Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15327
Revised: May 8, 2014
Accepted: June 13, 2014
Published online: November 7, 2014
AIM: To investigate inflammatory injury in the intestinal mucosa after intestinal ischemia-reperfusion (IIR) with Toll-like receptor (TLR)-mediated innate immunity.
METHODS: Ten macaques were randomized into control and IIR groups. The distribution and expression level of TLR2, TLR4, MD2, nuclear factor (NF)-κB p65 and interferon (IFN)-γ were measured by immunohistochemical stain and western blotting. The mRNA expression of TLR4, TLR2, MD2, interleukin (IL)-1β and tumor necrosis factor (TNF)-α were measured by reverse transcriptase-polymerase chain reaction. The cytokine levels in blood and intestinal tissues were measured by ELISA.
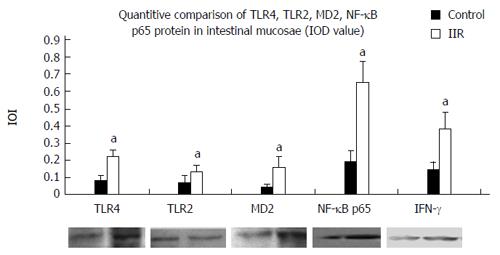
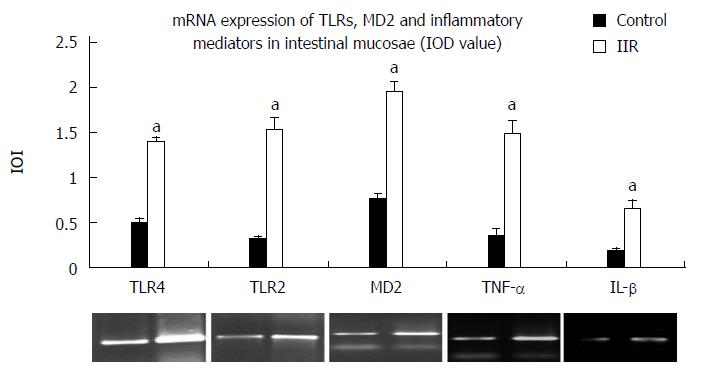
RESULTS: Obvious hemorrhage and erosion of mucosae were seen in the IIR group. Expression of TLR2, TLR4, MD2, NF-κB p65 and IFN-γ was significantly higher in the IIR group than in the control group (0.13 ± 0.04, 0.22 ± 0.04, 0.16 ± 0.06, 0.65 ± 0.12, 0.38 ± 0.10 vs 0.07 ± 0.04, 0.08 ± 0.03, 0.04 ± 0.02, 0.19 ± 0.06, 0.14 ± 0.05, P < 0.05). In addition, the expression of TLR2, TLR4, MD2, IL-1β and TNF-α mRNA in the IIR group were significantly higher than those of control group(1.52 ± 0.15, 1.39 ± 0.06, 1.94 ± 0.12, 1.48 ± 0.15, 0.66 ± 0.08 vs 0.31 ± 0.05, 0.5 ± 0.04, 0.77 ± 0.05, 0.35 ± 0.08, 0.18 ± 0.04, P < 0.05). Furthermore, IL-1β, IL-6 and TNF-α levels in the macaques ileum and plasma were significantly higher than in the control group (plasma: 86.3 ± 15.2, 1129 ± 248.3, 77.8 ± 16.2 vs 29.5 ± 7.3, 19.8 ± 8.2, 5.6 ± 1.7; ileum: 273.4. ± 44.7, 1636 ± 168.0, 205.5 ± 30.7 vs 76.8 ± 20.5, 663.4 ± 186.9, 49.0 ± 9.4; P < 0.05).
CONCLUSION: After IIR, general inflammatory injury in the intestinal mucosa is correlated with a strong innate immune response, mediated by activation of the TLR-NF-κB-cytokine pathway.
Core tip: After intestine ischemia-reperfusion, general inflammatory injury of the intestinal tract, and the ensuing multiple organ dysfunction syndrome, are correlated with a strong innate immune response, which is mediated by the extensive activation of the Toll-like receptor-nuclear factor-κB-cytokine pathway throughout the whole system.
- Citation: Wu H, Deng YY, Liu L, Tan QH, Wang CH, Guo MM, Xie YM, Tang CW. Intestinal ischemia-reperfusion of macaques triggers a strong innate immune response. World J Gastroenterol 2014; 20(41): 15327-15334
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15327
Multiple organ dysfunction syndrome (MODS) is the most severe complication of trauma, infection, or severe acute pancreatitis, and it has a high mortality rate. The intestinal tract is not only the target organ injured, but also the root of a systematic inflammatory reaction and a regulator of the internal environment under stress. For this reason, the gastrointestinal tract is thought to be an activating organ in the onset of MODS[1,2]. Damage to the intestinal mucosa barrier and bacteria translocation remain the chief mechanisms of MODS theory[3,4].
According to the theory of intestinal mucosa barrier injury and bacterial translocation, intestinal damage should occur in the colon. In a previous study we noticed general inflammatory damage to the intestine, but only slight damage to the right colon after occluding the superior mesenteric artery (SMA) during MODS caused by intestinal ischemia-reperfusion (IIR) of macaques[5]. As the SMA supplies both the intestine and the right colon[6], the difference in the tissue damage cannot be explained by the theory of local oxygen metabolism disturbance, oxygen free radical damage, or bacteria toxins. The intestine is the biggest immune organ in the whole body, and the fact that general inflammatory damage to the mucosa occurred rapidly after IIR reminds us of the possibility that the innate immune response may play a role in this damage.
The innate immune response acts rapidly, which can protect the body from injury from microorganisms and harmful substances. Widespread Toll-like receptors (TLRs) are thought to be signal recognition receptors for the innate immune system, which activate nuclear factor (NF)-κB and induce transcription and expression of immune-response genes and inflammatory cytokines after combining with pathogen-associated molecular patterns (PAMPs), and then start an inflammatory reaction[7,8]. Lipopolysaccharide (LPS) is one of the most widely recognized PAMPs. In previous studies, TLR4 and TLR2 were found to be the general receptors for LPS[9].
To investigate whether the general inflammatory damage following IIR and subsequent MODS is caused by an innate immune response mediated by TLRs in the intestine, we observed the change of TLR4 and TLR2 expression in the intestinal mucosa after inducing IIR in macaques, and analyzed the relationship between TLR4/TLR2 and NF-κB p65 and cytokines.
Ten adult rhesus macaques (body weight, 6.92 ± 1.67 kg, aged 4-7 years) were provided by the Experimental Animal Center of Sichuan University, and the study was approved by the Animal Ethics Committee of West China Hospital of Sichuan University. The animals were randomly divided into the IIR group and control group (n = 5 each, male/female ratio 3:2). Before surgery, all groups of animals were fasted for 12 h with free access to water and then anesthetized through xylazine (0.2 mL/kg, intramuscularly) and maintained with carbrital [20 mg/kg, intravenously (iv)] and diazepam (0.1 mL/kg, iv) as needed. Upon satisfactory anesthesia, the animal was fixed on an operating table for skin preparation of the abdominal surgical area, followed by routine disinfection and draping.
IIR group: An incision was made right in the middle of the upper abdomen of rhesus macaques. The duodenum was exposed, lifted, and pulled. The SMA was isolated and then occluded with a microsurgical clip. One hour later, the clip was removed, and intestinal perfusion was reestablished.
Control group: The same procedure as for the IIR group except for occlusion of SMA with a microsurgical clip.
After surgery, animals in both groups received infusion of saline and glucose (0.2 mL/kg per minute, iv glucose tolerance test) for 24 h. Venous blood samples were taken, the macaques were killed 24 h after surgery, and the vital organs was removed for further examination.
Terminal ileum and the right colon specimens, which were fixed in 4% paraformaldehyde, were dehydrated, embedded, and sectioned following routine procedure. After HE staining, tissue sections were examined by microscopy. For semiquantitative evaluation of lesions, 10 randomly selected microscopic fields were observed carefully in each sample. The scoring system was based on the area of the lesion as follows: +, < 1/3 total area of field; ++, 1/3-2/3 total area of field; and +++, > 2/3 total area of field. The remaining parts of the specimens were stored in a freezer at -70 °C.
The terminal ileum and the right colon tissue sections of macaques were used for the immunohistochemical examinations. Frozen 10-μm terminal ileum tissue sections were cut in a cryostat and placed on a glass slide. The sections were fixed in 100% methanol for 5 min at -20 °C, treated with 0.1% goat serum for 20 min at 20 °C, and then treated for 8 h at 4 °C with PBS containing 0.1% goat serum and the following primary antibodies: rabbit anti-human TLR4/TLR2 (1:100), rabbit anti-human MD2 (1:100), rabbit anti-human NF-κB p65 (1:100) and rabbit anti-human IFN-γ (1:100) (Santa Cruz Biotechnology, Santa Cruz, CA, United States). After treatment with primary antibodies the sections were washed three times in PBS for 10 min and stained for 0.5 h at 20 °C with a ready-to-use streptavidin-catalase immunohistochemical reagent system as a detection reagent. Color reactions were developed with diaminobenzidine (DAB) (Zhongshan Bioagent Company, Beijing, China). A semiquantitative immunohistochemical analysis of initial data with Image-Pro Plus 4.0 software was used to score integrated optical density (IOD) of the ileal epithelium. Each value that appears in the results is the mean of five visual fields in which duplicate measurements were made.
The quantities of TLR4, TLR2 and MD2 protein extracted from isolated ileum epithelial cells were measured by western blotting. Protein samples were separated by 12% SDS-PAGE. Samples were transferred to nitrocellulose membranes, which were then blocked for 2 h in 5% nonfat dry milk suspended in 0.1% Tween-20 Tris-buffered saline (TTBS, pH 7.4). Nitrocellulose membranes were incubated with rabbit polyclonal TLR4/TLR2 antibody (1:500), MD2 (1:500), NF-κB p65 (1:1000), IFN-γ (1:500), or β-actin (Cell Signaling Technology, Boston, MA, United States), at 4 °C overnight. Membranes were washed in TTBS, incubated with horseradish-peroxidase-conjugated secondary antibody (Univ-bio, Shanghai, China), and developed using enhanced chemiluminescence reagents. The objective bands were analyzed by a UVIB (ultraviolet irradiation band) imaging system and were normalized to 1 IOD unit.
Total RNA was extracted from terminal ileum tissue specimens using TRIzol reagent (Roche, Burlington, NC, United States). The purity of RNA extract was tested by spectrophotometry analysis of optical density (OD, OD260/OD280 = 1.95-2.0). Reverse transcription polymerase chain reaction (RT-PCR) amplification were conducted with PTC-100 PCR (Bio-Rad Laboratories, Hercules, CA, United States), in accordance with the illustrations of the RT-PCR core kit (TaKaRa, Shiga, Japan). The sequences of primers and PCR products are listed in Table 1[10,11]. The obtained cDNA (2 μL each) was used as a template for PCR amplification with β-actin as an internal reference. The PCR products (2 μL each) were electrophoresed on 2% agarose gel, and quantified by scanning the gel in an imaging system (Bio-Rad Gel Doc 2000). The data were standardized as a ratio of gray scale (IOD) of objective band to β-actin.
| mRNA | Sense | Antisense | Size (bp) |
| TLR4 | TGCAATGGATCAAGGACCAGAGGC | GTGCTGGGACACCACAACAATCACC | 449 |
| TLR2 | GGCCAGCAAATTACCTGTGTG | CTGAGCCTCGTCCATGGGCCACTCC | 637 |
| MD2 | GAAGCTCAGAAGCAGTATTGGGTC | GGTTGGTGTAGGATGACAAACTCC | 422 |
| IL-1β | AAACAGATGAAGTGGTCCTTCCAGG | TGGAGAACACCACTTGTTGCTCCA | 388 |
| TNF-α | AGGGCTCCAGGCGGTGCTTG | TGGTAGGAGACGGCGATGCGG | 418 |
| β-actin | CACCACACCTTCTACAATGAGC | GTGATCTCCTTCTGCATCCTGT | 695 |
The levels of IL-1β, IL-6, and TNF-α in plasma and intestinal were measured by ELISA (Senxiong Company, Shanghai, China), according to the manufacturer’s instructions. The plasma levels of cytokines were standardized as pg/mL. The ileal concentration of cytokines was standardized as pg/g protein.
All experimental data were processed in SPSS version 17.0 (SPSS, Chicago, IL, United States). Data were expressed as mean ± SD and subjected to tests for homogeneity of variance and normality (P > 0.05 for both). Intergroup comparisons were performed using one-way analysis of variance, and significant difference among means was identified by Fisher LSD test at the level of α = 0.05. P < 0.05 was considered statistically significant.
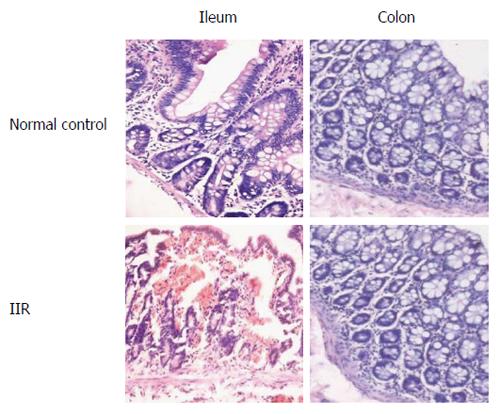
In the IIR group, rhesus macaques presented with obvious distension of the small intestine, pale mucous membrane, hemorrhage, and erosion in the small intestine, compared with the control group. No apparent changes were found in the colon of both groups. Marked mucosal inflammatory injury of the ileum, including increased macrophage and neutrophil infiltration into the mucosa, erosion or necrosis, and hemorrhage of the intestinal mucosa were observed under the microscope. In the IIR group, rhesus macaques showed significantly higher inflammatory lesion scores compared with the control group (P < 0.05) (Table 2). In contrast, inflammatory lesion scores of right colon were minor and no significant difference in both groups (Figure 1).
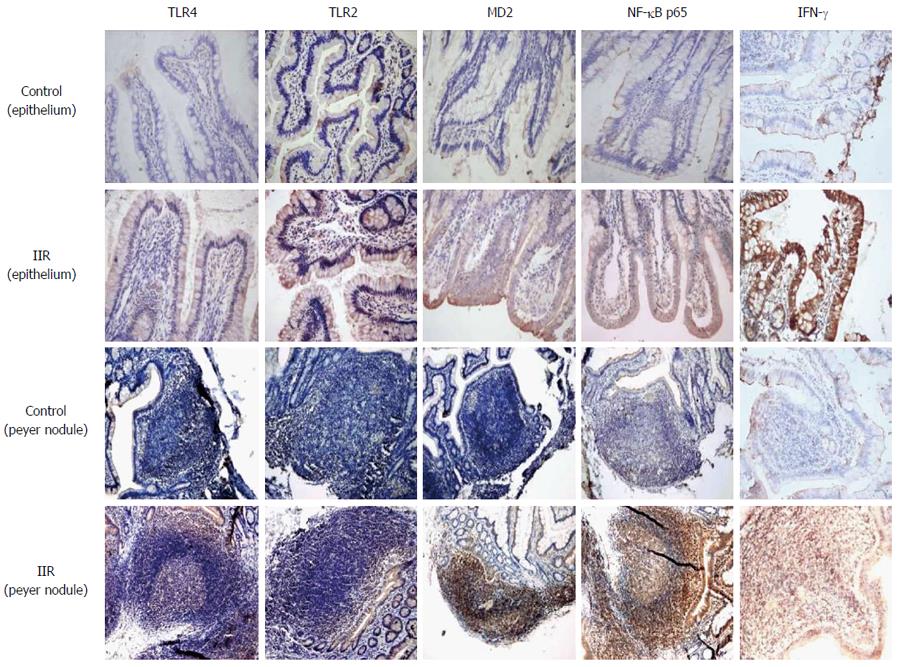
Only weak expression of TLR2, TLR4, MD2, NF-κB p65 and IFN-γ was observed by immunohistochemistry on normal epithelium and lamina propria. These protein molecules on the inflammation signal transcription pathway inside the whole intestinal mucosa, including the epithelium, lamina propria and Peyer’s nodules displayed strong expression after IIR (Figure 2).
Western blotting showed IOD values of TLR2, TLR4, MD2, NF-κB p65 and IFN-γ were significantly higher after IIR than in the control group (P < 0.05, Figure 3, Table 3).
After IIR, the expression of TLR2, TLR4, MD2, IL-1β and TNF-α mRNA in the macaques intestinal tissue were higher than in the control group (P < 0.05; Figure 4, Table 4).
IL-1β, IL-6 and TNF-α were significantly higher in peripheral plasma and small intestine mucosae after IIR than in the control group (P < 0.05; Table 5).
A balance is normally kept between the human body and the intestinal flora and microorganisms inside it. Few bacteria can survive in the small intestine because of the mechanical scouring due to bowel movements and the integrated bacteria-killing mechanisms of the upper gastrointestinal tract, especially bile[12]. Even though macaques eat more unclean food than humans, the germ-free rate measured in our previous study of healthy macaque ileum bacteria groups was as high as 40%[13]. TLR4, TLR2 and MD2 were found to be slightly expressed in macaque intestinal mucosal epithelia, but were almost negative in Peyer’s nodules. This low expression was because, due to the mild stimulus that microorganisms provide to normal intestinal mucosae, the TLR-NF-κB cytokine signal transduction pathway in intestinal epithelia and antigen-presenting cells was in a mildly activated state, which was observed as a physiological inflammatory reaction.
We have seen from observation of bacterial counts and spectra in the small intestine during IIR that ileal bacteria increase dramatically, by a factor of 106 after IIR. This increase is predominantly in aerobic bacteria, among which Escherichia coli (E. coli) increased by a factor of 105[13]. The abundant LPS of E. coli creates essential conditions for extensive activation of TLR4. We know that the recognition of LPS by TLR4 needs mediation of MD2 and CD14 on the cell surface and formation of an LPS recognition compound[14]. MD2 is one kind of secretory protein that exists on the surface of cell membranes, which helps TLR4 to recognize LPS/LBP/CD14 compounds and to lock LPS onto the combining site, so the presence of MD2 can increase the sensitivity of TLR4 to LPS[15-17]. Mutation of MD2 can induce non-response of TLR4-expressing cells to LPS[18], which is why MD2 plays an important role in the function of TLR4. We found in the present study that MD2 expression in the intestine following IIR increased dramatically, and assisted LPS in activating inactive TLR4. We thus draw the conclusion that the combined upregulation of TLR4-MD2 expression is one of the important factors in the innate immunity mediated by TLRs following IIR in macaques. TLR2 can identify broad-spectrum microbial products - mainly cell wall constituents of Gram-positive bacteria - such as peptidoglycan and lipoteichoic acid, and also LPS. Gram-negative bacteria, however, are predominant in the small intestines of macaques, and the amount of enterococci is also double. TLR2 in intestinal mucosae following IIR are activated, although the activation degree is not as intense as that of TLR4.
Previous studies have told us that immunological effect factors generated in the TLR-NF-κB cytokine signal transduction pathway, such as IL-1, IL-6, IL-8, TNF-α and IFN-γ, can further activate TLRs in addition to their bacterial activity and phagocytosis ability. This positive feedback continues in a cycle and amplifies inflammation of the mucosa[19,20]. Our study showed an obvious increase of TLR4 and TLR2 expression in intestinal epithelia and lymph tissues, either at the gene transcription or protein level, while at the same time, the expression of NF-κB and proinflammatory cytokines IL-1β, IL-6 and TNF-α also increased, and these increases showed good correlation. IIR accelerated the whole process of mRNA transcription, protein molecular expression, and functioning of immunological factors in the TLR-NF-κB-cytokine pathway in mucosal epithelia, which on the one hand fully displayed the characteristics of a fast reaction of the innate immune system, and on the other hand, the positive feedback of releasing inflammatory mediators of TLR expression induced a stream of inflammatory mediators. The dramatic increase in peripheral inflammatory mediators by a factor of 20-100, induced by the rapid and severe innate immune reaction in intestinal mucosae, should be the basis of MODS theory.
Organs and tissues may be severely harmed by inflammatory mediators produced by an innate immune response. The inter-reaction between inflammatory mediators and inflammatory cells increases vascular permeability, and causes tissue edema and reduction of vaso-quantity per unit volume, which aggravates cellular anoxia and induces tissue lesions; the large amount of enzymes and inflammatory products released by phagocytes directly injures tissue; also, increased cytokines tend to induce hypercoagulability, which is why extensive micro-thromboembolism can often be seen in the internal organs, especially the liver and lungs, of MODS patients[21-23]. The inhibition of cardiac contractility by abundant TNF-α, IL-1β and IL-6 causes changes in hemodynamics such as blood pressure decrease, tachycardia, and left ventricular ejection fraction decrease, and these are clinically manifest as hypotension and low cardiac output[24]. We observed that TLR-mediated inflammation following IIR damaged the integrity of the small intestinal mucosal barrier, and that ingression of propagative E. coli from the intestinal cavity into tissue or even the bloodstream led to bacterial translocation and bacteremia[13]. In addition, the integrity of the small intestinal mucosal barrier prevents contact of TLRs on B cells in Peyer’s nodules with bacteria, and expression of TLRs was low. Although this integrity is damaged because of a local innate immune response, an opportunity emerges for TLRs on B cells in Peyer’s nodules beneath the mucosal epithelium to come into contact with a large amount of pathogenic microorganisms. So, during IIR, not only the epithelium strongly expresses TLR4 and TLR2. Rather, TLR4 and TLR2 on B cells in Peyer’s nodules are also significantly activated. NF-κB shows a strong positive reaction, therefore, B cells are involved in the innate immune response, which aggravates local mucosal inflammatory reactions.
In conclusion, all macaques developed MODS following IIR, and this was related to the expansion of the severe innate immune response, which was mediated by the general activation of the TLR-NF-κB-cytokine pathway in the small intestinal mucosa throughout the whole system.
Intestinal ischemia-reperfusion (IIR) is the early pathophysiological process of multiple critical illnesses, such as severe acute pancreatitis or septic shock. IR may cause intense inflammatory reaction following by multiple organ function failure and even death, although the mechanism of this excess inflammatory reaction remains unclear.
The innate immune response mediated by Toll-like receptors (TLRs) results in rapid release of a large amount of inflammatory cytokines by activating the TLR-nuclear factor (NF)-κB cytokine signaling pathway. An increasing number of studies indicate that the innate immune response participates in the onset of various illnesses and plays a key role in excessive inflammatory reaction.
The change in intestinal flora after IIR activates the innate immune response mediated by TLRs, which leads to an excessive systemic inflammatory reaction.
Interruption of the TLR-NF-κB cytokine signaling pathway may adjust the intensity of systemic inflammatory reaction during IIR and protect the body from deterioration.
TLRs are thought to be signal recognition receptors for the innate immune system, which activate NF-κB and induce transcription and expression of immune-response genes and inflammatory cytokines after combining with pathogen-associated molecular patterns, and then start an inflammatory reaction.
The study revealed that after IIR, general inflammatory injury of the intestinal tract, and the ensuing multiple organ dysfunction syndrome, are correlated with a strong innate immune response, which is mediated by extensive activation of the TLR-NF-κB-cytokine pathway. The findings are interesting and novel. The methods used in the study properly corresponded to the task of the study. Immunohistochemistry was well performed, but the magnification of the images shown is too low to see the details of the distribution of the analyzed markers. High-resolution immunohistochemical images showing the expression of TLR4, TLR2, MD2, NF-κB and IFN-γ should be provided.
P- Reviewer: Bobryshev YV, Hershcovici T S- Editor: Ma YJ L- Editor: Kerr C E- Editor: Liu XM
| 1. | Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005;26:383-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Bahrami S, Redl H, Yao YM, Schlag G. Involvement of bacteria/endotoxin translocation in the development of multiple organ failure. Curr Top Microbiol Immunol. 1996;216:239-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Kannan KB, Colorado I, Reino D, Palange D, Lu Q, Qin X, Abungu B, Watkins A, Caputo FJ, Xu DZ. Hypoxia-inducible factor plays a gut-injurious role in intestinal ischemia reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;300:G853-G861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Tan Q, Hu B, Fan H, Wu H, Xu L, Tang C. Histopathological and functional changes of vital organs from macaques with intestinal ischemia-reperfusion. Zhongguo Shiyong Neike Zazhi. 2006;18:1407-1410. [DOI] [Cited in This Article: ] |
| 6. | Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J. 2005;98:217-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 524] [Cited by in F6Publishing: 505] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3900] [Cited by in F6Publishing: 3726] [Article Influence: 138.0] [Reference Citation Analysis (0)] |
| 9. | Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 591] [Cited by in F6Publishing: 588] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 10. | Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 248] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Isaacs KL, Sartor RB, Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992;103:1587-1595. [PubMed] [Cited in This Article: ] |
| 12. | Thadepalli H, Lou MA, Bach VT, Matsui TK, Mandal AK. Microflora of the human small intestine. Am J Surg. 1979;138:845-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 56] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Fan H, Wu H, Tan QH, Hu B, Tang CW. Relevance of Intestinal Mucosal Barrier Damage and Alteration of Luminal Microflora after Intestinal Ischemia Reperfusion Injury in Macaque. Weichangbingxue Zazhi. 2006;11:268-272. [DOI] [Cited in This Article: ] |
| 14. | Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777-1782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1571] [Cited by in F6Publishing: 1513] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 15. | Yang H, Young DW, Gusovsky F, Chow JC. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J Biol Chem. 2000;275:20861-20866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 216] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667-672. [PubMed] [Cited in This Article: ] |
| 17. | Konno K, Wakabayashi Y, Akashi-Takamura S, Ishii T, Kobayashi M, Takahashi K, Kusumoto Y, Saitoh S, Yoshizawa Y, Miyake K. A molecule that is associated with Toll-like receptor 4 and regulates its cell surface expression. Biochem Biophys Res Commun. 2006;339:1076-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Schromm AB, Lien E, Henneke P, Chow JC, Yoshimura A, Heine H, Latz E, Monks BG, Schwartz DA, Miyake K. Molecular genetic analysis of an endotoxin nonresponder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J Exp Med. 2001;194:79-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Campos MA, Rosinha GM, Almeida IC, Salgueiro XS, Jarvis BW, Splitter GA, Qureshi N, Bruna-Romero O, Gazzinelli RT, Oliveira SC. Role of Toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infect Immun. 2004;72:176-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Aldrich MB, Sevick-Muraca EM. Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine. 2013;64:362-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26:711-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Li A, Xiong J, Chen Z. IL-6, TNF-α, and iNOS is associated with decreased colonic contraction in rats with multiple organ dysfunction syndrome. J Surg Res. 2012;178:e51-e57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Johnson CD, Kingsnorth AN, Imrie CW, McMahon MJ, Neoptolemos JP, McKay C, Toh SK, Skaife P, Leeder PC, Wilson P. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Natanson C, Eichenholz PW, Danner RL, Eichacker PQ, Hoffman WD, Kuo GC, Banks SM, MacVittie TJ, Parrillo JE. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989;169:823-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 457] [Article Influence: 13.1] [Reference Citation Analysis (0)] |