Published online Aug 28, 2012. doi: 10.3748/wjg.v18.i32.4317
Revised: July 11, 2012
Accepted: July 18, 2012
Published online: August 28, 2012
AIM: To study the angle between the circular smooth muscle (CSM) and longitudinal smooth muscle (LSM) fibers in the distal esophagus.
METHODS: In order to identify possible mechanisms for greater shortening in the distal compared to proximal esophagus during peristalsis, the angles between the LSM and CSM layers were measured in 9 cadavers. The outer longitudinal layer of the muscularis propria was exposed after stripping the outer serosa. The inner circular layer of the muscularis propria was then revealed after dissection of the esophageal mucosa and the underlying muscularis mucosa. Photographs of each specimen were taken with half of the open esophagus folded back showing both the outer longitudinal and inner circular muscle layers. Angles were measured every one cm for 10 cm proximal to the squamocolumnar junction (SCJ) by two independent investigators. Two human esophagi were obtained from organ transplant donors and the angles between the circular and longitudinal smooth muscle layers were measured using micro-computed tomography (micro CT) and Image J software.
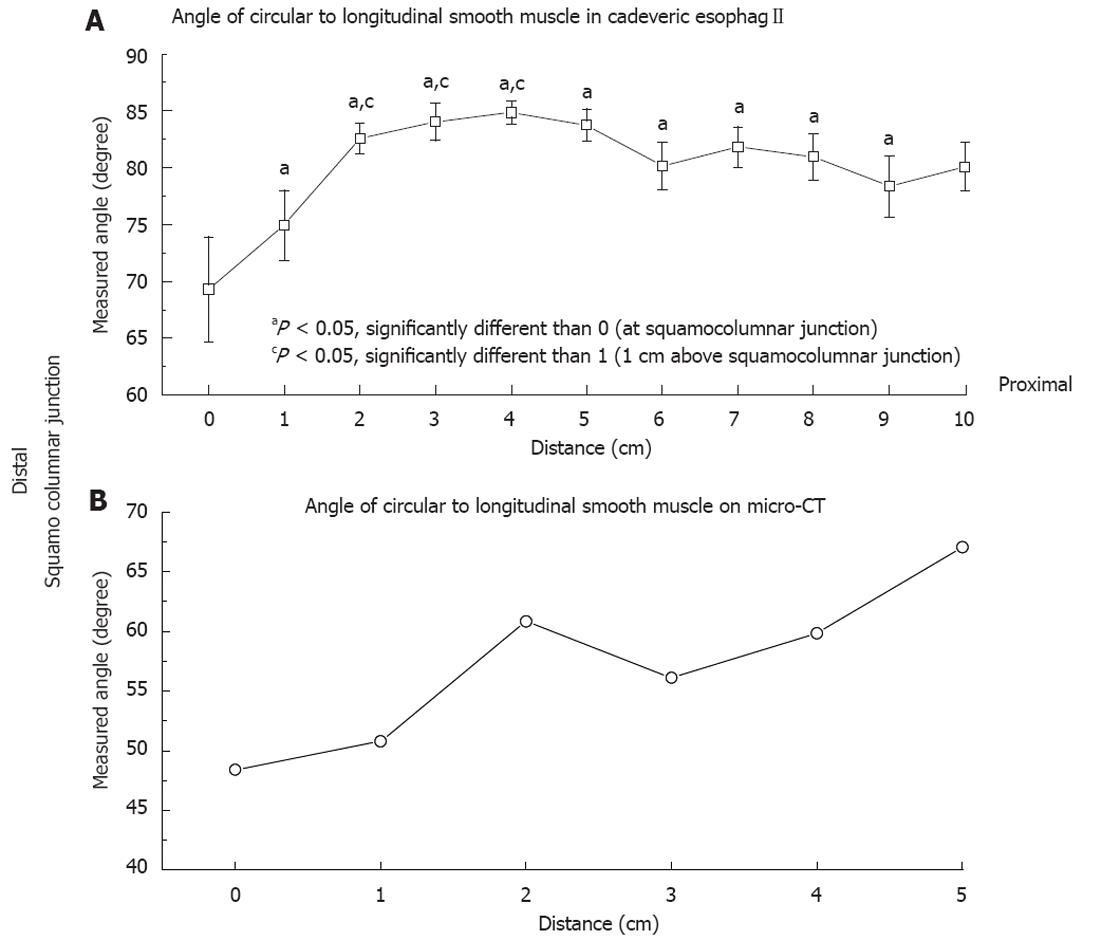
RESULTS: All data are presented as mean ± SE. The CSM to LSM angle at the SCJ and 1 cm proximal to SCJ on the autopsy specimens was 69.3 ± 4.62 degrees vs 74.9 ± 3.09 degrees, P = 0.32. The CSM to LSM angle at SCJ were statistically significantly lower than at 2, 3, 4 and 5 cm proximal to the SCJ, 69.3 ± 4.62 degrees vs 82.58 ± 1.34 degrees, 84.04 ± 1.64 degrees, 84.87 ± 1.04 degrees and 83.72 ± 1.42 degrees, P = 0.013, P = 0.008, P = 0.004, P = 0.009 respectively. The CSM to LSM angle at SCJ was also statistically significantly lower than the angles at 6, 7 and 8 cm proximal to the SCJ, 69.3 ± 4.62 degrees vs 80.18 ± 2.09 degrees, 81.81 ± 1.75 degrees and 80.96 ± 2.04 degrees, P = 0.05, P = 0.02, P = 0.03 respectively. The CSM to LSM angle at 1 cm proximal to SCJ was statistically significantly lower than at 3, 4 and 5 cm proximal to the SCJ, 74.94 ± 3.09 degrees vs 84.04 ± 1.64 degrees, 84.87 ± 1.04 degrees and 83.72 ± 1.42 degrees, P = 0.019, P = 0.008, P = 0.02 respectively. At 10 cm above SCJ the angle was 80.06 ± 2.13 degrees which is close to being perpendicular but less than 90 degrees. The CSM to LSM angles measured on virtual dissection of the esophagus and the stomach on micro CT at the SCJ and 1 cm proximal to the SCJ were 48.39 ± 0.72 degrees and 50.81 ± 1.59 degrees. Rather than the angle of the CSM and LSM being perpendicular in the esophagus we found an acute angulation between these two muscle groups throughout the lower 10 cm of the esophagus.
CONCLUSION: The oblique angulation of the CSM may contribute to the significantly greater shortening of distal esophagus when compared to the mid and proximal esophagus during peristalsis.
- Citation: Vegesna AK, Chuang KY, Besetty R, Phillips SJ, Braverman AS, Barbe MF, Ruggieri MR, Miller LS. Circular smooth muscle contributes to esophageal shortening during peristalsis. World J Gastroenterol 2012; 18(32): 4317-4322
- URL: https://www.wjgnet.com/1007-9327/full/v18/i32/4317.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i32.4317
The mechanisms of contraction and bolus transportation of the esophagus have been of great interest[1]. Peristalsis in the smooth muscle portion of the esophagus involves the interaction of central and peripheral neural mechanisms and the interaction between these neural mechanisms and the smooth muscle[2,3]. Different instruments have been devised to measure the motions of esophageal peristalsis[4,5]. The longitudinal muscle contraction of the human esophagus has been inferred from longitudinal shortening measured using widely spaced metal clips attached to the esophageal mucosa. The measured degrees of shortening vary depending upon how widely the clips are placed. The more widely spaced the clips, the more the measurement will under predict true longitudinal shortening. Furthermore, the relative motion between a clip on the mucosal surface and the underlying muscle layer introduces additional unknown errors into the estimate of longitudinal shortening[6-8]. We recently demonstrated that the mucosa shortens independent of the muscularis propria, upon cholinergic stimulation and with peristaltic contraction[9,10]. Nicosia et al[11] avoided this variability associated with mucosal clips, by developing a procedure to accurately measure local longitudinal shortening of the esophageal wall using simultaneous high-resolution endoluminal ultrasound and manometry during peristaltic contraction.
In a follow-up study we measured cross sectional area and manometry at four levels in the esophagus: 5 cm, 10 cm, 15 cm and 20 cm above the upper border of the distal esophageal high-pressure zone. We found that (1) the shortening of the circular smooth muscle (CSM) and longitudinal smooth muscle (LSM), at 5 cm was significantly greater than at 20 cm above the distal esophageal high pressure zone; and (2) the CSM and LSM both shortened in the longitudinal direction but the CSM contribution towards longitudinal esophageal shortening at the distal esophagus was greater than at the proximal esophagus[12]. This was surprising, as it was commonly assumed that the LSM of the esophagus was completely responsible for esophageal shortening, while the CSM was responsible for the narrowing of the lumen and the pressure generated within the esophageal lumen. Before this finding it was assumed that CSM contraction did not actively contribute to the longitudinal esophageal shortening and that any shortening within the CSM occurred by longitudinal smooth muscle drag.
We proposed several hypotheses to explain this physiologic phenomenon: (1) the LSM dragged the CSM with it during shortening causing the CSM to shorten. While this could account for some of the CSM shortening it could not account for the CSM shortening more than what is accounted for by the LSM. This also cannot explain the regional differences in shortening along the length of the esophagus; (2) the entire esophagus is tonically stretched at rest (under axial tension) and during swallowing there is a decrease in this tension due to deglutitive inhibition causing the circular smooth muscle to shorten, as a spring would shorten when the tension was released. Once again this could account for some of the CSM shortening but could not account for the differences in CSM shortening along the length of the esophagus; and (3) the CSM fibers actually spiral down the esophagus at an angle other than perpendicular to the longitudinal axis leading to subsequent shortening of the CSM during peristaltic contraction. This seemed the most likely explanation for the CSM shortening more than that contributed by LSM in the distal esophagus. In addition we hypothesized that if this was the case, one would observe that the distal spiral angle would be greater than the proximal spiral angle.
The aim of this study was to provide an explanation to the functional observation obtained by Dai et al[12], that the CSM shortens more than can be explained by longitudinal drag of the LSM alone during peristaltic contraction, especially in the distal esophagus.
This research study was approved by the Temple University Institutional review board. Data management, analysis and interpretation and preparation of the manuscript were performed by the authors who had full access to all the study data. All authors had responsibility for the decision to submit the manuscript for publication, and vouch for the accuracy, integrity, and completeness of the data as reported.
In the current study, we used fine dissection of cadaveric esophagi to define the LSM and CSM layers in detail. Angles of the CSM fibers relative to the axial direction (longitudinal muscle fibers) of the esophagus were measured. In addition, we used micro-computed tomography (micro CT) scan to virtually dissect the human esophagi obtained from organ transplant donors to evaluate the angle of the CSM relative to LSM.
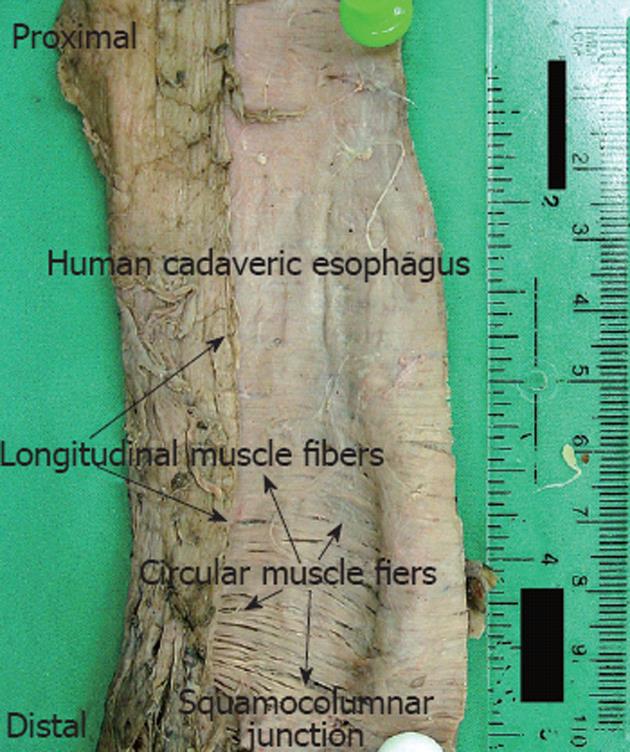
Nine human esophagi were procured from formaldehyde preserved cadavers previously used for medical education purposes. Each esophagus was removed from the pharnygo-esophageal junction to the cardia of the stomach. The outer longitudinal layer of the muscularis propria was exposed after carefully stripping the outer serosa. Minimal tension was applied to straighten the esophagus and the esophagus was then longitudinally cut open in a straight line following the orientation of the longitudinal muscles. The squamocolumnar junction (SCJ) was identified on the mucosa and its location was marked on the specimen. The inner circular layer of the muscularis propria was then revealed after careful dissection of the esophageal mucosa and the underlying muscularis mucosa. Photographs of each specimen were taken with half of the open esophagus folded back showing both the outer longitudinal and inner circular muscle layers. Although the entire esophagus is much longer only the distal 10 cm of the esophagus was used to make measurements. A ruler was also included in the pictures with a mark placed at the SCJ, which was identified earlier. The ruler was also used to mark the esophagus at every centimeter proximal to the SCJ. Special attention was given to ensure that the folded-back longitudinal layer was in its in vivo position relative to the circular layer by carefully aligning the marks made along the incision line on the esophagus before it was cut open (Figure 1).
Image Pro Plus (version 6.1, Media Cybernetics, Inc. MD) was used to analyze each digital image. Angles between the longitudinal and the circular muscles were measured by two independent investigators, blinded to the other investigators measurements. Measurements were made starting at the SCJ and moving proximally measuring every 1 cm for 10 cm.
Two human esophagi and stomachs were obtained from organ transplant donors (National Disease Research Institute and the International Institute for the Advancement of Medicine). The specimens were fixed by submersion in 4% paraformaldehyde for 10 d, followed by immersion in a 2% phosphotungstic acid, 0.02% potassium permanganate, 0.1% hematoxylin (PTAH) solution for 48 h after fixative wash out using running tap water for several hours. The specimen was removed carefully, washed and then dissected down in size so that a region of 5 cm diameter × 5 cm in height remained. This block of tissue was supported by a piece of Styrofoam that was shaped to match the shape of the specimen and wrapped in Parafilm and sealed closed to maintain hydration.
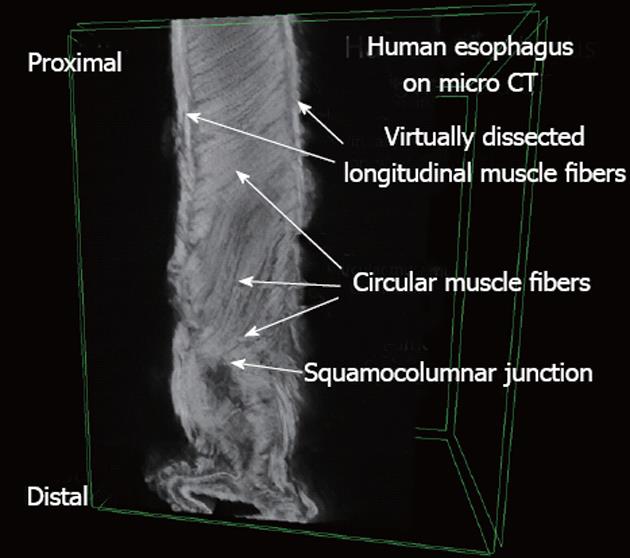
SkyScan 1172 high-resolution cone-beam micro-CT scanner (SkyScan, Ltd, Kontich, Belgium), with a Hamamatsu C9300 11Mp camera, was used to scan the specimens using the double-size and oversize sample options. The following settings were used: a camera pixel size of 8.76 μm, an image pixel size of 11.89 μm, voltage of 80 kV, current of 124 μA, aluminum 0.5 mm as well as copper filters, rotation step of 0.50 degrees, 180 degrees of rotation, a frame averaging of 11 335 rows, 3872 columns, and a scan duration of 3 h and 45 min. The image slices were reconstructed using cone-beam reconstruction software (SkyScan NRecon) based on the Feldkamp algorithm, on 3 linked servers, a process that yielded 3492 tomographic sections, each with a thickness of 11.89 μm in thickness, in the axial (transverse) plane, for each sample. A ring artifact correction of 10, and a beam hardening correction of 40% was applied to both samples.
Three dimensional (3D) image constructs were made using 3D imaging software (SkyScan CTVox). Virtual dissection of these 3D constructs was performed in order to image the circular smooth muscle and the longitudinal smooth muscle in the same plane, using sphere-shaped and cylinder-shaped virtual “cutting boxes” (Figure 2). Image J software was used to measure the angle between the muscle fibers of the LSM and the CSM. Although the entire esophagus is much longer only the distal 5 cm of the esophagus was used due to the limitations of the micro-CT scanner.
All data are presented as mean ± SE. Statistical analysis was performed using one way analysis of variance with post hoc pair wise comparisons using the Bonferroni method.
All data are presented as mean ± SE. The CSM to LSM angle at the SCJ and 1 cm proximal to SCJ on the autopsy specimens was 69.3 ± 4.62 degrees vs 74.9 ± 3.09 degrees, P = 0.32. The CSM to LSM angle at SCJ were statistically significantly lower than at 2 cm, 3 cm, 4 cm and 5 cm proximal to the SCJ, 69.3 ± 4.62 degrees vs 82.58 ± 1.34 degrees, 84.04 ± 1.64 degrees, 84.87 ± 1.04 degrees and 83.72 ± 1.42 degrees, P = 0.013, P = 0.008, P = 0.004, P = 0.009 respectively. The CSM to LSM angle at SCJ was also statistically significantly lower than the angles at 6, 7 and 8 cm proximal to the SCJ, 69.3 ± 4.62 degrees vs 80.18 ± 2.09 degrees, 81.81 ± 1.75 degrees and 80.96 ± 2.04 degrees, P = 0.05, P = 0.02, P = 0.03 respectively. The CSM to LSM angle at 1 cm proximal to SCJ was statistically significantly lower than at 3 cm, 4 cm and 5 cm proximal to the SCJ, 74.94 ± 3.09 degrees vs 84.04 ± 1.64 degrees, 84.87 ± 1.04 degrees and 83.72 ± 1.42 degrees, P = 0.019, P = 0.008, P = 0.02 respectively. At 10 cm above SCJ the angle was 80.06 ± 2.13 degrees which is close to being perpendicular but less than 90 degrees (Figure 3A). The CSM to LSM angles measured on virtual dissection of the esophagus and the stomach on micro CT at the SCJ and 1 cm proximal to the SCJ were 48.39 ± 0.72 degrees and 50.81 ± 1.59 degrees. Rather than the angle of the CSM and LSM being perpendicular in the esophagus we found an acute angulation between these two muscle groups throughout the lower 10 cm of the esophagus. (Figure 3B).
A number of prior studies looking at longitudinal shortening were performed using metallic clips or wires. Dodds et al[6] measured the axial motion of four tantalum wires inserted into the cat esophageal mucosa in vivo. Their results suggested the progression of a wave of longitudinal shortening into the lower esophagus roughly coincident with the bolus tail. Pouderoux et al[8] placed three clips at 4-cm intervals in the distal 8-10 cm of the human esophagus and concluded: “contracting longitudinal segment was advancing ahead of the contracting circular muscle segment.” An earlier study with mucosal clips by Edmundowicz et al[7] measured the change in length of upper and lower halves of the human esophagus and observed that early in the swallow the lower half lengthened while the upper half shortened, consistent with the clip motions in the cat esophagus measured by Dodds et al[6]. Their measurements were also consistent with the later clip data of Pouderoux et al[8], who observed that two adjacent 4-cm segments of the distal esophagus initially lengthen and later shorten in peristalsis-like fashion. Dai et al[13], Liu et al[14] and Miller et al[15-19] pioneered the use of endoluminal ultrasound to evaluate esophageal motility. Miller et al[20-22] combined the use of endoluminal ultrasound with manometry to study peristaltic contraction. Nicosia et al[11] used this new method to measure local longitudinal esophageal shortening in which we calculated shortening for both the circular CSM and LSM using the equation Cross Sectional Area rest/ Cross Sectional Area contract = Length contract/Length rest. It is thought that there is a mechanical advantage of local longitudinal shortening on peristaltic transport in the human esophagus. The peristaltic wave of local longitudinal muscle contraction coordinatedwith the circular muscle contraction wave has both a physiological advantage (concentrating circular muscle fibers), and a mechanicaladvantage (reducing the level of contractile force required to transportthe bolus), which combine to greatly reduce circular muscle toneduring esophageal peristalsis[23].
In a follow-up study evaluating local longitudinal shortening in different regions of the esophagus, we identified regional differences in local longitudinal shortening. The distal esophagus shortened more than the proximal esophagus. In addition the local longitudinal shortening of the CSM and total muscularis propria at 5 cm above the LES were significantly greater than at 20 cm. The shortening of LSM at 5 cm vs 20 cm was not statistically significant. These surprising results imply that the CSM was actually responsible for the greater shortening of the distal esophagus. We hypothesized that the increase in shortening of the CSM was due to the spiral nature of the muscle fibers within the distal CSM[12].
An extensive literature search yielded only a handful of publications that suggested the oblique/spiral nature of the CSM in the body of the esophagus. Netter illustrated in his atlas a distal circular smooth muscle layer oriented in a spiral fashion[24]. Floch in Netter’s Gastroenterology textbook described, “In the upper esophagus, the circular muscle closely approximates the encircling lower fibers of the cricopharyngeus muscle. The upper esophageal fibers are not circular but elliptical, with the anterior part of the ellipse at a lower level to the posterior part. The ellipses become more circular as the esophagus descends, until the start of its middle third, where the fibers run in a horizontal plane. In a 1-cm segment, the fibers are truly circular. Below this point, the fibers become elliptical once again, but they now have a reverse inclination - that is, the posterior part of the ellipse is located at a lower level than the anterior part. In the lower third of the esophagus, the fibers follow a spiral course down the esophagus”[25]. Gray’s anatomy text also mentioned the oblique nature of the CSM[26].
The study used formalin fixed specimens. The measurements might have been somewhat different if fresh non-contracted tissues were used. Formalin fixation can cause shortening in the length of the esophagus and the shortening associated with formalin fixation will change the angle measured between the CSM vs LSM. However, this does not change the validity of the comparisons between angles in different parts of the esophagus, but may alter the absolute numbers measured.
In the current study we found that rather than the angle of the circular smooth muscle and longitudinal smooth muscle being perpendicular to each other in the esophagus, a significant difference was found in the angle of the muscle fibers between these two smooth muscle groups. We also found a significant difference between the muscle angles of the CSM at and 1 cm above the SCJ when compared with the muscle angles further above the SCJ. In conclusion, we believe that this spiral or oblique angulation of the CSM to LSM along with drag from the LSM causes shortening of the esophagus which is greater than can be explained by shortening due to the LSM alone. Furthermore we believe that acute angulation of the CSM at the SCJ may be responsible for the greater distal esophageal shortening compared to the proximal esophagus during peristaltic contraction.
We like to thank the Department of Anatomy, Temple University school of Medicine for their support.
In a previous study, the distal esophagus shortened more than the proximal esophagus. In addition the local longitudinal shortening of the circular smooth muscle (CSM) and total muscularis propria at 5 cm above the lower esophageal sphincter were significantly greater than at 20 cm. It was concluded that the CSM shortened longitudinally more than that which was accounted for by the longitudinal smooth muscle (LSM) shortening. These surprising results implied that the CSM was actually responsible for the greater shortening of the distal esophagus. Authors hypothesized that the increase in shortening of the CSM was due to the spiral nature of the muscle fibers within the distal CSM.
It is thought that the LSM of the esophagus is completely responsible for esophageal shortening during peristaltic contraction. The role of CSM in esophageal shortening has not adequately been evaluated. In this study, the authors demonstrate that the CSM, which is at an oblique angle to the LSM, contributes to esophageal shortening.
Recent reports have highlighted the importance of longitudinal shortening of the esophagus in peristaltic contraction. This is the first study to evaluate the role of the CSM in longitudinal shortening due to the orientation of the CSM fibers.
By understanding how the CSM is oriented in the esophagus this study may increase the readers’ knowledge of the anatomy, physiology of peristaltic contraction. In the future, understanding of the mechanism of esophageal shortening may help in the explanation of peristaltic contractile dysfunction.
The esophageal wall consists of multiple layers. The main muscular component of the distal esophagus is the muscularis propria which is composed to two smooth muscle layers, the CSM and LSM. The CSM is the inner layer of muscularis propria which is closest to the lumen of the esophagus. The CSM is thought to generate the luminal pressure during peristaltic contraction. The LSM is the layer of muscularis propria which is separated from the CSM by an intramuscular connective tissue layer which contains neural tissue. The LSM is the outer layer of the muscularis propria which is thought to be responsible for esophageal shortening during peristaltic contraction.
The authors examined the relative angles of the CSM and LSM in the distal esophagus to explain how the CSM contributes to shortening of the distal esophagus. The authors found that the CSM in the distal esophagus is oriented at an oblique angle to the LSM, thus contributing to distal esophageal shortening. The results are interesting and may have a role in the pathophysiology of esophageal peristalsis.
Peer reviewer: David Ian Watson, Professor, Head, Department of Surgery, Flinders Medical Center, Flinders University, Room 3D211, Bedford Park, South Australia 5042, Australia
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42:610-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Crist J, Gidda JS, Goyal RK. Intramural mechanism of esophageal peristalsis: roles of cholinergic and noncholinergic nerves. Proc Natl Acad Sci USA. 1984;81:3595-3599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 86] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Diamant NE. Neuromuscular mechanisms of primary peristalsis. Am J Med. 1997;103:40S-43S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G988-G997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Pandolfino JE, Roman S. High-resolution manometry: an atlas of esophageal motility disorders and findings of GERD using esophageal pressure topography. Thorac Surg Clin. 2011;21:465-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Dodds WJ, Stewart ET, Hodges D, Zboralske FF. Movement of the feline esophagus associated with respiration and peristalsis. An evaluation using tantalum markers. J Clin Invest. 1973;52:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 88] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Edmundowicz SA, Clouse RE. Shortening of the esophagus in response to swallowing. Am J Physiol. 1991;260:G512-G516. [PubMed] [Cited in This Article: ] |
| 8. | Pouderoux P, Lin S, Kahrilas PJ. Timing, propagation, coordination, and effect of esophageal shortening during peristalsis. Gastroenterology. 1997;112:1147-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Vegesna AK, Tiwana MI, Braverman AS, Miller LS, Ruggieri MR. T1896 Contractile Response of Porcine Esophageal Muscularis Mucosa to Carbachol. Gastroenterology. 2010;138:S-601. [DOI] [Cited in This Article: ] |
| 10. | Vegesna AK, Weissman S, Patel A, Makipour K, Miller LS. Sa1426 Transabdominal Ultrasound to Evaluate the Gastroesophageal Junction in Normal Volunteers and Patients With GERD. Gastroenterology. 2012;142:S-302-S-303. [DOI] [Cited in This Article: ] |
| 11. | Nicosia MA, Brasseur JG, Liu JB, Miller LS. Local longitudinal muscle shortening of the human esophagus from high-frequency ultrasonography. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1022-G1033. [PubMed] [Cited in This Article: ] |
| 12. | Dai Q, Korimilli A, Thangada VK, Chung CY, Parkman H, Brasseur J, Miller LS. Muscle shortening along the normal esophagus during swallowing. Dig Dis Sci. 2006;51:105-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Dai Q, Liu JB, Brasseur JG, Thangada VK, Thomas B, Parkman H, Miller LS. Volume (3-dimensional) space-time reconstruction of esophageal peristaltic contraction by using simultaneous US and manometry. Gastrointest Endosc. 2003;58:913-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Liu JB, Miller LS, Goldberg BB, Feld RI, Alexander AA, Needleman L, Castell DO, Klenn PJ, Millward CL. Transnasal US of the esophagus: preliminary morphologic and function studies. Radiology. 1992;184:721-727. [PubMed] [Cited in This Article: ] |
| 15. | Miller L, Dai Q, Korimilli A, Levitt B, Ramzan Z, Brasseur J. Use of endoluminal ultrasound to evaluate gastrointestinal motility. Dig Dis. 2006;24:319-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Miller LS, Liu JB, Barbarevech CA, Baranowski RJ, Dhuria M, Schiano TD, Goldberg BB, Fisher RS. High-resolution endoluminal sonography in achalasia. Gastrointest Endosc. 1995;42:545-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Miller LS, Liu JB, Klenn PJ, Dhuria M, Feld RI, Goldberg BB. High-frequency endoluminal ultrasonography of the esophagus in human autopsy specimens. J Ultrasound Med. 1993;12:563-566. [PubMed] [Cited in This Article: ] |
| 18. | Miller LS, Liu JB, Klenn PJ, Holahan MP, Varga J, Feld RI, Troshinsky M, Jimenez SA, Castell DO, Goldberg BB. Endoluminal ultrasonography of the distal esophagus in systemic sclerosis. Gastroenterology. 1993;105:31-39. [PubMed] [Cited in This Article: ] |
| 19. | Miller LS, Schiano TD. The use of high frequency endoscopic ultrasonography probes in the evaluation of achalasia. Gastrointest Endosc Clin N Am. 1995;5:635-647. [PubMed] [Cited in This Article: ] |
| 20. | McCray WH, Chung C, Parkman HP, Miller LS. Use of simultaneous high-resolution endoluminal sonography (HRES) and manometry to characterize high pressure zone of distal esophagus. Dig Dis Sci. 2000;45:1660-1666. [PubMed] [Cited in This Article: ] |
| 21. | Miller LS, Dai Q, Sweitzer BA, Thangada V, Kim JK, Thomas B, Parkman H, Soliman AM. Evaluation of the upper esophageal sphincter (UES) using simultaneous high-resolution endoluminal sonography (HRES) and manometry. Dig Dis Sci. 2004;49:703-709. [PubMed] [Cited in This Article: ] |
| 22. | Miller LS, Liu JB, Colizzo FP, Ter H, Marzano J, Barbarevech C, Helwig K, Leung L, Goldberg BB, Hedwig K [corrected to Helwig K. Correlation of high-frequency esophageal ultrasonography and manometry in the study of esophageal motility. Gastroenterology. 1995;109:832-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Pal A, Brasseur JG. The mechanical advantage of local longitudinal shortening on peristaltic transport. J Biomech Eng. 2002;124:94-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Netter FH. Atlas of human anatomy. Colacino S, editor. White Plains, NY: Ciba-Geigy Corp 1989; . [Cited in This Article: ] |
| 25. | Flochm MH, Floch NR. Netter’s gastroenterology. 2nd ed. Kowdley KV, Pitchumoni CS, Scolapio J, Rosenthal R, editors. Philadelphia, PA: Saunders (Elsevier) 2010; 6-8. [Cited in This Article: ] |
| 26. | Standring S, editor . Gray's anatomy: the anatomical basis of clinical practice. 40th ed. Edinburgh: Churchill Livingstone (Elsevier) 2008; 1111-1125. [Cited in This Article: ] |