Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8745
Revised: October 20, 2013
Accepted: November 1, 2013
Published online: December 14, 2013
AIM: To construct normal values for the tests of the psychometric hepatic encephalopathy score (PHES) and to evaluate its usefulness in the diagnosis of minimal hepatic encephalopathy (MHE) among Chinese individuals with cirrhosis.
METHODS: The five tests of PHES, number connection test-A (NCT-A), number connection test-B, serial dotting test, line tracing test and digit symbol test (DST), were administered to all enrolled subjects in a quiet room with sufficient light. Cirrhotic subjects with overt HE were excluded by the West-Haven criteria and a detailed neurological examination. Based on the nomograms of healthy volunteers, the patients were classified as having MHE when their PHES was less than -4.
RESULTS: In total, 146 healthy volunteers completed all the PHES tests. Age and education years were confirmed to be predictors of all five tests. In total, 53 patients with liver cirrhosis completed the PHES. Of the patients with liver cirrhosis, 24 (45.3%), 22(41.5%) and 7(13.2%) had Child-Pugh grades A, B and C, respectively. MHE was diagnosed in 26 patients (49.1%). Compared with compensated cirrhotic patients (Child A), decompensated cirrhotic patients (Child B and C) had a higher proportion of MHE (65.5% vs 29.2%). No differences in age and education years were found between the MHE and non-MHE groups. NCT-A and DST were able to diagnose MHE with a sensitivity of 76.9% and a specificity of 96.3% (AUC = 0.866, K = 0.735).
CONCLUSION: The proportion of MHE is associated with liver function. NCT-A and DST are simple tools that can be used for the diagnosis of MHE in China.
Core tip: The psychometric hepatic encephalopathy score (PHES) has been standardized in several countries, but requires further validation in China. The authors aimed to evaluate the usefulness of PHES for the diagnosis of minimal hepatic encephalopathy (MHE) among Chinese patients with liver cirrhosis. In China, the results of the five neuropsychological tests of PHES were influenced by age and educational status. In total, 49.1% of the patients with cirrhosis were classified as having MHE, and the proportion of MHE was associated with the severity of liver function. Number connection test-A and digit symbol test are simple and useful tools that can be used for the diagnosis of MHE in China.
- Citation: Li SW, Wang K, Yu YQ, Wang HB, Li YH, Xu JM. Psychometric hepatic encephalopathy score for diagnosis of minimal hepatic encephalopathy in China. World J Gastroenterol 2013; 19(46): 8745-8751
- URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8745.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8745
Minimal hepatic encephalopathy (MHE) is a highly prevalent asymptomatic disturbance in patients with liver cirrhosis. MHE is associated with impaired health-related quality of life and driving capability and can predict the development of overt hepatic encephalopathy (OHE)[1-4]. MHE is not detectable by routine physical or neurological examinations, and a specific neuropsychological/neurophysiological test is needed for its diagnosis[5-7]. The psychometric hepatic encephalopathy score (PHES) is internationally recommended as the gold standard for the diagnosis of MHE[8,9].
The PHES is composed of five tests, number connection test-A (NCT-A), number connection test-B (NCT-B), serial dotting test (SDT), line tracing test (LTT) and digit symbol test (DST). PHES can be used to assess motor speed, motor accuracy, concentration, attention, visual perception, visual-spatial orientation, visual construction and memory[10], which are related to most of neuropsychological impairments in MHE. The PHES has been standardized in several countries, such as Germany, Italy, Spain, India, Korea and Mexico. However, in China further validation is needed. The aims of this study were to construct and validate a dataset of normal values for the PHES in a healthy Chinese population and to evaluate the usefulness of PHES for the diagnosis of MHE among Chinese patients with liver cirrhosis.
Healthy volunteers: The healthy volunteers that were recruited for the control group included people who visited the Health Promotion Center at the First Affiliated Hospital of Anhui Medical University in Hefei, China, for routine health examinations, and through word-of-mouth referrals. The following exclusion criteria were applied for the control group: (1) Presence of chronic liver diseases, neurological or psychiatric diseases, or other diseases that can affect cognitive function; (2) A past history of chronic liver disease, neurologic or psychiatric disorders; (3) Consumption of psychotropic drugs; (4) Alcohol consumption > 50 g/d within the past 3 mo; and (5) Inability to read and write.
Liver cirrhosis group: Consecutive inpatients from the Department of Gastroenterology and Hepatology were recruited. Patients with OHE, which was defined according to the West-Haven criteria[11], were excluded. The diagnosis of liver cirrhosis was based on a combination of physical examination, laboratory tests, medical imaging and endoscopic evidence or on liver histology, if available. The following exclusion criteria were applied for the liver cirrhosis group: (1) A history of OHE, upper gastrointestinal hemorrhage or spontaneous bacterial peritonitis during the past 2 wk; (2) Consumption of lactulose, psychoactive drugs or any antibiotics during the past 2 wk; (3) Presence of neurological or psychiatric diseases, such as Alzheimer’s disease, Parkinson’s disease and nonhepatic metabolic encephalopathy, or a mini-mental status examination (MMSE) score < 25 points; (4) Presence of significant comorbidity, such as heart, respiratory, or renal failure; (5) Presence of hepatocellular carcinoma or other malignancy, previous TIPS or shunt surgery; (6) Alcohol consumption > 50 g/d within the past 3 mo; and (7) Inability to read and write.
All the subjects, both healthy volunteers and patients with liver cirrhosis, were required to have a fair knowledge of numbers and the Chinese alphabet. The research protocol was approved by the ethics committee of the hospital in accordance with the ethical guidelines of the Declaration of Helsinki. Written informed consent to participate was obtained from each subject.
All the five tests of PHES were administered to all the enrolled subjects in the same sequence. The tests were conducted on a one-to-one basis in a quiet room with sufficient light. A specially trained medical doctor assisted the enrolled subjects in finishing these tests.
As some of our enrolled subjects were not familiar with the English alphabet, we replaced the alphabet in NCT-B with the Chinese alphabet in the same order[12]. The results of the NCT-A, NCT-B, and SDT were measured as seconds, including the time needed to correct any errors, and the result of DST was measured as points. The results of the LTT were measured as both the time needed to complete the test (LTTt, seconds) and as the error score (LTTe), LTT = (1 + LTTe/100) × LTTt[13]. Accordingly, a higher result of DST equals better performance, and lower results on the other tests equal better performance. Formulas were constructed to predict the expected results of the five neuropsychological tests. These values were then used as references to which the results from the patients with liver cirrhosis were compared.
The result of DST within ± 1SD from the mean of the control performance was scored as 0 points. Results between -1 and -2SD, between -2 and -3SD and worse than -3SD were scored as -1, -2 and -3, respectively. A result better than mean + 1SD was scored as +1.
The results (NCT-A, NCT-B, SDT and LTT) within ± 1SD from the mean of the control performance were scored as 0 points. Results between +1 and +2SD, between +2 and +3SD, and worse than +3SD were scored as -1, -2 and -3 points, respectively. Those better than mean -1SD were scored as +1 point[10]. The final score of PHES was generated from the sum of the scores of five tests, which ranged between +5 and -15.
On the day of neuropsychological testing, venous blood was taken for routine liver function tests, hematologic parameters and venous ammonia concentration. Venous ammonia was measured within 30 min after blood sampling.
Statistical analyses were performed using the statistical package for the social science (SPSS version 11.0; SPSS, Chicago, IL, United States). Data are expressed as mean ± SD or as proportion. ROC analysis was performed with results of NCT-A, NCT-B and DST comparing to PHES. Continuous and categorical variables were compared using the t test, the one-way ANOVA test, the Mann-Whitney U-test and the χ2-test, respectively. Levene’s test was used in the evaluation of differences in variance. Non-parametric tests were applied if homogeneity of variance assumptions were not met. Multiple liner regression models were used to predict the value of each test for patients with liver cirrhosis. The difference between the expected and observed results for each test was divided by the corresponding SD of the healthy reference population. Kappa statistics were used to study the agreement between the PHES and NCT-A, NCT-B, DST. A two-sided P value < 0.05 was considered significant.
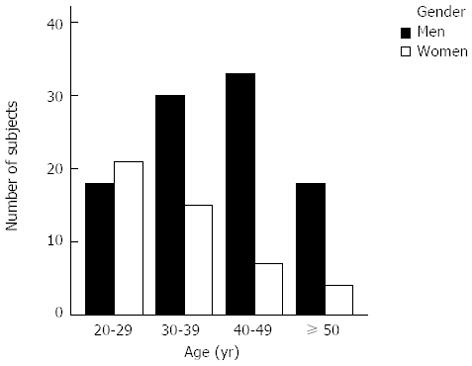
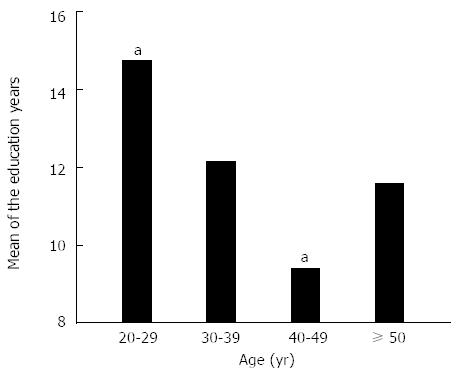
Of 154 healthy volunteers who were recruited, 8 were not able to complete NCT-B and as such, only the remaining 146 volunteers were included. The age and education years of the 146 volunteers were 37.3 ± 10.5 (range 20-67) and 12.0 ± 4.0 (range 2-19) years, respectively, and 99 were men (67.8%). The distribution of subjects according to age was as follows: 20-29 years, 39 (26.7%); 30-39 years, 45 (30.8%); 40-49 years, 40 (27.4%); and ≥ 50 years, 22 (15.1%) (Figure 1). The education years according to age are presented in Figure 2.
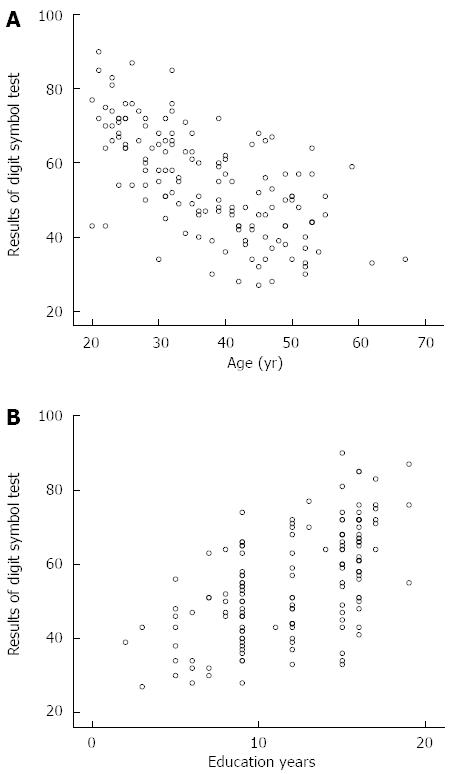
The results of NCT-A, NCT-B, LTT, SDT and DST were 38.289 ± 13.694, 55.846 ± 17.798, 33.287 ± 8.286, 38.035 ± 5.774 and 55.0 ± 14.3, respectively. The results of the five tests were significantly correlated with age and education, and the Pearson’s correlation coefficients are shown in Table 1. In all age categories, the results of all five tests were not significantly correlated with gender (P > 0.05). The variables that affected the results of a neuropsychological test were included in the multiple liner regression models, and the final formulas are shown in Table 2. As shown in Table 2, age and education years were predictors of the results of the five neuropsychological tests in healthy volunteers. As shown in Figure 3, younger age and better education were associated with better DST results.
| NCT-A | NCT-B | LTT | SDT | DST | |
| Age | 0.510 | 0.478 | 0.336 | 0.322 | -0.647 |
| Education | -0.409 | -0.355 | -0.358 | -0.374 | 0.585 |
| Test | Equation | SD |
| NCT-A | 27.861 + 0.548 × age - 0.821 × education | 7.581 |
| NCT-B | 42.816 + 0.672 × age - 0.971 × education | 9.173 |
| SDT | 38.937 + 0.113 × age - 0.423 × education | 2.408 |
| LTT | 33.242 + 0.182 × age - 0.559 × education | 3.455 |
| DST | 63.020 - 0.672 × age + 1.421 × education | 10.608 |
In the healthy volunteer group, the score of PHES was not correlated with education years (P = 0.992) or age (P = 0.595). Additionally, the PHES did not differ between men and women (P = 0.589).
Of 56 inpatients with liver cirrhosis that were enrolled, 3 were not able to complete NCT-B and thus were not considered further. All tests of the PHES were completed by 53 patients with cirrhosis whose age and education years were 45.6 ± 8.2 years (range 27-62) and 8.2 ± 3.6 years (range 0-15), respectively. The study group comprised 50 (94.3%) men.
The score of PHES in the healthy volunteer group was -0.6 ± 3.7 (median, 0; range -11 to +5). The score of PHES in the liver cirrhosis group was -5.6 ± 4.9 (median, -4; range -13 to +4), significantly lower than that in the volunteer group (Mann-Whitney U = 1476.00, P = 0.000). In the healthy volunteer group, the lower boundary of the 95% range between mean - 2SD and mean + 2SD was -4.0. Using a cutoff for MHE of < -4, 26 of the 53 patients with liver cirrhosis were diagnosed with MHE (49.1%).
The proportion of patients with MHE increased with the increase in the Child-Pugh grade. Specifically, 7 had Child-Pugh grade A (7/24, 29.2%), 14 had Child-Pugh grade B (14/22, 63.6%) and 5 had Child-Pugh grade C (5/7, 71.4%). Compared with compensated cirrhotic patients (Child A), decompensated cirrhotic patients (Child B and C) had a higher proportion of MHE (19/29 vs 7/24; χ2 = 6.943, P = 0.008). No differences in age and education years were found between the MHE and non-MHE groups (P > 0.05). Venous ammonia concentration was measured in 26 cirrhotic patients and was found to be similar between the MHE and non-MHE groups (t = 1.086, P = 0.288). In 37 patients with cirrhosis who underwent endoscopic examination, the prevalence of MHE was not associated with esophageal varices (P = 0.584 by Fisher’s exact test). In total, 38 of 53 cirrhotic patients were hepatitis B virus (HBV) positive, while 15 were HBV negative. The prevalence of MHE was similar between the HBV positive and negative groups (χ2 = 0.048, P = 0.827) and the prevalence of MHE was not influenced by antiviral therapy. Table 3 shows the characteristics of patients with or without MHE.
| MHE | Non-MHE | P value | ||
| Age (yr) | 45.3 ± 8.0 | 45.9 ± 8.5 | t = 0.289 | 0.774 |
| Education (yr) | 8.3 ± 4.4 | 8.4 ± 2.8 | Mann-Whitney U = 348.000 | 0.956 |
| Ammonia (μmol/L) | 74.2 ± 64.2 | 52.9 ± 24.2 | t = 1.086 | 0.288 |
| Child-Pugh grade | χ2 = 6.943 | 0.008 | ||
| Child A | 7 | 17 | ||
| Child B/C | 19 | 10 | ||
| Esophageal varices | 0.5841 | |||
| With esophageal varices | 15 | 19 | ||
| Without esophageal varices | 2 | 1 | ||
| HBV | χ2 = 0.048 | 0.827 | ||
| HBV positive | 19 | 19 | ||
| HBV negative | 7 | 8 | ||
| Antiviral therapy | > 0.051 | |||
| With antiviral therapy | 4 | 4 | ||
| Without antiviral therapy | 15 | 15 |
International consensus recommends that at least two of the NCT-A, NCT-B, DST and block-design test (BDT) should be used for the diagnosis of MHE[8]. Because the BDT is not easy to use, we compared PHES assessment using NCT-A, NCT-B and DST. Based on the normal range of healthy volunteers, the result of a single test was classified to be abnormal if the score was less than -1 point[10]. Using the NCT-A and DST, we were able to diagnose MHE with a sensitivity of 76.9% and a specificity of 96.3% (AUC = 0.866, K = 0.735), if at least one of the two tests was abnormal (Table 4).
| Sensitivity | Specificity | AUC | K value | ||
| Both of the two tests were abnormal | NCT-A + NCT-B | 0.538 | 0.963 | 0.751 | 0.505 |
| NCT-A + DST | 0.077 | 1.000 | 0.538 | 0.078 | |
| NCT-B + DST | 0.154 | 1.000 | 0.577 | 0.156 | |
| All of the three tests were abnormal | NCT-A + NCT-B+DST | 0.077 | 1.000 | 0.538 | 0.078 |
| At least one of the two tests was abnormal | NCT-A/NCT-B | 0.923 | 0.741 | 0.832 | 0.661 |
| NCT-A/DST | 0.769 | 0.963 | 0.866 | 0.735 | |
| NCT-B/DST | 0.769 | 0.741 | 0.755 | 0.510 | |
| At least one of the three tests was abnormal | NCT-A/NCT-B/DST | 0.923 | 0.741 | 0.832 | 0.661 |
| At least two of the three tests were abnormal | NCT-A/NCT-B/DST | 0.615 | 0.963 | 0.789 | 0.582 |
MHE refers to the cognitive defects in patients with cirrhosis and/or portal-systematic shunting that can be diagnosed after the exclusion of OHE and alternative diagnoses for neuropsychological impairment[8,11]. Despite the impact of MHE, most cirrhotic patients are not routinely tested for MHE and remain untreated, because of the lack of standardization of normal values, simple tools and expertise to administer tests[14]. Validations of reference norms for neuropsychological tests may increase the likelihood for detection of MHE. The PHES is a neuropsychological test that was specifically designed and recommended for diagnosis of MHE[8,15]. The PHES has been validated in Germany, Italy, Spain and other countries. To date, the number of studies focused on the prevalence of MHE in Chinese patients with liver cirrhosis is limited, and the validation of PHES in China is needed. Due to the high prevalence of liver cirrhosis and the impact of MHE, it is important to screen for MHE in China. As such, we sought to construct a normative dataset for PHES in healthy Chinese volunteers and to evaluate the value of PHES in the diagnosis of MHE among Chinese patients with liver cirrhosis. In our study, we found that age and education years were predictors of all five tests included in PHES. However, no differences in age and education years were found between the MHE and non-MHE groups. The proportion of patients with MHE was associated with the severity of liver function.
Age and educational status are widely recognized to be associated with the results of neuropsychological tests and accordingly age- and -education -matched normal values of healthy controls are recommended[8]. In the study from Spain, the results of NCT-A and NCT-B were better in males than in females. In our present study, all five neuropsychological tests of the PHES were influenced by age and education. However, they did not differ between males and females in all age categories. As such, age and education, which affected the results of the neuropsychological tests, were included in the multiple linear regression model and formulas used to establish the expected values. In the healthy volunteer group, the PHES was not affected by age, education and gender. In this study, normative data that were matched for age and education years were used, and no differences were found between patients with and without MHE. Therefore, we conclude that in the Chinese population, age and education influence the neuropsychological tests included in the PHES, but are not associated with the score of PHES and the presence or absence of MHE.
When the cutoff was set at -4, PHES had good sensitivity and specificity for diagnosing MHE[10]. This is the same cutoff that was used by the majority of studies focusing on the use of PHES for screening of MHE[10,12,13,15-21]. In this study, the lower boundary of the 95% range between mean-2SD and mean+2SD in the volunteer group was -4.0. Accordingly, patients with liver cirrhosis were diagnosed with MHE on the basis of PHES scores lower than -4. MHE was diagnosed in 49.1% of patients with liver cirrhosis. This is similar to a study from India, in which 48% of cirrhotic patients were diagnosed with MHE[20]. However, a lower incidence of MHE (25.6%) was reported in a study from Korea[12]. One reason might be that the liver function of patients in the studies was different. While 80.6% had Child A in the Korean study, the proportion of Child A in our study and the Indian study were 45.3% and 22.0%, respectively. This higher proportion of Child A may account for the low incidence of MHE diagnosed.
In our study, the proportion of patients with MHE increased with the increase in the Child-Pugh grade as follows: 7 of 24 patients (29.2%) with compensated liver cirrhosis (Child-Pugh grade A) and 19 of 29 patients (65.5%) with decompensated liver cirrhosis (Child-Pugh grades B and C) (P = 0.008). This finding is consistent with those of previous studies[17,22]. MHE was further confirmed to be affected by liver function. The pathogenesis of HE is multifactorial, and ammonia is considered an important risk factor[23]. However, the relationship between blood ammonia concentration and MHE is still controversial[12,24-26]. Ammonia reaches the systemic circulation and accumulates in the central nervous system via esophageal varices[27]. In the present study, we found that MHE did not correlate with the presence of esophageal varices and venous ammonia levels. In MHE patients, the blood brain barrier may be breached[23], enabling ammonia to diffuse across the blood-brain barrier into the brain more freely[28]. As such, the venous ammonia concentration of patients with MHE may be similar to patients without MHE.
International consensus meetings have recommended the use of the PHES for diagnosing MHE[8,9]. The Vienna consensus has also recommended that at least two of four tests (NCT-A, NCT-B, DST and BDT) should be used for the diagnosis of MHE[8]. Three of the four tests, NCT-A, NCT-B and DST, have been commonly used for the detection of MHE. The result of a single test was regarded to be abnormal if the result was beyond the 2 SD range of the control norms[10]. In some studies, MHE was diagnosed when both of the two tests were abnormal[1,29,30]. In others, MHE was diagnosed when at least one of the two tests was abnormal[24,31,32]. The present study compared PHES with NCT-A, NCT-B and DST for the diagnosis of MHE. The diagnosis of MHE on the basis of NCT-A and DST showed good agreement with PHES. If at least one of the NCT-A and DST tests was abnormal, MHE could be diagnosed with a sensitivity of 76.9% and a specificity of 96.3% with respect to PHES (AUC = 0.866, K = 0.735). Based on our study, we conclude that NCT-A and DST, which can be completed in minutes, are simple tools for screening MHE among Chinese inpatients with liver cirrhosis.
In summary, the preliminary normal values for all five tests of PHES in Chinese healthy volunteers have been constructed and are influenced by age and educational level. On the basis of a PHES score lower than -4, MHE was detected in 49.1% of the Chinese inpatients with liver cirrhosis. The combination of NCT-A and DST might be a simple and useful tool for the diagnosis of MHE in China.
Minimal hepatic encephalopathy (MHE) is widely prevalent in patients with cirrhosis. MHE is associated with impaired health-related quality of life, driving capability and can predict the development of overt hepatic encephalopathy. MHE is not detectable by routine physical or neurological examinations, and a specific neuropsychological/neurophysiological test is needed.
International consensus recommends use of the psychometric hepatic encephalopathy score (PHES) for diagnosing MHE. The PHES has been standardized in Germany, Italy, Spain, India and Korea, but not in China.
This study constructed normal values for the PHES test in healthy Chinese volunteers and evaluated the usefulness of PHES for the diagnosis of MHE in Chinese patients with liver cirrhosis. In the present study, approximately 49% of patients with liver cirrhosis were classified as MHE. Compared to PHES, NCT-A and DST were able to diagnose MHE with a sensitivity of 76.9% and a specificity of 96.3% (AUC = 0.866, K = 0.735).
The results of the five neuropsychological tests of PHES are influenced by age and educational status. Age- and education-corrected nomograms can be used for MHE screening in patients with liver cirrhosis. The proportion of patients with MHE is associated with the severity of liver function. NCT-A and DST are simple and useful tools for the diagnosis of MHE in China.
This is a single-center study from China aiming to validate the use of the PHES for the diagnosis of MHE in cirrhotic patients without overt hepatic encephalopathy. The study, which has created age- and education level-corrected values for the Chinese population, will enable other authors to diagnose MHE in patients with liver cirrhosis and to evaluate interventions.
P- Reviewers: Kapoor S, Mihaila RG, Savopoulos CG, Zhong JH S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 369] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 2. | Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, Stravitz RT, Luketic V, Fuchs M, White MB. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646-1653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 3. | Bajaj JS. Minimal hepatic encephalopathy matters in daily life. World J Gastroenterol. 2008;14:3609-3615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 107] [Cited by in F6Publishing: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Schomerus H, Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis. 2001;16:37-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Zhan T, Stremmel W. The diagnosis and treatment of minimal hepatic encephalopathy. Dtsch Arztebl Int. 2012;109:180-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, Morgan MY. Review article: the design of clinical trials in hepatic encephalopathy--an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 7. | Córdoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011;54:1030-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1468] [Cited by in F6Publishing: 1364] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 9. | Randolph C, Hilsabeck R, Kato A, Kharbanda P, Li YY, Mapelli D, Ravdin LD, Romero-Gomez M, Stracciari A, Weissenborn K. Neuropsychological assessment of hepatic encephalopathy: ISHEN practice guidelines. Liver Int. 2009;29:629-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Weissenborn K, Ennen JC, Schomerus H, Rückert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 528] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Dhiman RK, Saraswat VA, Sharma BK, Sarin SK, Chawla YK, Butterworth R, Duseja A, Aggarwal R, Amarapurkar D, Sharma P. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian National Association for Study of the Liver. J Gastroenterol Hepatol. 2010;25:1029-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Seo YS, Yim SY, Jung JY, Kim CH, Kim JD, Keum B, An H, Yim HJ, Lee HS, Kim CD. Psychometric hepatic encephalopathy score for the detection of minimal hepatic encephalopathy in Korean patients with liver cirrhosis. J Gastroenterol Hepatol. 2012;27:1695-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Amodio P, Campagna F, Olianas S, Iannizzi P, Mapelli D, Penzo M, Angeli P, Gatta A. Detection of minimal hepatic encephalopathy: normalization and optimization of the Psychometric Hepatic Encephalopathy Score. A neuropsychological and quantified EEG study. J Hepatol. 2008;49:346-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Bajaj JS, Etemadian A, Hafeezullah M, Saeian K. Testing for minimal hepatic encephalopathy in the United States: An AASLD survey. Hepatology. 2007;45:833-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Kappus MR, Bajaj JS. Assessment of minimal hepatic encephalopathy (with emphasis on computerized psychometric tests). Clin Liver Dis. 2012;16:43-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Weissenborn K. Psychometric tests for diagnosing minimal hepatic encephalopathy. Metab Brain Dis. 2013;28:227-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Romero-Gómez M, Córdoba J, Jover R, del Olmo JA, Ramírez M, Rey R, de Madaria E, Montoliu C, Nuñez D, Flavia M. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology. 2007;45:879-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50:2014-2021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 20. | Dhiman RK, Kurmi R, Thumburu KK, Venkataramarao SH, Agarwal R, Duseja A, Chawla Y. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci. 2010;55:2381-2390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Duarte-Rojo A, Estradas J, Hernández-Ramos R, Ponce-de-León S, Córdoba J, Torre A. Validation of the psychometric hepatic encephalopathy score (PHES) for identifying patients with minimal hepatic encephalopathy. Dig Dis Sci. 2011;56:3014-3023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Wang JY, Zhang NP, Chi BR, Mi YQ, Meng LN, Liu YD, Wang JB, Jiang HX, Yang JH, Xu Y. Prevalence of minimal hepatic encephalopathy and quality of life evaluations in hospitalized cirrhotic patients in China. World J Gastroenterol. 2013;19:4984-4991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 23. | McPhail MJ, Bajaj JS, Thomas HC, Taylor-Robinson SD. Pathogenesis and diagnosis of hepatic encephalopathy. Expert Rev Gastroenterol Hepatol. 2010;4:365-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Tan HH, Lee GH, Thia KT, Ng HS, Chow WC, Lui HF. Minimal hepatic encephalopathy runs a fluctuating course: results from a three-year prospective cohort follow-up study. Singapore Med J. 2009;50:255-260. [PubMed] [Cited in This Article: ] |
| 25. | Ong JP, Aggarwal A, Krieger D, Easley KA, Karafa MT, Van Lente F, Arroliga AC, Mullen KD. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114:188-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 331] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Bajaj JS, Thacker LR, Heuman DM, Fuchs M, Sterling RK, Sanyal AJ, Puri P, Siddiqui MS, Stravitz RT, Bouneva I. The Stroop smartphone application is a short and valid method to screen for minimal hepatic encephalopathy. Hepatology. 2013;58:1122-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 27. | Chatauret N, Butterworth RF. Effects of liver failure on inter-organ trafficking of ammonia: implications for the treatment of hepatic encephalopathy. J Gastroenterol Hepatol. 2004;19:S219-S223. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Lockwood AH, Yap EW, Wong WH. Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab. 1991;11:337-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 224] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Romero-Gómez M. Critical flicker frequency: it is time to break down barriers surrounding minimal hepatic encephalopathy. J Hepatol. 2007;47:10-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Sharma P, Sharma BC, Sarin SK. Critical flicker frequency for diagnosis and assessment of recovery from minimal hepatic encephalopathy in patients with cirrhosis. Hepatobiliary Pancreat Dis Int. 2010;9:27-32. [PubMed] [Cited in This Article: ] |
| 31. | Bajaj JS, Saeian K, Verber MD, Hischke D, Hoffmann RG, Franco J, Varma RR, Rao SM. Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. Am J Gastroenterol. 2007;102:754-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Marić D, Klasnja B, Filipović D, Brkić S, Ruzić M, Bugarski V. Minimal hepatic encephalopathy in patients with decompensated liver cirrhosis. Acta Clin Croat. 2011;50:375-380. [PubMed] [Cited in This Article: ] |