let-7c | 882 | 542-3p | 877 | |||
| let-7e | 1554 | 544-3p | 921 | |||
| let-7f | 3473 | 544ab | 939 | |||
| let-7g | 3529 | 1268 | ||||
| let-7i | 4306 | 1269 | ||||
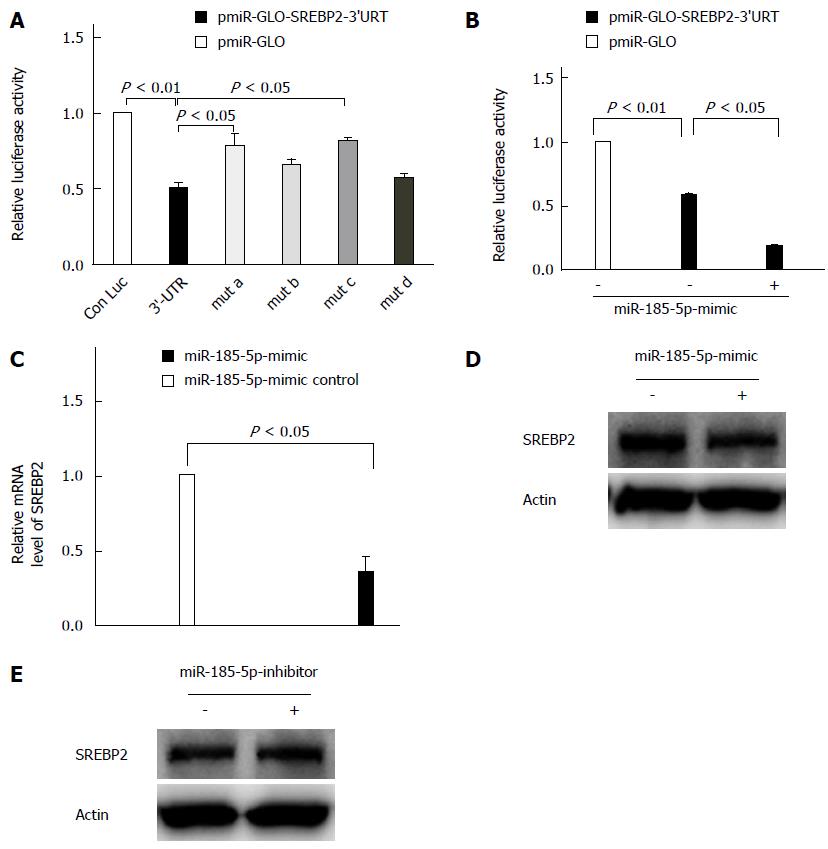
miRNAs can pair imperfectly to MREs within 3’-UTRs of target mRNAs to suppress gene expression post-transcriptionally. Bioinformatics analyses revealed that there are at least four putative MREs for miR-185-5p within the SREBP2 3’-UTR. A mutagenesis assay indicated that mutation of the seed sequence binding sites for miR-185-5p in the SREBP2 3’-UTR (SREBP2 3’-UTR-mut a and c) caused a restoration of luciferase activity, even approaching that of the wild-type 3’-UTR (P = 0.033; Figure 3A). Thus, a specific interaction between the SREBP2 3’-UTR and miR-185-5p was confirmed. In contrast, compared with the luciferase activity of wild-type SREBP2 3’-UTR, overexpression of miR-185-5p caused a more drastic repression (P = 0.021; Figure 3B). Finally, we investigated the influence of miR-185-5p on SREBP2 mRNA levels by qRT-PCR in the HepG2 cells. Overexpression of miR-185-5p mimics resulted in significantly reduced SREBP2 mRNA levels (P = 0.022; Figure 3C). Western blot analysis indicated that SREBP2 protein expression was also reduced in miR-185-5p-overexpressing cells (Figure 3D). However, the miR-185-5p inhibitor enhanced SREBP2 protein expression (Figure 3E), indicating that the SREBP2 mRNA and protein expression are regulated by miR-185-5p in HepG2 cells. In the HepG2 cell line, the effect of the miR-185-5p inhibitor was not as strong as the mimic, because the endogenous miR-185-5p expression level was low in this cell line[14]. Overall, miR-185-5p negatively regulated SREBP2 expression as a result of interaction with the SREBP2 mRNA 3’-UTR, which lead to reduced SREBP2 mRNA and protein levels.
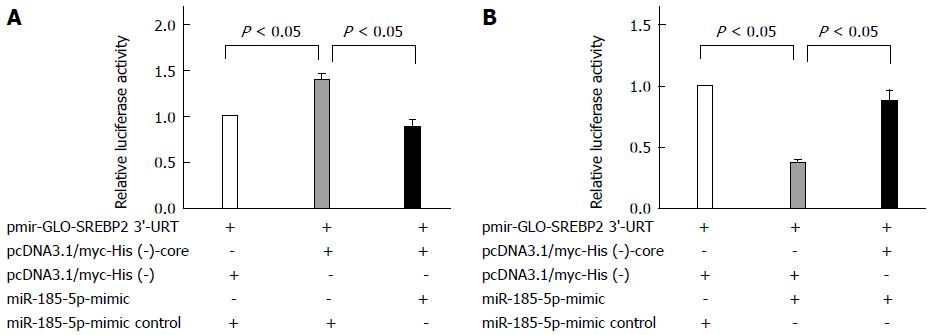
In addition, the miR-185-5p mimic inhibited the luciferase activity, which was enhanced by HCV core protein (P = 0.024; Figure 4A). Moreover, the HCV core protein upregulated the expression of SREBP2, which was repressed by the miR-185-5p mimic (P = 0.034; Figure 4B), further indicating that miR-185-5p involves the upregulation of SREBP2 by the HCV core protein.
HCV utilizes host lipids to complete its life cycle, so chronic HCV infection is closely associated with lipid disorder[15]. Accordingly, it is necessary to understand the lipid disturbance in patients with CHC in order to develop new strategies to eradicate this virus completely. Results from HCV core transgenic mice have revealed that the core protein induces steatosis[6]. The pathogenic mechanism of HCV-induced lipid accumulation is multifactorial. As reported, HCV disturbs lipid homeostasis mainly in three aspects: impairing secretion of lipoprotein, increasing lipogenesis, and impairing fatty acid degradation[16]. However, how HCV modulates hepatic cholesterol metabolism remains largely unknown. It is possible that the lipid droplets contain large amounts of cholesterol, but research data regarding the cholesterol content in livers with HCV infection are limited.
In order to investigate the changes of cholesterol metabolism caused by HCV infection, researchers have studied many key enzymes involved in the cholesterol and fatty acid metabolism. It was reported that HCV core protein enhanced the transcriptional activity of SREBP1 and PPARγ, inducing hepatic lipid accumulation in cultured cells[17]. Data from Fujino et al[18] revealed that in HCV-infected human liver, the mRNA levels of HMGCR, HMGCS, and squalene synthase were all significantly upregulated compared with controls. SREBP2 is the primary means to regulate de novo cholesterol synthesis in the liver[7,19]. In our study, it was found that HCV core protein activates transcription activity of the SREBP2 gene promoter. HMGCR is the key target regulated by SREBP2. The level of HMGCR mRNA is increased in transgenic mice overexpressing SREBPs[10]. Thus, we speculate that HMGCR expression level in core-expressing cells could be affected. It is clearly demonstrated that the mRNA and protein levels of HMGCR were significantly upregulated in HepG2 cells expressing the HCV core protein, while SREBP2 mRNA and protein levels were also significantly enhanced. At the same time, intracellular TC concentration was significantly elevated compared with control HepG2 cells. Similarly, Woodhouse et al[20] reported firstly that cholesterol content in an HCV-infected Huh-7.5 cell line was increased by 56% compared with controls. We concluded that the HCV core protein upregulated SREBP2 and HMGCR expression, resulting in increased intracellular cholesterol synthesis. The present study clearly demonstrates that HCV core protein plays an important role in cholesterol metabolism, and may be one of the mechanisms of steatosis induced by HCV infection. The transcription factor SREBP2 and the rate-limiting enzyme for cholesterol synthesis HMGCR are involved in the regulation of enhanced cholesterol synthesis induced by the HCV core protein.
Recent researchers have identified miRNAs as potent regulators of lipid metabolism genes, including cholesterol homeostasis[21]. These new data suggest that miRNAs play important roles in the regulation of cholesterol metabolism. miR-185-5p is described firstly as a regulator in cancer progression. For instance, overexpression of miR-185-5p suppressed the migration and invasiveness of LNCaP prostate cancer cells[22]. While more and more evidence reveals the connection between miR-185-5p and cancer, its role in regulating lipid metabolism remains poorly understood. More recently, it has been reported that in human hepatic cells, HDL-cholesterol uptake could be repressed by miR-185-5p selectively by inhibiting the expression of scavenger receptor B I[23]. This is the only report linking miR-185-5p to lipid metabolic regulation. On the other hand, miRNA-dependent SREBP2 post-transcriptional regulation is being explored. Yang et al[14] reported that miR-185 is involved in de novo cholesterol biosynthesis and LDL uptake, and miR-185-dependent regulation of SREBP2 requires SREBP1c for function. Consistently, we identified in our study SREBP2 as a target of miR-185-5p from multiple aspects (including at the mRNA level, protein expression, and structural basis). In other words, miR-185-5p negatively regulates SREBP2 expression as a result of interaction with the SREBP2 mRNA 3’-UTR, reducing SREBP2 mRNA and protein expression. These results complement and support each other, albeit with different focus points.
As mentioned above, HCV induces liver steatosis to enhance its replication. A series of evidence has been reported that HCV replication is suppressed in vitro by statins, competitive inhibitors of HMGCR[24]. Data from Kapadia et al[25] and Sidorkiewicz et al[26] also revealed that host cholesterol metabolism was indispensable for HCV to complete its life cycle. Therefore, for CHC patients, it is necessary to monitor and control cholesterol metabolism to tackle this viral infection. In this study, we demonstrate that the HCV core protein controls cholesterol homeostasis by regulating SREBP2 expression, and in this course, miR-185-5p plays an important role. The downregulation of miR-185-5p expression by the core protein will be confirmed further by in vitro HCV infection models (HCVcc model) or clinical samples in the future work. Best of all, the results presented in this study provide a new insight into the mechanism of hepatic steatosis by HCV infection.
Liver steatosis is the accumulation of lipid in the liver and is a frequent histologic finding in chronic hepatitis C (CHC). Hepatitis C virus (HCV) infection is a major cause of hepatic steatosis. The HCV core protein is known as an inducer of steatosis. The sterol response element binding proteins (SREBPs) play an important role in regulation of lipid synthesis and cholesterol metabolism. The influence of the HCV core protein on this transcription factor could play an important role in the development of steatosis during HCV infection. However, the pathogenesis of liver steatosis induced by HCV and its implications in the prevention and treatment of CHC remain largely unknown.
In this study, the authors investigated the regulation of expression of host genes by HCV core protein to reveal the molecular pathogenesis of HCV-induced steatosis.
This paper focuses on the intracellular cholesterol regulation by HCV core protein via SREBP2. The regulation relationship between HCV core protein and SERBP2 was established. The microRNA miR-185-5p was confirmed in the regulation of SERBP2 expression, and miR-185-5p was the target for HCV core protein. The authors also established the relationship among the HCV core protein, miR-185-5p, and SREBP2 at transcriptional, post-transcriptional, and translational levels.
HCV core protein controls cholesterol homeostasis through regulating SREBP2 expression, and in this course, miR-185-5p plays an important role. The results suggest that SREBP2 and miR-185-5p may represent a new potential target in the pathogenesis of HCV infection.
Liver steatosis is defined as accumulation of lipid in the liver. Epidemiologic studies revealed that HCV infection is a primary cause of liver steatosis. miRNAs are about 22 nucleotide single-stranded small noncoding RNAs.
In this manuscript, the author analyzed the mechanism that HCV core protein can increase intracellular cholesterol synthesis. HCV can regulate SREBP2 expression via miRNA-185-5p. That is, HCV core decreased miR-185-5p, which was suggested to be responsible for the augmentation of SREBP2 levels. The overall findings are novel and represent a new approach to tackle HCV-induced steatosis
P- Reviewer: Bo YK, Watashi K S- Editor: Qi Y L- Editor: AmEditor E- Editor: Liu XM
| 1. | Bach N, Thung SN, Schaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology. 1992;15:572-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 306] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstål R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002;37:837-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Khan M, Jahan S, Khaliq S, Ijaz B, Ahmad W, Samreen B, Hassan S. Interaction of the hepatitis C virus (HCV) core with cellular genes in the development of HCV-induced steatosis. Arch Virol. 2010;155:1735-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Reed KE, Rice CM. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Curr Top Microbiol Immunol. 2000;242:55-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 250] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001;61:4365-4370. [PubMed] [Cited in This Article: ] |
| 6. | Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527-1531. [PubMed] [Cited in This Article: ] |
| 7. | Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2786] [Cited by in F6Publishing: 2788] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 8. | Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028-30032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 545] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 9. | Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA. 2003;100:12027-12032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1021] [Cited by in F6Publishing: 1075] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 10. | Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996;98:1575-1584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 649] [Cited by in F6Publishing: 639] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3661] [Cited by in F6Publishing: 3827] [Article Influence: 239.2] [Reference Citation Analysis (0)] |
| 12. | Fernández-Hernando C, Suárez Y, Rayner KJ, Moore KJ. MicroRNAs in lipid metabolism. Curr Opin Lipidol. 2011;22:86-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 13. | Imam JS, Buddavarapu K, Lee-Chang JS, Ganapathy S, Camosy C, Chen Y, Rao MK. MicroRNA-185 suppresses tumor growth and progression by targeting the Six1 oncogene in human cancers. Oncogene. 2010;29:4971-4979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Yang M, Liu W, Pellicane C, Sahyoun C, Joseph BK, Gallo-Ebert C, Donigan M, Pandya D, Giordano C, Bata A. Identification of miR-185 as a regulator of de novo cholesterol biosynthesis and low density lipoprotein uptake. J Lipid Res. 2014;55:226-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Ye J. Reliance of host cholesterol metabolic pathways for the life cycle of hepatitis C virus. PLoS Pathog. 2007;3:e108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | de Gottardi A, Pazienza V, Pugnale P, Bruttin F, Rubbia-Brandt L, Juge-Aubry CE, Meier CA, Hadengue A, Negro F. Peroxisome proliferator-activated receptor-alpha and -gamma mRNA levels are reduced in chronic hepatitis C with steatosis and genotype 3 infection. Aliment Pharmacol Ther. 2006;23:107-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Kim KH, Hong SP, Kim K, Park MJ, Kim KJ, Cheong J. HCV core protein induces hepatic lipid accumulation by activating SREBP1 and PPARgamma. Biochem Biophys Res Commun. 2007;355:883-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Fujino T, Nakamuta M, Yada R, Aoyagi Y, Yasutake K, Kohjima M, Fukuizumi K, Yoshimoto T, Harada N, Yada M. Expression profile of lipid metabolism-associated genes in hepatitis C virus-infected human liver. Hepatol Res. 2010;40:923-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA. 1999;96:11041-11048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1019] [Cited by in F6Publishing: 998] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 20. | Woodhouse SD, Narayan R, Latham S, Lee S, Antrobus R, Gangadharan B, Luo S, Schroth GP, Klenerman P, Zitzmann N. Transcriptome sequencing, microarray, and proteomic analyses reveal cellular and metabolic impact of hepatitis C virus infection in vitro. Hepatology. 2010;52:443-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Moore KJ, Rayner KJ, Suárez Y, Fernández-Hernando C. microRNAs and cholesterol metabolism. Trends Endocrinol Metab. 2010;21:699-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Qu F, Cui X, Hong Y, Wang J, Li Y, Chen L, Liu Y, Gao Y, Xu D, Wang Q. MicroRNA-185 suppresses proliferation, invasion, migration, and tumorigenicity of human prostate cancer cells through targeting androgen receptor. Mol Cell Biochem. 2013;377:121-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Wang L, Jia XJ, Jiang HJ, Du Y, Yang F, Si SY, Hong B. MicroRNAs 185, 96, and 223 repress selective high-density lipoprotein cholesterol uptake through posttranscriptional inhibition. Mol Cell Biol. 2013;33:1956-1964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Delang L, Paeshuyse J, Vliegen I, Leyssen P, Obeid S, Durantel D, Zoulim F, Op de Beeck A, Neyts J. Statins potentiate the in vitro anti-hepatitis C virus activity of selective hepatitis C virus inhibitors and delay or prevent resistance development. Hepatology. 2009;50:6-16. [PubMed] [Cited in This Article: ] |
| 25. | Kapadia SB, Barth H, Baumert T, McKeating JA, Chisari FV. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J Virol. 2007;81:374-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Sidorkiewicz M, Józwiak B, Durys B, Majda-Stanislawska E, Piekarska A, Kosciuk N, Ciechowicz J, Majewska E, Bartkowiak J. Mevalonate pathway modulation is associated with hepatitis C virus RNA presence in peripheral blood mononuclear cells. Virus Res. 2009;145:141-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |