Published online May 7, 2011. doi: 10.3748/wjg.v17.i17.2211
Revised: November 10, 2010
Accepted: November 17, 2010
Published online: May 7, 2011
AIM: To study intestinal permeability (IP) and its relationship to the disease activity in patients with inflammatory bowel diseases (IBD) - Crohn’s disease (CD) and ulcerative colitis (UC).
METHODS: Fifty-eight patients with active IBD (32 with CD and 26 with UC) and 25 healthy controls consented to participate in the study. The clinical activity of CD was estimated using the Crohn’s Disease Activity Index (CDAI), and the endoscopic activity of UC using the Mayo scoring system. IP was assessed by the rise in levels of iohexol, which was administered orally (25 mL, 350 mg/mL) 2 h after breakfast. Three and six hours later serum (SIC mg/L) and urine (UIC g/mol) iohexol concentrations were determined by a validated HPLC-UV technique.
RESULTS: In the CD group, SIC values at 3 h (2.95 ± 2.11 mg/L) and at 6 h after ingestion (2.63 ± 2.18 mg/L) were significantly higher compared to those of healthy subjects (1.25 ± 1.40 mg/L and 1.11 ± 1.10 mg/L, respectively, P < 0.05). UIC (g/mol) values were also higher in patients, but the differences were significant only for UIC at 6 h. Significant positive correlation (P < 0.05) was found between the CDAI and IP, assessed by SIC at 3 h (r = 0.60) and 6 h (r = 0.74) after the ingestion. In comparison to controls, SIC and UIC of UC patients were higher in the two studied periods, but the differences were significant at 6 h only. Significantly higher values of SIC (P < 0.05) were found in patients with severe endoscopic activity of UC compared to those of patients with mild and moderate activity (3.68 ± 3.18 vs 0.92 ± 0.69 mg/L).
CONCLUSION: Serum levels of iohexol at 3 h and 6 h after its ingestion reflect increased IP, which is related to the disease activity in patients with IBD.
- Citation: Gerova VA, Stoynov SG, Katsarov DS, Svinarov DA. Increased intestinal permeability in inflammatory bowel diseases assessed by iohexol test. World J Gastroenterol 2011; 17(17): 2211-2215
- URL: https://www.wjgnet.com/1007-9327/full/v17/i17/2211.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i17.2211
Crohn’s disease (CD) and ulcerative colitis (UC), the two principal forms of inflammatory bowel disease (IBD), are characterized by a chronic inflammation of the gastrointestinal tract. Over the past decade there has been increasing recognition of the importance of both epithelial barrier function and innate immunity in the genesis of intestinal inflammation[1-5].
Mucosal barrier function in IBD has been investigated in numerous studies and many of them, but not all[6], have demonstrated increased intestinal permeability (IP) in some of the patients[7-11]. Alterations of IP can be evaluated using several different probes such as disaccharides (lactulose, cellobiose), monosaccharides (mannitol), polyethylenglycols of different molecular mass and 51Cr-ethylenediaminetetraacetate (51Cr-EDTA). Studies have utilized different methods for the assessment of permeability, both in regard to administration procedures of the different probes and to outcome measures[12,13].
So far, IP assessment in patients has been performed by measuring urine recovery of ingested permeability substrates. The urine test can be relatively uncomfortable and can lead to potential inaccuracy[14,15] due to incomplete urine collection, renal dysfunction and variable hydration status of the tested subjects. In addition, some analytical difficulties in quantifying carbohydrates in urine have limited the widespread use of those permeability markers. Furthermore, urinary tract infections may compromise the recovery of sugar permeability markers[16]. Measurement of the permeability substrates in plasma or serum could eventually reduce the problems with urine recovery and could potentially have a valuable role, particularly in pediatric patients.
The aim of the present study was to evaluate IP in patients with active IBD (CD and UC) by measuring serum and urine levels of water-soluble contrast medium iohexol, to assess the relationship of IP to the disease activity, and to compare the reliability of serum vs urine levels of iohexol as an IP disease marker.
The study included 58 patients with active IBD: 32 patients with CD (16 males, 16 females, mean age 38.9 years: range 18-70 years) and 26 patients with UC (11 males, 15 females, mean age 41.5 years: range 21-70 years), hospitalized in the Clinical Centre of Gastroenterology, University Hospital Queen Joanna, Sofia. Diagnosis was based on the commonly accepted clinical, endoscopic and histological criteria. Disease activity of CD patients was estimated using the Crohn’s Disease Activity Index (CDAI); values greater than 150 were accepted as a marker for clinical activity[17]. The endoscopic activity (EA) of UC was assessed using the Mayo scoring system (findings on endoscopy): 0: normal or inactive disease; 1: mild disease (erythema, decreased vascular pattern, mild friability, erosions); 2: moderate disease (marked erythema, lack of vascular pattern, friability); 3: severe disease (spontaneous bleeding, ulceration) (Table 1). CD patients were divided in two subgroups with regard to the endoscopic activity score: “EA 3” - severe and “EA 1+2” - mild and moderate endoscopic activity.
| Variables | CD (n = 32) | UC (n = 26) |
| Males/females | 16/16 | 11/15 |
| Age (yr), mean (range) | 38.9 (18-70) | 41.5 (21-70) |
| Duration of the disease (yr), mean (range) | 6 (1-31) | 6 (1-26) |
| Crohn’s disease | ||
| Area of involvement of the GI tract | ||
| Small intestine only (L1) | 4 | |
| Large intestine only (L2) | 7 | |
| Ileo-colonic (L3) | 21 | |
| UGIT involvement (L4) | 0 | |
| Clinical activity | ||
| CDAI, median (range) | 211 (156-344) | |
| CDAI 151-220 | 17 | |
| CDAI 221-400 | 15 | |
| CDAI > 400 | 0 | |
| Ulcerative colitis | ||
| Extent (E) | ||
| Proctitis (Е 1) | 2 | |
| Left-side (distal) colitis (Е 2) | 12 | |
| Total colitis (Е 3) | 12 | |
| Endoscopic activity-Mayo scoring system (EA) | ||
| Endoscopic remission (EA 0) | 0 | |
| Mild (EA 1) | 6 | |
| Moderate (EA 2) | 5 | |
| Severe (EA 3) | 15 |
The location and behavior of CD, and extent and severity of UC, were classified using the modified Montreal classification[18]. According to CDAI, all CD patients had active disease (CDAI > 150). All patients with UC had endoscopic features of an active disease. Twenty-five healthy persons (18 males, 7 females, mean age 40.6 years: range 25-68 years) were recruited as a control group. None of them had signs or symptoms of gastrointestinal disorders or renal diseases. None of the investigated subjects took alcohol, non-steroidal anti-inflammatory drugs or any other medications (antidepressants, anticholinergics, metoclopramide, lactulose) that have the potential to affect gastrointestinal motility, for at least 2 wk before the test. Renal function, assessed by serum creatinine levels and calculated Glomerular Filtration Rate, was normal in all investigated subjects (Table 1).
The study was approved by the Ethics Committee of University Hospital Queen Joanna. Written informed consent was obtained from all patients and control subjects.
Iohexol (Omnipaque™, Nycomed-General Electric) was administered orally (25 mL of 350 mg/mL injection solution) in the morning, 2 h after breakfast, immediately after voiding. Drinking was not allowed for the next 3 h. Food was permitted after 5 h. Blood and urine samples were collected at 3 and 6 h after the iohexol ingestion. Serum was separated within 45 min after the blood withdrawal by centrifugation at 1 500 g for 10 min. Serum and urine were kept frozen at -20oC until analysis. Iohexol concentrations were determined by a validated high pressure liquid chromatography technique. Briefly, sample preparation consisted of protein precipitation; separation was performed on a C8 column with a mobile phase of 5% aqueous acetonitrile and detection at 240 nm. Selectivity was confirmed by comparing the signal in blank and spiked samples in 12 individual sources of human serum and urine. Accuracy and precision (within-run and between-runs) were within 12%; extraction recovery was over 90%; linearity range was 0.25-10.00 mg/L for serum samples and 2.50-700.00 mg/L for urine samples; R2 > 0.998. Stability was also validated accordingly. Urine results were presented as iohexol/creatinine ratios (g/mol). Results of the control group (mean + 2SD) were used as a cut-off level for increased IP at 95% confidence interval, and values exceeding that cut-off were considered abnormal.
Statistical methods used: descriptive statistics, nonparametric ANOVA (Kruskal-Wallis test), nonparametric Mann-Whitney U test for overall testing the difference between subject groups, nonparametric Wilcoxon test for the difference between pairs of groups, Pearson’s test (r) for correlation. Each hypothesis was tested at a level of significance of 0.05.
All permeability tests were well tolerated and no side effects were reported. Based on the results obtained from healthy subjects, values of SIC over 4.0 and 3.3 mg/L at 3 and 6 h respectively, as well as values of UIC over 35.5 and 31.4 g/mol at 3 and 6 h after ingestion, were accepted as abnormal (cut-offs). One control subject had slightly increased IP at 3 h, and two control subjects at 6 h, post-iohexol ingestion.
The results from applied Kruskal-Wallis test showed that the factor “group” is significant (P < 0.05). Mann-Whitney U test results indicated that in the group of CD patients the mean values of SIC at 3 h (2.95 ± 2.11 mg/L) and at 6 h (2.63 ± 2.18 mg/L) post-ingestion were significantly higher than those in the control group (1.25 ± 1.40 mg/L and 1.11 ± 1.10 mg/L, respectively). Abnormal IP was found in 10 patients at 3 h (31%) and in 16 patients at 6 h (50%) after iohexol intake. Urine recovery of iohexol was also higher in patients 3 h (18.09 ± 13.13 g/mol) and 6 h (36.92 ± 27.68 g/mol) post-ingestion compared to the control group (14.64 ± 10.44 g/mol and 14.18 ± 7.78 g/mol, respectively), but the difference was significant only for the mean UIC values at 6 h.
In the UC group, the mean serum levels (1.57 ± 1.55 mg/L and 2.49 ± 2.80 mg/L) and urine recovery of iohexol (9.86 ± 9.26 g/mol and 27.76 ± 25.18 g/mol) at 3 and 6 h after its ingestion, respectively, were higher than those for healthy controls, but the differences were significant only at 6 h for both parameters. IP was established as abnormal in 3 (11%) and 8 (31%) patients with UC at 3 and 6 h post-iohexol ingestion, respectively.
There was a significant positive correlation between the parameters SIC and CDAI in the group of CD patients at 3 h (r = 0.60) and at 6 h (r = 0.74) post-ingestion, while the correlation between CDAI and UIC was insignificant both at 3 h (r = 0.48) and at 6 h (r = 0.32) post-ingestion of iohexol.
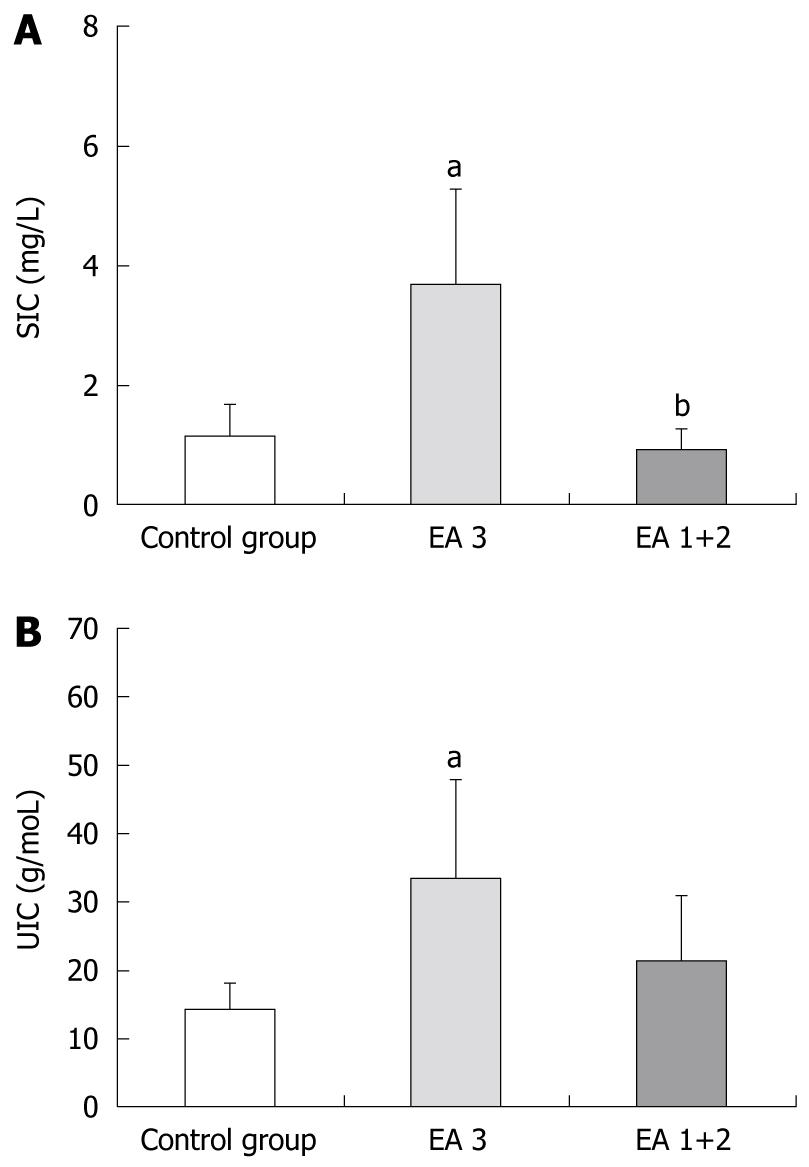
In the subgroup “ЕА 3” of patients with severe endoscopic activity of UC (n=15), the mean values of SIC at 6 h were significantly higher (3.68 ± 3.18 mg/L), compared to those of the control group (1.11 ± 1.10 mg/L), while the values (0.92 ± 0.69 mg/L) for the patient subgroup “ЕА 1+2” (n = 11) with mild and moderate endoscopic activity did not differ from the controls (Figure 1A). The mean UIC values for patient subgroup “ЕА 3” at 6 h were also significantly higher (33.60 ± 28.42 g/mol) compared to those of the control group (14.18 ± 7.78 g/mol), and mean UIC values of the subgroup “EA 1+2” (19.78 ± 18.25 g/mol) were slightly but insignificantly higher from the control values (Figure 1B).
The permeability disturbances were five-fold more frequent in the subgroup of patients with severe activity (47%) in comparison to the subgroup of patients with mild and moderate activity (9%).
Altered barrier function in IBD is documented in experimental and clinical studies, but it is difficult to compare the results of IP due to the existing variability in the chemical nature of the chosen candidate markers in the experimental design and methodology. It is considered that the larger molecular weight markers reflect the changes predominantly in paracellular permeability, while smaller ones register transcellular transfer changes. Paracellular permeability across the epithelial cell monolayers is regulated primarily by the tight junctions (TJs) that encircle the apical poles of the epithelial cells[1,7]. The epithelium in the inflamed intestinal segments of patients with CD is characterized by a reduction of the TJ strands, strand breaks, and alterations of the TJ proteins. In patients with UC the epithelial leaks appear early due to micro-erosions resulting from upregulated epithelial apoptosis and in addition to a prominent increase of claudin-2[3]. Immune regulation of the epithelial functions by cytokines may cause a barrier dysfunction not only by the TJ impairments but also by apoptotic leaks, transcytotic mechanisms and mucosal gross lesions[4].
In our study, IP in patients with IBD was examined by measuring of the serum levels and urine recovery of iohexol, following oral administration. Andersen et al[19] demonstrated that water-soluble radiographic contrast media could be of use for evaluation of the altered intestinal barrier function. The contrast agent iohexol is a moderately large molecule (molecular weight 821 Dalton) with a low absorption under normal conditions, and enhanced absorption through the inflamed intestinal mucosa. It does not bind to serum proteins and is filtered through the glomerulus without indications of tubular secretion or reabsorption. Until now, to the best of our knowledge, serum levels of iohexol have not been used for the assessment of IP. The results of this study demonstrate that iohexol permeation through the intestinal mucosa is significantly increased in both CD and UC patients. Using different probes, similar findings have also been reported in numerous studies both in adult and pediatric populations with IBD[7-11,20-24]. The findings of this study support the understanding that patients with active IBD (including both CD and UC) have a mucosal barrier dysfunction, which can be assessed by measurement of IP. The permeability alterations were more frequent in CD than in UC patients: increased iohexol absorption as a marker for the abnormal IP was established in 50% of CD and in 31% of UC patients, figures which were significantly more frequent in comparison to the healthy subjects. Taking into account the fact that 3 h after ingestion iohexol is located in the large intestine[7], the higher SIC and UIC at 6 h after ingestion in patients with UC in our research suggest that this test can be used for evaluation of the colon permeability also.
The relationship between increased IP and disease activity in IBD has been established in some studies[9,11,20,21]. IP in CD patients is increased proportionally to the disease activity; it can be used to predict the clinical relapse of the disease (due to subclinical mucosal inflammation) and to assess prognosis[25,26]. However, the data in the literature are contradictory, most probably due to the usage of different permeability probes as mentioned above. Our study demonstrates that the permeation of iohexol through the intestinal mucosa, evaluated by serum concentrations, correlates positively with the disease activity in CD patients. These results are in agreement with data reported by Halme et al[7,20] for increased IP of iohexol (measured by its urinary recovery) and its correlation with the clinical disease activity indices. Furthermore, Halme et al[27] concluded that the iohexol test is a superior activity marker compared to the lactulose-mannitol test in patients with IBD. We established a relationship between serum levels of iohexol and the endoscopic activity score for UC patients. Using different permeability markers, Miki et al[11],
Arslan et al[23] and Casellas et al[24] found a relationship between IP and disease activity in IBD patients. However, other investigators did not establish such a correlation[8,28,29]. Our data for significant positive correlation between iohexol penetration through the intestinal mucosa and disease activity support the hypothesis of the important role of the impaired intestinal barrier in the pathogenesis of active IBD[2,3,5].
In conclusion, the water-soluble contrast medium iohexol is a suitable marker for assessing the gut barrier function as its penetration through the intestinal mucosa is increased in patients with active IBD (both CD and UC) and is related to the disease activity. In our study, serum iohexol concentration appears to be a superior marker of altered IP, compared to urinary iohexol level. Measurement of a single serum sample of iohexol 6 h following its oral administration makes the proposed permeability test more convenient and provides a possibility for the assessment of altered barrier function in both small and large intestine.
Increased intestinal permeability (IP) has been implicated in the pathogenesis of the inflammatory bowel diseases (IBD). Mucosal barrier function has been investigated by the measuring of urine recovery of ingested permeability substrates, which is relatively uncomfortable and could lead to potential inaccuracy. To clarify the role of barrier dysfunction and to introduce better diagnostic procedure, the authors decided to evaluate IP in patients with active IBD by measuring serum and urine levels of water-soluble contrast medium iohexol, to assess the relationship of IP to disease activity, and to compare the reliability of serum vs urine levels of iohexol as an IP disease marker.
In the present study the relationship between IP and disease activity in patients with active IBD was investigated by using the iohexol test. The findings support the hypothesis that, by affecting the penetration of macromolecules and pathogens, the permeability disorders are linked to intestinal inflammation and play a role in mucosal damage in IBD.
Mucosal barrier function in IBD has been investigated in numerous studies which differ with respect to methods for the permeability assessment, both in regard to administration procedures of the different probes and to outcome measures. Measurement of permeability substrates in plasma or serum may reduce the problems with urine recovery and might potentially have a valuable role.
The assessment of permeability alterations with the suitable, easy to perform, and convenient iohexol test provides a platform for therapeutic modulation of the gut barrier function for better control of IBD patients.
IP reflects the integrity of the intestinal mucosa to prevent penetration of macromolecules and bacterial antigens from the gut lumen. Tight junctions are continuous, circumferential, belt-like structures that encircle the apical poles of the epithelial cells. They are a key regulator of paracellular permeability across the intestinal epithelium. Iohexol is a non-ionic, water-soluble contrast agent.
The authors found that patients with active ulcerative colitis and Crohn’s disease had increased serum and urinary concentration of iohexol, indicating disruptive IP. There was a correlation between severity of intestinal leakiness and disease activity. The authors concluded urinary and serum iohexol are acceptable methods of assessing IP. The authors concluded that IP disrupted in patients with active IBD. It is an interesting study and data appear to support the conclusion.
Peer reviewer: Ali Keshavarzian, MD, Josephine M. Dyrenforth Professor of Medicine, Professor of Pharmacology and Molecular Biophysics and Physiology Director, Digestive Diseases and Nutrition Vice Chairman of Medicine for Academic and Research Affairs, Rush University Medical Center 1725 W. Harrison, Suite 206, Chicago, IL 60612, United States
S- Editor Sun H L- Editor Logan S E- Editor Zheng XM
| 1. | Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3-20; quiz 21-22. [Cited in This Article: ] |
| 2. | McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100-113. [Cited in This Article: ] |
| 3. | Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379-383. [Cited in This Article: ] |
| 4. | Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M, Fromm M. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci. 2009;1165:294-300. [Cited in This Article: ] |
| 5. | Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401-407. [Cited in This Article: ] |
| 6. | Munkholm P, Langholz E, Hollander D, Thornberg K, Orholm M, Katz KD, Binder V. Intestinal permeability in patients with Crohn's disease and ulcerative colitis and their first degree relatives. Gut. 1994;35:68-72. [Cited in This Article: ] |
| 7. | Halme L, Edgren J, von Smitten K, Linden H. Increased urinary excretion of iohexol after enteral administration in patients with ileal Crohn's disease. A new test for disease activity. Acta Radiol. 1993;34:237-241. [Cited in This Article: ] |
| 8. | Benjamin J, Makharia GK, Ahuja V, Kalaivani M, Joshi YK. Intestinal permeability and its association with the patient and disease characteristics in Crohn's disease. World J Gastroenterol. 2008;14:1399-1405. [Cited in This Article: ] |
| 9. | Welcker K, Martin A, Kölle P, Siebeck M, Gross M. Increased intestinal permeability in patients with inflammatory bowel disease. Eur J Med Res. 2004;9:456-460. [Cited in This Article: ] |
| 10. | Miele E, Pascarella F, Quaglietta L, Giannetti E, Greco L, Troncone R, Staiano A. Altered intestinal permeability is predictive of early relapse in children with steroid-responsive ulcerative colitis. Aliment Pharmacol Ther. 2007;25:933-939. [Cited in This Article: ] |
| 11. | Miki K, Moore DJ, Butler RN, Southcott E, Couper RT, Davidson GP. The sugar permeability test reflects disease activity in children and adolescents with inflammatory bowel disease. J Pediatr. 1998;133:750-754. [Cited in This Article: ] |
| 12. | Sun Z, Wang X, Andersson R. Role of intestinal permeability in monitoring mucosal barrier function. History, methodology, and significance of pathophysiology. Dig Surg. 1998;15:386-397. [Cited in This Article: ] |
| 13. | Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512-1520. [Cited in This Article: ] |
| 14. | Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566-1581. [Cited in This Article: ] |
| 15. | Cox MA, Lewis KO, Cooper BT. Measurement of small intestinal permeability markers, lactulose, and mannitol in serum: results in celiac disease. Dig Dis Sci. 1999;44:402-406. [Cited in This Article: ] |
| 16. | Milnes JP, Walters AJ, Andrews DJ, Low-Beer TS. Urinary infection may invalidate the double-sugar test of intestinal permeability. Scand J Gastroenterol. 1988;23:885-890. [Cited in This Article: ] |
| 17. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. [Cited in This Article: ] |
| 18. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5-36. [Cited in This Article: ] |
| 19. | Andersen R, Laerum F. Intestinal permeability measurements - a new application for water soluble contrast media? Acta Radiol Suppl. 1995;399:247-252. [Cited in This Article: ] |
| 20. | Halme L, Edgren J, Turpeinen U, von Smitten K, Stenman UH. Urinary excretion of iohexol as a marker of disease activity in patients with inflammatory bowel disease. Scand J Gastroenterol. 1997;32:148-152. [Cited in This Article: ] |
| 21. | Resnick RH, Royal H, Marshall W, Barron R, Werth T. Intestinal permeability in gastrointestinal disorders. Use of oral [99mTc]DTPA. Dig Dis Sci. 1990;35:205-211. [Cited in This Article: ] |
| 22. | Issenman RM, Jenkins RT, Radoja C. Intestinal permeability compared in pediatric and adult patients with inflammatory bowel disease. Clin Invest Med. 1993;16:187-196. [Cited in This Article: ] |
| 23. | Arslan G, Atasever T, Cindoruk M, Yildirim IS. (51)CrEDTA colonic permeability and therapy response in patients with ulcerative colitis. Nucl Med Commun. 2001;22:997-1001. [Cited in This Article: ] |
| 24. | Casellas F, Aguadé S, Soriano B, Accarino A, Molero J, Guarner L. Intestinal permeability to 99mTc-diethylenetriaminopentaacetic acid in inflammatory bowel disease. Am J Gastroenterol. 1986;81:767-770. [Cited in This Article: ] |
| 25. | Takeuchi K, Maiden L, Bjarnason I. Genetic aspects of intestinal permeability in inflammatory bowel disease. Novartis Found Symp. 2004;263:151-158; discussion 159-163, 211-218. [Cited in This Article: ] |
| 26. | Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000;35:1163-1169. [Cited in This Article: ] |
| 27. | Halme L, Turunen U, Tuominen J, Forsström T, Turpeinen U. Comparison of iohexol and lactulose-mannitol tests as markers of disease activity in patients with inflammatory bowel disease. Scand J Clin Lab Invest. 2000;60:695-701. [Cited in This Article: ] |
| 28. | Ukabam SO, Clamp JR, Cooper BT. Abnormal small intestinal permeability to sugars in patients with Crohn's disease of the terminal ileum and colon. Digestion. 1983;27:70-74. [Cited in This Article: ] |
| 29. | Turck D, Ythier H, Maquet E, Deveaux M, Marchandise X, Farriaux JP, Fontaine G. Intestinal permeability to [51Cr]EDTA in children with Crohn's disease and celiac disease. J Pediatr Gastroenterol Nutr. 1987;6:535-537. [Cited in This Article: ] |