Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6658
Revised: February 25, 2014
Accepted: March 12, 2014
Published online: June 7, 2014
AIM: To identify the influence of the surgery type and prognostic factors in middle and distal bile duct cancers.
METHODS: Between August 1990 and June 2011, data regarding the clinicopathological factors of 194 patients with surgical and pathological confirmation were collected. A total of 133 patients underwent resections (R0, R1, R2; n = 102, 24, 7), whereas 61 patients underwent nonresectional surgery. Either pancreaticoduodenectomy (PD) or bile duct resection (BDR) was selected according to the sites of tumors and co-morbidities of the patients after confirming resection margin by the frozen histology in all cases. Univariate and multivariate analyses of clinicopathologic factors were performed, utilizing the Kaplan-Meyer method and Cox hazard regression analysis.
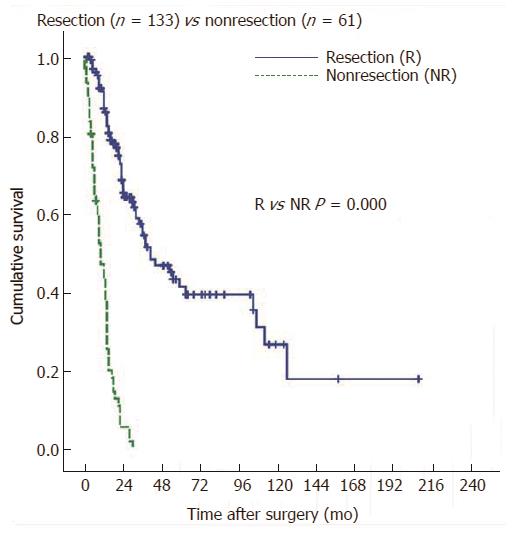
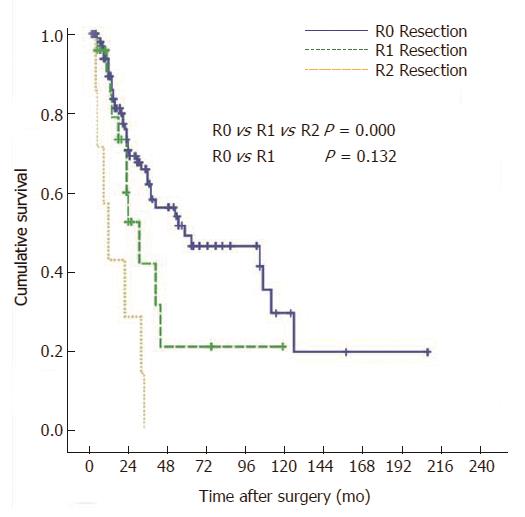
RESULTS: The overall 5-year survival rate for the 133 patients who underwent resection (R0, R1, and R2) was 41.2%, whereas no patients survived longer than 3 years among the 61 patient who underwent nonresectional surgeries. The 5-year survival rate of the patients who underwent a PD (n = 90) was higher than the rate of those who underwent BDR (n = 43), although the difference was not statistically significant (46.6% vs 30.0% P = 0.105). However, PD had a higher rate of R0 resection than BDR (90.0% vs 48.8%, P < 0.0001). If R0 resection was achieved, PD and BDR showed similar survival rates (49.4% vs 46.5% P = 0.762). The 5-year survival rates of R0 and R1 resections were not significantly different (49.0% vs 21.0% P = 0.132), but R2 resections had lower survival (0%, P = 0.0001). Although positive lymph node, presence of perineural invasion, presence of lymphovascular invasion (LVI), 7th AJCC-UICC tumor node metastasis (TNM) stage, and involvement of resection margin were significant prognostic factors in univariate analysis, multivariate analysis identified only TNM stage and LVI as independent prognostic factors.
CONCLUSION: PD had a greater likelihood of curative resection and R1 resection might have some positive impact. The TNM stage and LVI were independent prognostic factors.
Core tip: The prognosis in bile duct cancer is unfavorable and varies according to the type of surgery, curability, and pathological factors. We analyzed data collected over a period of 22 years that provide valuable information regarding the prognosis. We show that pancreaticoduodenectomy (PD) has a higher chance of curative resection and suggest that BDR should be applied only to tumors located around the cystic duct or in patients with comorbidity precluding PD. Tumor node metastasis stage and lymphovascular invasion are independent prognostic factors. We believe this study to be of great value for the physician and surgeon treating patients with these rare tumors.
- Citation: Kwon HJ, Kim SG, Chun JM, Lee WK, Hwang YJ. Prognostic factors in patients with middle and distal bile duct cancers. World J Gastroenterol 2014; 20(21): 6658-6665
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6658.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6658
Cholangiocarcinoma is a rare malignant tumor of the biliary tree that can arise anywhere from the intrahepatic to the extrahepatic bile duct just proximal to the duodenal ampulla, excluding the gallbladder. Extrahepatic bile duct cancer, defined as the presence of malignant tumors arising at the biliary tree distal to second-order branches, accounts for 80% to 90% of all cholangiocarcinomas. Extrahepatic bile duct cancer can be further divided into hilar or middle/distal bile duct cancers. Among these extrahepatic bile duct cancers, middle and distal bile duct cancers comprise approximately 20% to 30%[1-4].
The site of an extrahepatic bile duct cancer has clinical importance because it affects the selection of the appropriate type of surgical resection and the outcomes after surgery. Compared to hilar bile duct cancer, which requires concomitant bile duct and liver resection, the surgical resection for a middle and distal bile duct cancer requires either segmental bile duct resection or pancreaticoduodenectomy with lymph node dissection. The type of surgery selected depends on the possibility of achieving tumor-free resection margins, and the prognostic influence of the type of resection on long-term prognosis has been controversial[5,6].
In some patients, the superficial spreading nature of the disease, as well as comorbid diseases, can make it hard to achieve R0 resection, inevitably resulting in R1 resection. Because such cases have been anecdotal, the effect of R1 resection on long-term prognoses must be investigated.
Factors such as tumor node metastasis (TNM) stage, perineural invasion, and lymphovascular invasion have been reported to affect survival, but their influences on prognosis have not always been universal. Tumors with higher T stages tend to run a greater risk of distant metastasis and poorer prognosis, but in some cases, tumors are confined to the bile duct without lymph node metastasis, showing early recurrence in distant areas, such as the liver, lungs, or multiple bones. The unpredictable prognoses of such cases might be explained by bile duct cancer progressing not only by directly invading into the depths of the bile duct wall but also into the perineural, vascular, and lymphatic spaces[7]. Nagahashi et al[8] recently insisted that the presence of lymphovascular invasion resulted in poor prognosis, comparing a pT1 tumor invading the fibromuscular layer to a pT1 tumor confined to the mucosa. The role of such factors as perineural invasion (PNI) and low viscosity index (LVI) must be investigated.
In this study, we analyzed the clinicopathological data of 133 patients who underwent surgical resection for middle and distal bile duct cancers, to identify the influence of the type of surgery selected and of clinicopathological factors on the long-term prognoses of the patients.
Between August 1990 and June 2011, a total of 194 patients with middle and distal bile duct cancers underwent surgery and were pathologically diagnosed in the Department of Surgery, Kyungpook National University Hospital, Daegu, South Korea. Data regarding the clinicopathological factors of the patients were obtained by retrospective review of medical records.
The preoperative diagnosis of middle and distal bile duct cancer was made by imaging studies, including an abdominal computed tomography scan, magnetic resonance imaging, and positron emission tomography. Preoperative endoscopic retrograde cholangiopancreatography was performed to decompress jaundice and to obtain tissue diagnosis whenever possible. The sites of tumors were determined by imaging studies. Middle and distal bile duct cancer was defined by imaging studies and by the operative findings of tumors with the main lesions located at middle and distal third of extrahepatic bile duct. Tumors that were grossly identified to extend toward the hilar bifurcation during surgery were excluded from this study.
In all of the cases, the resection margins were sent for frozen biopsy. Resection of the remnant tissue with microscopic involvement of carcinoma in situ or with invasive carcinoma was defined as R1 resection, and resection of remnant tissue with gross involvement was defined as R2 resection.
Pancreaticoduodenectomy was performed for tumors located in the distal third of the extrahepatic bile duct. When proximal resection margins were microscopically negative, we proceeded to perform PD. If positive, the proximal bile duct was repeatedly resected until a negative margin was achieved. If a negative margin could not be achieved from the uppermost bile duct, we tried to perform an R1 or R2 PD in selected cases.
Either PD or BDR was performed for tumors in the middle third of extrahepatic bile duct. When the proximal resection margin was microscopically negative, we proceeded to resect the bile duct, including the tumor. If the lowest distal resection margin was negative after BDR, no additional resection was performed; however, if the lowest resection margin was positive, PD was added unless the patient had a serious comorbidity precluding an additional PD. When the proximal resection margin was microscopically positive, the proximal bile duct was repeatedly resected until a negative margin was achieved. If a negative margin could not be achieved from the uppermost bile duct, we performed R1 or R2 BDR. Lymph node dissection was routinely performed around the hepatoduodenal ligament, common hepatic artery, and retropancreas when resection was possible.
Nonresectional surgeries, including exploration only, simple cholecystectomy, and bypass surgery, were indicated for patients with very advanced bile duct carcinoma. These cases included tumors directly invading the common hepatic artery, superior mesenteric artery, inferior vena cava, long segment, or more than half the circumference of the portal vein, as well as tumors with peritoneal or liver metastases.
Follow-up examinations were performed based on abdominal ultrasonography, computed tomography, and measurement of the serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels every 3 to 6 mo None of the patients received chemotherapy before or after surgery. Information on long-term outcomes after surgery was collected by personal interview or on the telephone. If a patient died, we recorded the survival time after surgery and the cause of death. For surviving patients, the postoperative length of survival and status of recurrence were recorded.
A prognostic analysis was performed using the data from 133 patients who underwent resectional surgery including R0, R1 and R2 resection. For the survival analysis after surgical resection, the patients with non-resectional surgery or mortality cases were excluded from this study. The survival data were processed using the Kaplan-Meier method and were compared using the log-rank test. A P value less than 0.05 was considered statistically significant. Multivariate analysis was performed using the clinicopathologic factors that were statistically significant in univariate analysis or other marginal predictors, which were obtained using Cox proportional hazards regression.
In total, the study enrolled 120 men and 74 women, and the mean age was 66.4 ± 8.6 years old. Among these 194 patients, 133 patients received resection, and the resection rate was 68.6%. Ninety patients (67.7%, 90/133) underwent PD, and 43 (32.3%) patients underwent BDR. The remaining 61 patients underwent nonresectional surgeries, such as bypass, cholecystectomy, or exploration only.
The numbers of patients with R0, R1, and R2 resections were 102, 24, and seven, respectively. The PD group had a higher rate of R0 resection than the BDR group [90% (81/90) vs 48.8% (21/43) P < 0.0001]. In detail, the PD group included eight patients with R1 and one patient with R2 PD, whereas the patients who underwent BDR included 16 patients with R1 and six patients with R2 BDR. The reason for R1 PD and R1 BDR was a microscopic positive margin at the uppermost resection margin in eight and 12 patients, respectively. The remaining four patients with R1 BDR had positive margins at their lowermost resection margins, but the co-morbidity of the patients precluded an additional PD. In one patient with portal vein invasion, a combined portal vein wedge resection was performed.
According to the 7th AJCC-UICC classification, the frequencies of TIS, T1, T2, and T3 were 1.6%, 28.1%, 19.5% and 50.8%, respectively. Lymph node dissection was performed routinely whenever R0 or R1 resection was possible. The average number of lymph nodes which were harvested was 10.9 ± 4.5. Lymph node metastasis was present in 25%, and perineural invasion was present in 41.4%. The rates of perineural invasion for T1, T2 and T3 tumors were 18.4%, 60% and 46.2%, respectively, showing significant associations (P = 0.017).
Lymphovascular invasion was present in 25 of 128 (19.5%). The frequencies of lymphovascular invasion in T1, T2, and T3 were 5.3%, 20.0%, and 26.2%, respectively; invasion was associated with T staging at a statistically significant level (P = 0.013). Lymphovascular invasion was not significantly associated with nodal metastasis (P = 0.071).
Well, moderately, and poorly differentiated cases were found in 33.6%, 55.5% and 10.9% of patients, respectively.
The overall 1-, 3-, and 5-year survival rates for the 133 patients who underwent resection (R0, R1, and R2) were 86.8%, 54.4% and 41.2%, respectively, whereas no patients survived longer than 3 years among the 61 who underwent nonresectional surgeries (Figure 1).
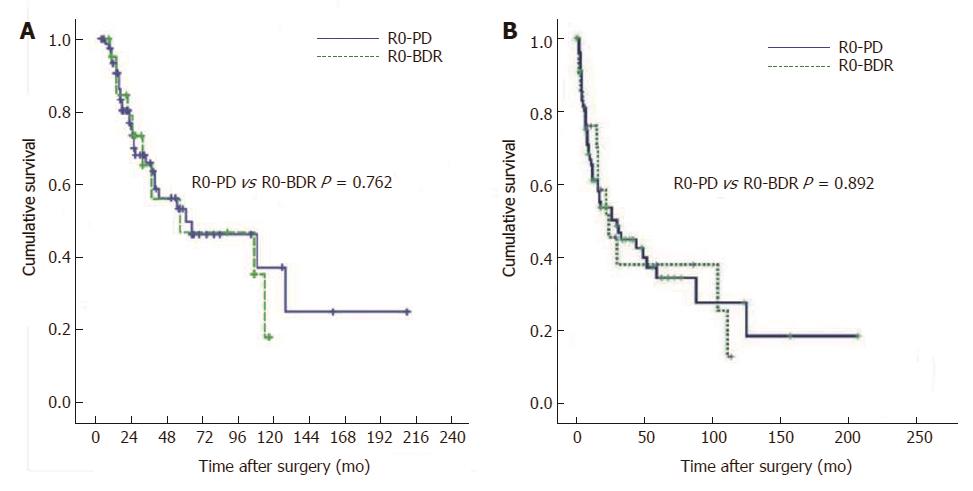
To elucidate the factors influencing long-term survival after resection, 10 clinicopathologic factors for 133 patients who underwent R0, R1 and R2 resection were entered into univariate analysis (Table 1). The 5-year survival rates, according to the type of resection, were 46.6% for PD and 30.0% for BDR, although the differences between the two groups did not reach statistical significance (P = 0.105). The 5-year survival rates according to margin status were 49.0% for R0 resections (n = 102), 21.0% for R1 resections (n = 24), and 0% for R2 resections (n = 7) (P < 0.0001) (Figure 2). When an R0 resection was achieved, the 5-year survival rates after PD and BDR were similar (49.4% vs 46.5%, P = 0.762) (Figure 3A).
| Variables | Total (n = 133) | ||||
| No. | 5 yr survival | Median | P value | ||
| Age | Mean 65.8 | ||||
| ≤ 70 | 86 (64.7) | 39.1 | 41.0 | 0.917 | |
| > 70 | 47 (35.3) | 44.8 | 38.0 | ||
| Sex | M | 81 (60.9) | 33.6 | 36.0 | 0.359 |
| F | 52 (39.1) | 59.4 | 105.0 | ||
| CA19-9 (U/mL) | ≤ 35 | 27 (30) | 57.9 | 85.0 | 0.734 |
| > 35 | 63 (70) | 44.9 | 37.0 | ||
| Type of resection | PD | 90 (67.7) | 47.2 | 59.0 | 0.105 |
| BDR | 43 (32.3) | 30.0 | 32.0 | ||
| T classification | Tis, T1 | 38 (29.7) | 52.3 | 63.0 | 0.144 |
| T2 | 25 (19.5) | 56.8 | - | ||
| T3 | 65 (50.8) | 34.1 | 36.0 | ||
| N classification | N0 | 96 (75.0) | 51.3 | 63.0 | 0.033 |
| N1 | 32 (25.0) | 17.0 | 30.0 | ||
| M classification | M0 | 130 (97.7) | 42.2 | 41.0 | 0.242 |
| M1 | 3 (2.3) | 0 | 16.0 | ||
| TNM stage | 0, I | 51 (39.5) | 64.5 | 105.0 | 0.006 |
| II | 75 (58.1) | 30.1 | 36.0 | ||
| III | 0 (0) | - | - | ||
| IV | 3 (2.3) | 0 | 16.0 | ||
| PNI | - | 75 (58.6) | 55.8 | 105.0 | 0.022 |
| + | 53 (41.4) | 17.5 | 38.0 | ||
| LVI | - | 103 (80.5) | 52.3 | 63.0 | 0.000 |
| + | 25 (19.5) | 6.7 | 23.0 | ||
| Resection margin | R0 | 102 (76.7) | 49.0 | 59.0 | 0.000 |
| R1 | 24 (18.0) | 21.0 | 31.0 | ||
| R2 | 7 (5.3) | 0 | 12.0 | ||
| Differentiation | Papillary | 4 (3.2) | 66.7 | 105.0 | 0.409 |
| W/D | 40 (32.5) | 48.8 | 59.0 | ||
| M/D | 66 (53.7) | 36.7 | 36.0 | ||
| P/D | 13 (10.6) | 56.1 | 63.0 | ||
According to the 7th edition of the AJCC T-staging system, the 5-year survival rates of Tis/T1, T2, and T3 were 52.3%, 56.8% and 34.1%, respectively (P = 0.144).
The 5-year survival rate of patients without lymph node metastasis was higher than that of patients with lymph node metastasis (51.3% vs 17.0%, P = 0.033).
The five-year survival rates of the patients at TNM stage 0 and 1 were higher than those of patients with TNM stage 2 and 4 respectively (64.5% vs 30.1%, and 0%; P = 0.006).
The 5-year survival rate for patients with perineural invasions was worse than that for patients without perineural invasion in univariate analysis (17.5% vs 55.8%; P = 0.022).
Lymphovascular invasion was present in 25 patients (19.5%). The presence of lymphovascular invasion unfavorably affected long-term survival. The 5-year survival rate of the 103 patients without lymphovascular invasion was 52.3%, compared to 6.7% for the 25 patients with lymphovascular invasion.
The pathological grading of differentiation was not associated with prognosis in this study (P = 0.409).
Recurrence after surgery occurred in 58.8% (60/102) of the patients with R0 resection during the period of follow up. The most common site of first recurrence was abdominal lymph nodes only (38.3%, periportal, around Superior mesenteric artery, paraaortic) followed by liver only (35%), anastomosis site (8.3%), and lung (1.7%). The abdominal lymph node metastasis was present in combination with local recurrence and liver metastasis at the time of first recurrence in 5% and 1.7% respectively. Lymph node metastases were found in 55.0% of the patients with first recurrence in the pattern of lymph node metastasis only (38.3%) or combination with other organ metastases (16.7%). The long term disease free survival of R0 PD was similar to R0 BDR (Figure 3B).
In summary, univariate analysis revealed that lymph node metastasis (P = 0.033), TNM stage (P = 0.006), perineural invasion (P = 0.022), lymphovascular invasion (P < 0.0001), and resection margins (P < 0.0001) were significant factors for long-term survival.
A multivariate analysis was performed using the seven clinicopathologic factors that had been proved to be significant, or at least marginally predictive, in univariate analysis: type of resection; T classification; N classification; TNM stage; perineural invasion; lymphovascular invasion; and resection margin. In multivariate analysis, the TNM stage and lymphovascular invasion were identified as independent prognostic factors associated with poor survival (Table 2).
| Variable | Hazard ratio | 95%CI | P value | |
| AJCC stage | 0, I | 1 | 0.023 | |
| II | 4.572 | 1.473-14.183 | ||
| III | - | - | ||
| IV | 11.185 | 1.061-117.922 | ||
| LVI | - | 1 | 0.002 | |
| + | 2.942 | 1.510-5.733 |
Lymphovascular invasion was present in 25 of 128 cases (19.5%), unfavorably affecting the 5-year survival rate (52.3% vs 6.7%, P < 0.0001). pT1 tumors without lymphovascular invasion showed significantly better survival, compared to pT1 tumors with lymphovascular invasion (5-year survival rate, 57.40% vs 0%; P < 0.0001).
The presence of lymphovascular invasion in patients with nodal metastasis did not affect survival, whereas the presence of lymphovascular invasion in patients without nodal metastasis strongly affected the 5-year survival rate (0% vs 60.4%; P < 0.0001).
Middle and distal bile duct cancers are rare malignancies with poor prognoses, and only complete surgical resection of bile duct cancer offers a chance for long-term survival. The prognosis of middle and distal bile duct cancers remains poor, even with radical resection, due to the high incidence of recurrence. Furthermore, it is difficult to determine the optimal extent of resection and to identify the prognostic factors. Although several prognostic factors, such as surgical radicality, nodal status, depth of invasion, differentiation, perineural invasion, and lymphovascular invasion, have been reported for middle and distal extrahepatic bile duct cancers[9-13], the prognostic values of these factors have not been consistent. Among the aforementioned factors, nodal metastasis, differentiation, and R0 resection have been widely recognized to be associated with long-term survival[3,14-17].
In this study, the overall survival was longer in patients with resection, compared to patients without resection. However, the prognosis of patients with R2 resection was much poorer compared that of patients with R0 or R1 resection. As shown in the results, patients with R0 and R1 resections showed 5-year survival rates of 49.0% and 21.0%, respectively, whereas none of the patients with nonresection or R2 resection survived longer than 3 years (medians of 10 mo and 12 mo , respectively).
Many studies have reported that R0 resection is necessary for long-term survival[5,17-19]. However, it is sometimes very difficult to achieve a tumor-free margin of the bile duct due to superficial microscopic spreading and multifocal tumors. In such cases, R1 resection is inevitable, and some authors have reported that patients with R1 resections have survived longer than expected[3,5,6,20].
In our study, R1 resection was performed in 24 patients, including eight patients with R1 PD and 16 patients with R1 BDR. Positive uppermost bile duct margins accounted for the 20 patients with R1 resections (8 PD and 12 BDR), and positive lowermost bile duct margins accounted for the four patients with R1 BDR resections, for whom the addition of further PD was precluded by the presence of co-morbidities. The 5-year survival rates after R0-resection were higher than those after R1-resection, but the difference did not reach statistical significance (49.0% vs 21.0%; P = 0.132) (Lymphovascular. 2). Thus, it can be assumed that R1 resection, if inevitable, should be recognized as prolonging survival, according to our data, which reflected that no patients with R2 resections or nonresections survived longer than 36 mo The definition of RI resection in this study was a microscopically positive margin of the resected bile duct. The major drawbacks of this study were that the pathological description was not made by a single pathologist and that the invasiveness of the positive margin was not sub-classified into carcinoma in situ or invasive carcinoma. These means of analysis will be further investigated in the near future.
According to the primary site and the extent of the tumor, different surgical procedures can be applied. PD is most commonly performed for tumors because bile duct resection is sometimes not sufficient to achieve adequate surgical margins, compared to PD[5,6]. In our study as well, the microscopic involvement of margins was more frequent after bile duct resection, compared to PD [37.2% (16/43) vs 8.9% (8/90); P < 0.0001]. The 5-year survival rates of patients with PD and BDR were 46.6% and 30.0% (P = 0.105), respectively; this difference, although not statistically significant, can be attributed to a higher rate of positive resection margins in patients with bile duct resection. The lack of statistical significance in the difference between the two groups might have been due to the limited number of patients. However, if R0 resection had been achieved, the 5-year survival rates after bile duct resection would have been similar after PD (46.5% vs 49.4%; P = 0.762) (Figure 3A). Thus, it is possible that PD offers a greater likelihood of complete resection and, consequently, better survival. Therefore, PD is recommended unless patient co-morbidities preclude its implementation. BDR is an option only if the co-morbidities of patients preclude PD or if an R0 resection seems to be achieved for tumors limited to the area surrounding the cystic duct.
The frequency of lymph node metastasis has been reported to range from 23.8% to 68%, and lymph node involvement has been determined to be an important predictor of survival in patients with middle and distal bile duct cancers[3,6,16,19,21,22]. In our study, the frequency of nodal metastasis was 25%, and patients with nodal metastasis had worse survival than patients without nodal metastasis in univariate analysis (5-year survival rate 17.0% vs 51.3%; P = 0.033)
Perineural invasion is one of the pathways through which local infiltrations spread and metastasize. Although perineural invasion has recently been accepted as a prognostic factor in a number of different malignancies, its clinical significance for mid-distal extrahepatic cholangiocarcinoma remains unclear. According to Bhuiya et al[7], perineural invasion had a profound impact on the survival of patients with extrahepatic bile duct cancer. The 5-year survival rate for patients with perineural invasion was 32%, compared to 67% for patients without invasion. A distinct and significant correlation was potentially reported between the depth of tumor invasion and perineural invasion. In our study as well, the frequency of perineural invasion was 41.4%, and the rates of perineural invasion for T1, T2 and T3 tumors were 18.4%, 60% and 46.2%, respectively. Thus, a significant association with depth of invasion was shown (P = 0.017). The survival of patients with perineural invasion was worse than that of patients without perineural invasion in univariate analysis (5-year survival rate 17.5% vs 55.8%; P = 0.022).
Lymphovascular channel invasion is one of many different manners by which tumors spread. The clinical significance of LVI was first described as far back as 1967, when studies reported higher recurrence and poorer survival in cervical cancer patients with lymphovascular invasion[23]. Nevertheless, few reports have been published regarding the significance of lymphovascular invasion, and its clinical significance has not been established in middle and distal bile duct cancers[8,24]. Our study showed that the presence of lymphovascular invasion was an independent prognostic factor and unfavorably influenced long-term survival. Lymphovascular invasion was present in 19.5% of patients, and the overall 5-year survival rates of these patients with lymphovascular invasion were poor, compared to the rates of patients without lymphovascular invasion (6.7% vs 52.3%, P < 0.0001).
Additionally, lymphovascular invasion was significantly associated with the depth of invasion (T classification) (P = 0.013). The frequency of lymphovascular invasion, according to the depth of the invasion (T1, T2 and T3), was 5.3%, 20% and 26.2%, respectively. Although pathologic T1 (pT1) tumors generally have favorable prognoses after resection, the presence of lymphovascular invasion, even in pT1 tumors, affected survival unfavorably, compared to pT1 tumors without lymphovascular invasion (5-year survival rate, 0% vs 57.4%; P < 0.0001). Our results were similar to those from a study published by Nagahashi et al[8], who reported poorer prognoses in patients with invasion into the fibromuscular layer, compared to those with mucosal layer invasion. These authors insisted that the cause of these adverse effects was related to lymphovascular invasion. Contrary to our expectations, the present study did not find that lymphovascular invasion was strongly associated with nodal metastasis (P = 0.071). This lack of association has also been reported in other studies examining nodal status and lymphovascular invasion in breast cancer[25,26]. A direct correlation between the presence of lymphovascular invasion and nodal metastasis might not always be apparent. The presence of lymphovascular invasion in patients with nodal metastasis did not affect survival, but in patients without nodal metastasis, the presence of lymphovascular invasion affected 5-year survival (0% vs 60.4%; P < 0.0001). This result suggests that lymphovascular invasion has a profound impact on the survival of patients with lymph node-negative middle and distal extrahepatic bile duct cancers.
Pancreaticoduodenectomy had higher rate of R0 resection than bile duct resection, although the long-term survival between the two groups was not significantly different. BDR can be an applied when only R0 resection is possible or if the co-morbidity of the patient precludes PD. R1 resection, if inevitable, can be performed because it offers better survival than R2 or no resection. The TNM stage and LVI were identified as independent factors influencing the survival of patients with middle and distal bile duct cancers.
Cholangiocarcinoma is a rare malignant tumor of the biliary tree that can arise anywhere from the intrahepatic to the extrahepatic bile duct just proximal to the duodenal ampulla, excluding the gallbladder. The prognosis in bile duct cancer is unfavorable and varies according to the type of surgery, curability, and pathological factors.
Authors analyzed the clinicopathological data of 133 patients who underwent surgical resection for middle and distal bile duct cancers, to identify the influence of the type of surgery selected and of clinicopathological factors on the long-term prognoses of the patients.
This is a well-designed retrospective study with a quite big sample size. The conclusions are reasonable and credible.
P- Reviewers: Matsumoto I, Rerknimitr R, Yang YM S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
| 1. | Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009;16:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Patel T, Singh P. Cholangiocarcinoma: emerging approaches to a challenging cancer. Curr Opin Gastroenterol. 2007;23:317-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-473; discussion 473-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 897] [Cited by in F6Publishing: 829] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 4. | Longmire WP Jr. Tumor of the extrahepatic biliary radicals. Curr Probl Cancer. 1976;1:1-45. [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Sakamoto Y, Kosuge T, Shimada K, Sano T, Ojima H, Yamamoto J, Yamasaki S, Takayama T, Makuuchi M. Prognostic factors of surgical resection in middle and distal bile duct cancer: an analysis of 55 patients concerning the significance of ductal and radial margins. Surgery. 2005;137:396-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Jang JY, Kim SW, Park DJ, Ahn YJ, Yoon YS, Choi MG, Suh KS, Lee KU, Park YH. Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann Surg. 2005;241:77-84. [PubMed] [Cited in This Article: ] |
| 7. | Bhuiya MR, Nimura Y, Kamiya J, Kondo S, Fukata S, Hayakawa N, Shionoya S. Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann Surg. 1992;215:344-349. [PubMed] [Cited in This Article: ] |
| 8. | Nagahashi M, Shirai Y, Wakai T, Sakata J, Ajioka Y, Nomura T, Tsuchiya Y, Hatakeyama K. Depth of invasion determines the postresectional prognosis for patients with T1 extrahepatic cholangiocarcinoma. Cancer. 2010;116:400-405. [PubMed] [Cited in This Article: ] |
| 9. | Blom D, Schwartz SI. Surgical treatment and outcomes in carcinoma of the extrahepatic bile ducts: the University of Rochester experience. Arch Surg. 2001;136:209-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Gibby DG, Hanks JB, Wanebo HJ, Kaiser DL, Tegtmeyer CJ, Chandler JG, Jones RS. Bile duct carcinoma. Diagnosis and treatment. Ann Surg. 1985;202:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 299] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Tompkins RK, Thomas D, Wile A, Longmire WP. Prognostic factors in bile duct carcinoma: analysis of 96 cases. Ann Surg. 1981;194:447-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 211] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Nimura Y, Hayakawa N, Kamiya J, Maeda S, Kondo S, Yasui A, Shionoya S. Combined portal vein and liver resection for carcinoma of the biliary tract. Br J Surg. 1991;78:727-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 142] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, Nagakawa T, Nakayama T. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685-1695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 472] [Cited by in F6Publishing: 426] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 15. | Pitt HA, Nakeeb A, Abrams RA, Coleman J, Piantadosi S, Yeo CJ, Lillemore KD, Cameron JL. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995;221:788-797; discussion 797-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 246] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Fong Y, Blumgart LH, Lin E, Fortner JG, Brennan MF. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996;83:1712-1715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 148] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Yoshida T, Matsumoto T, Sasaki A, Morii Y, Aramaki M, Kitano S. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg. 2002;137:69-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Wakai T, Shirai Y, Moroda T, Yokoyama N, Hatakeyama K. Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer. 2005;103:1210-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Choi SB, Park SW, Kim KS, Choi JS, Lee WJ. The survival outcome and prognostic factors for middle and distal bile duct cancer following surgical resection. J Surg Oncol. 2009;99:335-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, Sueda T. Prognostic significance of lymph node metastasis and surgical margin status for distal cholangiocarcinoma. J Surg Oncol. 2007;95:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Tajima H, Miwa K. Role of nodal involvement and the periductal soft-tissue margin in middle and distal bile duct cancer. Ann Surg. 1999;229:76-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Friedell GH, Steiner G, Kistner RW. Prognostic value of blood-vessel invasion in cervical cancer. Obstet Gynecol. 1967;29:855-857. [PubMed] [Cited in This Article: ] |
| 24. | Patel SH, Kooby DA, Staley CA, Sarmiento JM, Maithel SK. The prognostic importance of lymphovascular invasion in cholangiocarcinoma above the cystic duct: a new selection criterion for adjuvant therapy? HPB (Oxford). 2011;13:605-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Ragage F, Debled M, MacGrogan G, Brouste V, Desrousseaux M, Soubeyran I, de Lara CT, Mauriac L, de Mascarel I. Is it useful to detect lymphovascular invasion in lymph node-positive patients with primary operable breast cancer? Cancer. 2010;116:3093-3101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Woo CS, Silberman H, Nakamura SK, Ye W, Sposto R, Colburn W, Waisman JR, Silverstein MJ. Lymph node status combined with lymphovascular invasion creates a more powerful tool for predicting outcome in patients with invasive breast cancer. Am J Surg. 2002;184:337-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |