Published online Sep 7, 2010. doi: 10.3748/wjg.v16.i33.4187
Revised: May 4, 2010
Accepted: May 11, 2010
Published online: September 7, 2010
AIM: To compare the imaging results with histology and to evaluate the diagnostic sensitivity of imaging modalities for hepatocellular carcinoma (HCC) smaller than 2 cm.
METHODS: Nodules smaller than 2 cm (n = 34) revealed by ultrasonography (US) in 29 patients with liver cirrhosis were analyzed. Histological diagnosis of HCC was performed by ultrasonographic guidance: moderately-differentiated HCC (n = 24); well-differentiated HCC (n = 10). The patterns disclosed by the four imaging modalities defined the conclusive diagnosis of HCC: (1) contrast-enhanced computed tomography (CECT), hypervascularity in the arterial phase and washout in the equilibrium phase; (2) Sonazoid contrast-enhanced US (CEUS), hypervascularity in the early vascular phase and defect in the Kupffer phase; (3) gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI), hypervascularity in the arterial phase and/or defect in the hepatobiliary phase; and (4) CT arterioportal angiography: hypervascularity by CT during arteriography and/or perfusion defect by CT during arterial portography.
RESULTS: Overall, the sensitivity of diagnosing HCC smaller than 2 cm was 52.9% (18/34) (95% CI: 35.1-70.2) by CECT; 67.6% (23/34) (95% CI: 49.5-82.6) by Sonazoid CEUS; 76.5% (26/34) (95% CI: 58.8-89.3) by Gd-EOB-DTPA MRI; and 88.2% (30/34) (95% CI: 72.5-96.7) by CT arterioportal angiography. The diagnostic sensitivity of detecting moderately-differentiated HCC by CECT, Sonazoid CEUS, Gd-EOB-DTPA MRI and CT arterioportal angiography was 62.5% (15/24) (95% CI: 40.6-81.2), 79.2% (19/24) (95% CI: 57.8-92.9), 75.0% (18/24) (95% CI: 53.3-90.2) and 95.8% (23/24) (95% CI: 78.9-99.9), respectively. A significant difference (P < 0.05) was observed between CECT and CT arterioportal angiography in all nodules. There was no difference between Sonazoid CEUS, Gd-EOB-DTPA MRI, and CT arterioportal angiography. The combined sensitivity of Sonazoid CEUS and Gd-EOB-DTPA MRI was 94.1% (32/34).
CONCLUSION: Changing the main diagnostic modality for HCC smaller than 2 cm from CT arterioportal angiography to Sonazoid CEUS and Gd-EOB-DTPA MRI is recommended.
- Citation: Mita K, Kim SR, Kudo M, Imoto S, Nakajima T, Ando K, Fukuda K, Matsuoka T, Maekawa Y, Hayashi Y. Diagnostic sensitivity of imaging modalities for hepatocellular carcinoma smaller than 2 cm. World J Gastroenterol 2010; 16(33): 4187-4192
- URL: https://www.wjgnet.com/1007-9327/full/v16/i33/4187.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i33.4187
The definitive diagnosis of nodular lesions, detected by imaging techniques in the liver with cirrhosis, remains a critical challenge for clinicians. The issue is particularly complicated for small (1-2 cm) nodules, many of which may be preneoplastic with uncertain malignant potential[1], such as macroregenerative nodules, low-grade dysplastic nodules (LGDN) or high-grade dysplastic nodules (HGDN), or more rarely, hemangiomas that are found in up to 42% of explanted livers[2-4].
Recently, clinicians have been able to conduct computed tomography (CT) scanning during angiography, thereby acquiring data on lesions and intranodular blood flow simultaneously[5,6]. To resolve the areas of uncertainty, we have previously reported on the superiority of CT arterioportal angiography [including CT during arteriography (CTA) and CT during arterial portography (CTAP)], concluding that it is superior to contrast-enhanced CT (CECT) and magnetic resonance imaging (MRI) in the diagnosis of hepatocellular carcinoma (HCC) nodules smaller than 2 cm[7].
Moreover, development of the newly introduced diagnostic imaging techniques, Sonazoid contrast-enhanced ultrasonography (CEUS) and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI have provided higher degrees of detectability for small HCC. In this study, we compared the diagnostic sensitivity of CECT, Sonazoid CEUS, Gd-EOB-DTPA MRI, and CT arterioportal angiography in diagnosing HCC in nodules smaller than 2 cm.
From April 2008 to December 2009, we analyzed 34 nodules smaller than 2 cm [8-20 mm; mean ± SD 12.7 ± 3.71 mm; the interquartile range (IQR) 10-15 mm] detected by US in 29 patients (13 men and 16 women; aged 55-84 years; mean ± SD 70.5 ± 7.96 years; IQR 67-76 years) with liver cirrhosis related to hepatitis B virus in 1, hepatitis C virus (HCV) in 24, and alcohol in 4. α-fetoprotein (AFP) measured less than 20 ng/mL in 21 patients and was above 21 ng/mL in 8 (Table 1). In this study, one nodule that was not histologically diagnosed as HCC irrespective of compatibility by imaging studies was excluded, and two nodules were excluded because of inconsistency between readers in the imaging results. The study was approved by the Ethics Committee in Kobe Asahi Hospital.
| Age (yr), range (mean ± SD) | 55-84 (70.5 ± 7.96) IQR 67-76 |
| Sex (M/F) | 13/16 |
| Cause | |
| HBV | 1 |
| HCV | 24 |
| Alcohol | 4 |
| AFP (ng/mL) | |
| < 20 | 21 |
| > 21 | 8 |
| Nodule characteristics (mm), range (mean ± SD) | 8-20 (12.7 ± 3.71) IQR 10-15 |
| Histological diagnosis of the 34 nodules | |
| Moderately-differentiated HCC | 24 |
| Well-differentiated HCC | 10 |
CECT was conducted with the use of helical CT (Siemens, Germany) with precontrast and postcontrast triple-phase (arterial, portal, venous, and equilibrium phases) scans, after the injection of 120 mL of nonionic contrast medium at 3 mL/s; the scans were carried out in a craniocaudal direction with a 5 mm collimation in the other phases. Acquisition of the arterial and equilibrium phases was automatically started at 30 and 180 s, respectively, after the intravenous injection.
Ultrasonography was performed using a SSA-660A (Toshiba Medical Systems, Tochigi, Japan). The vascular findings on phase-inversion harmonic US were shown as tumor vessel flow in the early vascular phase about 15-40 s after injection of Sonazoid (GE HealthCare, Piscataway, NJ, USA). The real-time replenishing images were obtained during the vascular phase (< 2 min after the injection) by release burst imaging. Images of the liver parenchyma were obtained in the postvascular Kupffer phase, at least 10 min after the intravenous injection of Sonazoid. Hepatic malignances were visualized as defects in the postvascular phase. An additional contrast agent was injected to confirm tumor vessel flow in the defect, a technique known as defect reperfusion imaging[8].
Images by MRI scans (Phillips, Netherlands) were obtained by the 1.0-T superconducting system (Gyroscan 10T-NT, Phillips, Netherlands). Enhanced MRI was used to obtain coronal images by the gradient-echo technique (FFG) at 150/3.5 ms TR/TE, 80° flip angle, and 168 × 256 matrix. In each sequence, the respiration suspension time was 20-30 s. Gd-EOB-DTPA (Primovist; Bayer HealthCare, Osaka, Japan) at a dose of 0.025 mmol/kg body weight was injected intravenously as a rapid bolus at 2 mL/s. Dynamic contrast-enhanced MRI was initiated at 30 s, 70 s, 2-3 min and 20 min after the start of the bolus injection to obtain multiphasic (arterial, portal, late, and hepatobiliary) images.
CTA: At angiography, 45 mL of diluted contrast medium was injected through a catheter at 2 mL/s into the common hepatic artery. The whole liver was then scanned at intervals of 5 to 10 mm.
CTAP: At angiography, 115 mL of diluted contrast medium was injected through a catheter at 2 mL/s into the superior mesenteric artery, according to the scanning time of the entire liver using a power injector during sequential scanning of the liver with incremental changes in the position of the table. Infusion of contrast material was initiated 20 s before CTAP. The whole liver was then scanned at intervals of 5 to 10 mm.
US-guided biopsy was carried out with the use of a 21 gauge Majima needle (Top, Japan). The diagnosis of HCC was made by two operators [a physician (K.S.) and a pathologist (Y.H.)] using the same specimen.
Specimens were routinely processed and stained with hematoxylin and eosin and by the Masson trichromatic method. The diagnosis of HCC was made according to the criteria of the International Working Party[1].
The following patterns disclosed by the four imaging modalities were defined as the conclusive diagnosis of HCC. (1) CECT: hypervascularity in the arterial phase and washout in the equilibrium phase; (2) Sonazoid CEUS: hypervascularity in the early vascular phase and defect in the Kupffer phase; (3) Gd-EOB-DTPA MRI: hypervascularity in the arterial phase and/or defect in the hepatobiliary phase; and (4) CT arterioportal angiography: hypervascularity by CTA and/or perfusion defect by CTAP (Table 2).
| Modality | Imaging pattern |
| Contrast-enhanced CT | Hypervascularity in the arterial phase and washout in the equilibrium phase |
| Sonazoid contrast-enhanced ultrasonography | Hypervascularity in the early vascular phase and defect in the Kupffer phase |
| Gd-EOB-DTPA magnetic resonance imaging | Hypervascularity in the arterial phase and/or defect in the hepatobiliary phase |
| CT arterioportal angiography | Hypervascularity by CTA and/or perfusion defect by CTAP |
To minimize differences in the results between the operators, imaging studies were carried out and reviewed by two operators [a physician (M.K.) and a radiologist (T.M.)] using the same examination protocol.
The sensitivity for detecting tumors was indicated by the 95% CI. The 95% CI was estimated by F distribution. The level of significance was set at P < 0.05.
The 34 nodules were histologically diagnosed as moderately-differentiated (24 nodules) and well-differentiated (10 nodules) HCC (Table 1). For HCC smaller than 2 cm, the overall diagnostic sensitivity was 52.9% (18/34) (95% CI: 35.1-70.2) by CECT; 67.6% (23/34) (95% CI: 49.5-82.6) by Sonazoid CEUS; 76.5% (26/34) (95% CI: 58.8-89.3) by Gd-EOB-DTPA MRI; and 88.2% (30/34) (95% CI: 72.5-96.7) by CT arterioportal angiography, with a significant difference (P < 0.05) between CECT and CT arterioportal angiography. The combined sensitivity of Sonazoid CEUS and Gd-EOB-DTPA MRI was 94.1% (32/34). In diagnosing moderately-differentiated HCC, the diagnostic sensitivity of CECT, Sonazoid CEUS, Gd-EOB-DTPA MRI and CT arterioportal angiography was 62.5% (15/24) (95% CI: 40.6-81.2), 79.2% (19/24) (95% CI: 57.8-92.9), 75.0% (18/24) (95% CI: 53.3-90.2) and 95.8% (23/24) (95% CI: 78.9-99.9), respectively. There was no difference between CECT, Sonazoid CEUS, Gd-EOB-DTPA MRI, and CT arterioportal angiography in moderately differentiated HCC. The sensitivity of well-differentiated HCC was not analyzed because of the paucity of cases (Table 3).
| Modality | Diagnostic sensitivity | |||
| All nodules (n = 34) | Moderately-differentiated HCC (n = 24) | |||
| n (%) | 95% CI | n (%) | 95% CI | |
| Contrast-enhanced computed tomography | 18 (52.9) | 35.1-70.2 | 15 (62.5) | 40.6-81.2 |
| Sonazoid contrast-enhanced ultrasonography | 23 (67.6) | 49.5-82.6 | 19 (79.2) | 57.8-92.9 |
| Gd-EOB-DTPA magnetic resonance imaging | 26 (76.5) | 58.8-89.3 | 18 (75.04) | 53.3-90.2 |
| Computed tomography arterioportal angiography | 30 (88.2) | 72.5-96.7 | 23 (95.8) | 78.9-99.9 |
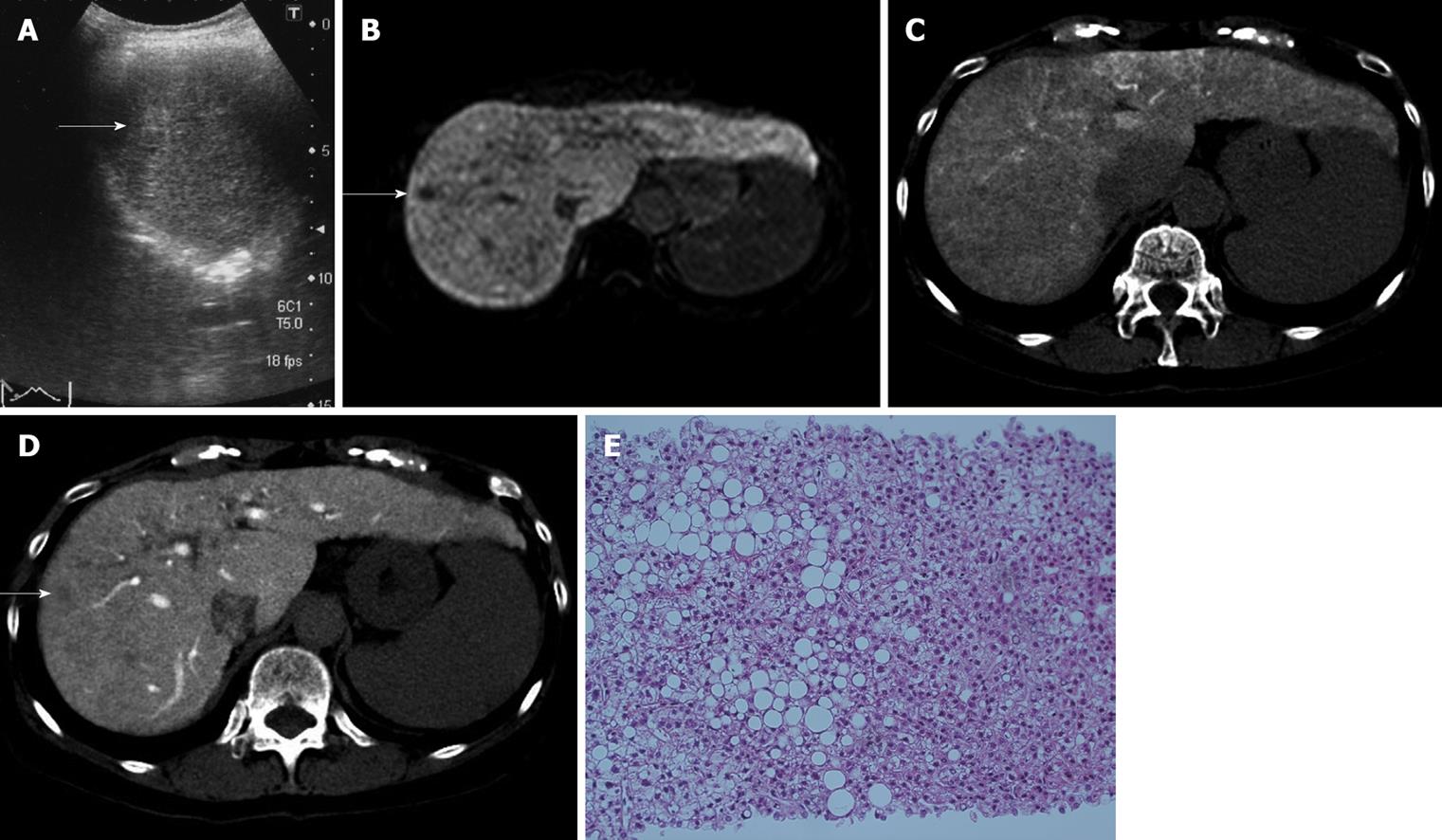
Case No. 1: Detection by Gd-EOB-DTPA MRI and arterioportal angiography: In a 67-year-old woman with HCV-related liver cirrhosis (AFP 9.0 ng/mL; PIVKAII 21 mAU/mL), US revealed a 12 mm hyperechoic nodule in segment eight (Figure 1A). Sonazoid CEUS revealed no hypervascularity in the early vascular phase and no defect in the Kupffer phase. CECT revealed no hypervascularity in the arterial phase and washout in the equilibrium phase. Gd-EOB-DTPA MRI revealed no hypervascularity in the arterial phase, but a defect in the hepatobiliary phase (Figure 1B). CTA revealed isodensity (Figure 1C), and CTAP a perfusion defect (Figure 1D). US-guided biopsy revealed moderately-differentiated HCC (Figure 1E).
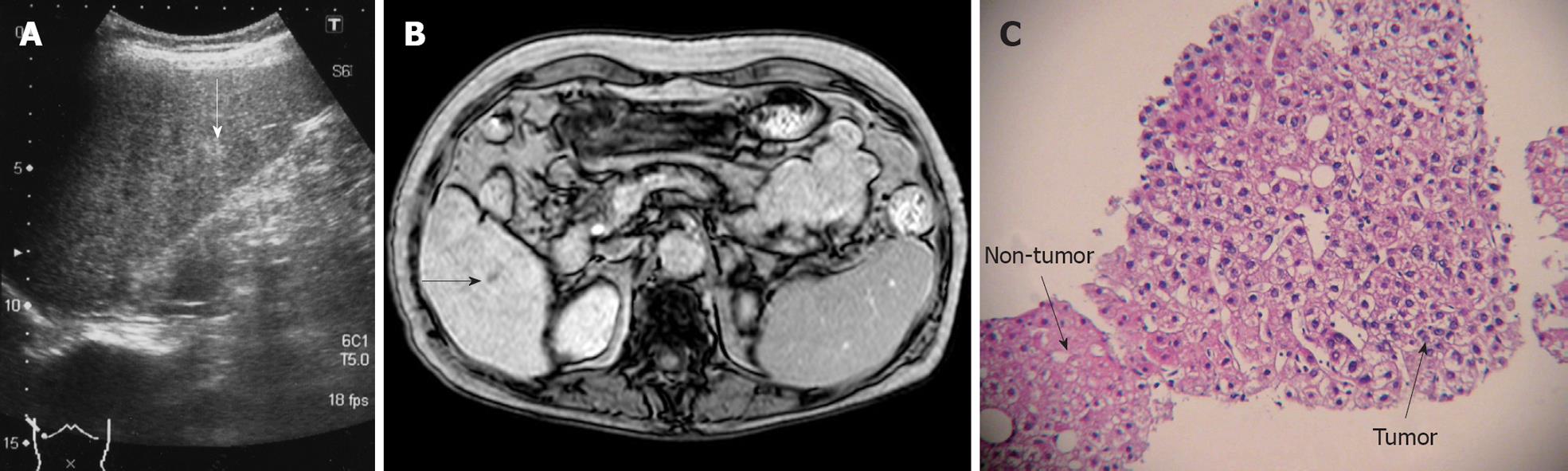
Case No. 2: Detection by Gd-EOB-DTPA MRI: In a 74-year-old woman with HCV-related liver cirrhosis (AFP 7.1 ng/mL, PIVKA II 42 mAU/mL), US revealed an 8 mm hyperechoic nodule in segment six (Figure 2A). Sonazoid CEUS revealed no hypervascularity in the early vascular phase and no defect in the Kupffer phase. CECT revealed isodensity in both the arterial phase and the equilibrium phase. MRI revealed isointensity. Gd-EOB-DTPA MRI revealed no hypervascularity in the early phase, but disclosed a defect in the hepatobiliary phase (Figure 2B). CTA revealed no hypervascularity and CTAP no perfusion defect. US-guided biopsy revealed well-differentiated HCC (Figure 2C).
Confirmation of arterial hypervascularity by three imaging modalities (triphasic CT, triphasic MRI, and CEUS), even in the absence of a significant (> 400 ng/mL) rise in AFP, is recommended by the European Association for the Study of the Liver (EASL) as diagnostic criteria for HCC nodules larger than 2 cm in patients with cirrhosis[9]. These recommendations for the management of HCC provide a rational approach to the problem but leave some areas of uncertainty, particularly those regarding the interpretation of discordant vascularity, the use of imaging techniques in nodules smaller than 2 cm, the meaning of truly hypovascular nodules, and the management of those diagnosed with LGDN or HGDN at guided biopsy.
The American Association for the Study of Liver Diseases[2] recommends that the diagnosis of HCC should be made without biopsy when characteristic arterial vascularization and venous washout are observed on three imaging modalities: triphasic CT scan, triphasic MRI and contrast-enhanced harmonic US.
Nevertheless, these recommendations have not been tested and validated except by Bolondi et al[10] and Forner et al[11]. According to Bolondi et al[10], the noninvasive EASL criteria with CEUS and CECT for the diagnosis of HCC are satisfied in only 44% of nodules smaller than 2 cm in cirrhosis. Forner et al[11] reported that the diagnostic sensitivity of MRI and CEUS in the diagnosis of HCC (smaller than 2 cm) is 67%.
The main characteristics of Sonazoid, a newly introduced second-generation US contrast agent exclusively approved in Japan in 2007, are that it facilitates real-time blood flow images at low acoustic power and stable Kupffer phase imaging from 10 to 120 min after its injection. In vascular imaging, Sonazoid is considered more effective than Levovist and easy to use; it allows visualization, even with the use of non-high-end equipment and, therefore, reduces dependence on the operator’s skills/equipment, all of which may promote the widespread use of CEUS. As stated earlier, Sonazoid CEUS provides very stable postvascular phase images for up to 60-120 min[12], which has resulted in the invention of the breakthrough method, defect reperfusion imaging that is an innovative technology that will greatly change the daily practices of HCC management. In our study, the diagnostic sensitivity of Sonazoid CEUS was 67.6% in all HCC, and 79.2% in moderately-differentiated HCC.
Kudo et al[8,13,14] have recently developed defect reperfusion imaging (using the properties of very stable Kupffer phase images and real-time fine blood flow images obtained with Sonazoid) for typical HCC, which is depicted by CT but not by B mode scanning. The method is a breakthrough for accurate localization and treatment guidance[8]: dramatic resolution of many limitations in the diagnosis and treatment of HCC, such as detection of small HCCs[15], evaluation of treatment response[16], and needle insertion guidance; additionally, detection is even more sensitive than with MDCT[15].
A newly introduced contrast agent, Gd-EOB-DTPA, approved in Japan in 2008, is a hepatocyte-specific MRI contrast medium with a different mechanism that utilizes neither dynamic nor Kupffer cell imaging. It is useful in cases which would be difficult to diagnose by techniques such as dynamic MRI or SPIO-MRI. Typical HCC shows high intensity with Gd-EOB-DTPA in the arterial-dominant phase and low intensity in the portal-dominant phase and thereafter. The imaging diagnosis of HCC can be made approximately 10-20 min after the injection of Gd-EOB-DTPA. In our study, the diagnostic sensitivity of Gd-EOB-DTPA MRI was 76.5% in all nodules and 75.0% in moderately-differentiated HCC.
Previously, we had concluded that CT arterioportal angiography was superior to CECT and Gadolinium-enhanced MRI for diagnosing HCC in nodules smaller than 2 cm[7]. In this study, the diagnostic sensitivity of CT arterioportal angiography was 88.2% in all nodules and 95.8% in moderately-differentiated HCC. We observed a significant difference between CECT and CT arterioportal angiography (P < 0.05) in all nodules. However, there was no difference between Sonazoid CEUS, Gd-EOB-DTPA MRI, and CT arterioportal angiography. The combined sensitivity of Sonazoid CEUS and Gd-EOB-DTPA MRI in all nodules was 94.1%, due to improvement in the diagnostic capabilities of Sonazoid CEUS and Gd-EOB-DTPA MRI. This improvement in these two imaging modalities with the use of the newly introduced contrast agents provided higher sensitivity for the diagnosis of nodules smaller than 2 cm with Sonazoid CEUS and Gd-EOB-DTPA MRI than with Sonovue CEUS and CECT reported by Bolondi et al[10], or with Sonovue CEUS and Gadolinium-enhanced MRI reported by Forner et al[11].
These results, considered together with the invasiveness of CT arterioportal angiography, suggest that the principal diagnostic modality for HCC smaller than 2 cm should be changed from CT arterioportal angiography to Sonazoid CEUS and Gd-EOB-DTPA MRI.
In spite of the recent advances in imaging techniques, the definitive diagnosis of nodular lesions detected by imaging modalities in the liver with cirrhosis remains a critical challenge for clinicians. The issue is particularly complicated for small (1-2 cm) nodules, many of which may be preneoplastic with uncertain malignant potential. We undertook this study to evaluate the effectiveness of imaging techniques in the diagnosis of hepatocellular carcinoma (HCC) smaller than 2 cm on the basis of histologic findings. Four imaging modalities were compared: contrast-enhanced computed tomography (CECT), Sonazoid contrast-enhanced ultrasonography (CEUS), gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) magnetic resonance imaging (MRI), and CT arterioportal angiography.
The authors compared the imaging results with histology and evaluated the diagnostic sensitivity of the 4 imaging modalities.
Previously, the authors had concluded that CT arterioportal angiography was superior to CECT and gadolinium-enhanced MRI for diagnosing HCC in nodules smaller than 2 cm. In this study, the sensitivity of diagnosing 34 HCCs smaller than 2 cm was 52.9% by CECT; 67.6% by Sonazoid CEUS; 76.5% by Gd-EOB-DTPA MRI; and 88.2% by CT arterioportal angiography. A significant difference was observed between CECT and CT arterioportal angiography (P < 0.05). There was no difference between Sonazoid CEUS, Gd-EOB-DTPA MRI, and CT arterioportal angiography, and the combined sensitivity of Sonazoid CEUS and Gd-EOB-DTPA MRI was 94.1%, due to improvement in the diagnostic sensitivity of Sonazoid CEUS and Gd-EOB-DTPA MRI. This improvement in these two imaging modalities with the use of the newly introduced contrast agents provided higher sensitivity for the diagnosis of nodules smaller than 2 cm with Sonazoid CEUS and Gd-EOB-DTPA MRI than with Sonovue CEUS and CECT reported by Bolondi et al, or with Sonovue CEUS and Gadolinium-enhanced MRI reported by Forner et al.
These results, considered together with the invasiveness of CT arterioportal angiography, suggest that the principal diagnostic modality for HCC smaller than 2 cm should be changed from CT arterioportal angiography to Sonazoid CEUS and Gd-EOB-DTPA MRI.
The major strength of the study is that there are many patients with small tumors. The patients have also been applied to new equipment and new contrast substances. It’s a very interesting paper.
Peer reviewers: Søren Rafaelsen, MD, Consultant Radiologist, Associate Professor, Department of Radiology, Vejle Hospital, Vejle, 7100, Denmark; Bernardo Frider, MD, Professor, Department of Hepatology, Hospital General de Agudos Cosme Argerich, Alte Brown 240, Buenos Aires 1155, Argentina
S- Editor Wang JL L- Editor Webster JR E- Editor Zheng XM
| 1. | Terminology of nodular hepatocellular lesions. International Working Party. Hepatology. 1995;22:983-993. [Cited in This Article: ] |
| 2. | Theise ND, Schwartz M, Miller C, Thung SN. Macroregenerative nodules and hepatocellular carcinoma in forty-four sequential adult liver explants with cirrhosis. Hepatology. 1992;16:949-955. [Cited in This Article: ] |
| 3. | Mion F, Grozel L, Boillot O, Paliard P, Berger F. Adult cirrhotic liver explants: precancerous lesions and undetected small hepatocellular carcinomas. Gastroenterology. 1996;111:1587-1592. [Cited in This Article: ] |
| 4. | Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034-1042. [Cited in This Article: ] |
| 5. | Hayashi M, Matsui O, Ueda K, Kawamori Y, Gabata T, Kadoya M. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology. 2002;225:143-149. [Cited in This Article: ] |
| 6. | Tajima T, Honda H, Taguchi K, Asayama Y, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Shimada M, Masuda K. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002;178:885-897. [Cited in This Article: ] |
| 7. | Kim SR, Ando K, Mita K, Fuki S, Ikawa H, Kanbara Y, Imoto S, Matsuoka T, Hayashi Y, Kudo M. Superiority of CT arterioportal angiography to contrast-enhanced CT and MRI in the diagnosis of hepatocellular carcinoma in nodules smaller than 2 cm. Oncology. 2007;72 Suppl 1:58-66. [Cited in This Article: ] |
| 8. | Kudo M, Hatanaka K, Chung H, Minami Y, Maekawa K. A proposal of novel treatment-assist technique for hepatocellular carcinoma in the sonazoid-enhanced ultrasonography: value of defect re-perfusion imaging (in Japanese). Kanzo. 2007;48:299-301. [Cited in This Article: ] |
| 9. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [Cited in This Article: ] |
| 10. | Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27-34. [Cited in This Article: ] |
| 11. | Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104. [Cited in This Article: ] |
| 12. | Inoue T, Kudo M, Hatanaka K, Takahashi S, Kitai S, Ueda T, Ishikawa E, Hagiwara S, Minami Y, Chung H. Imaging of hepatocellular carcinoma: qualitative and quantitative analysis of postvascular phase contrast-enhanced ultrasonography with sonazoid. Comparison with superparamagnetic iron oxide magnetic resonance images. Oncology. 2008;75 Suppl 1:48-54. [Cited in This Article: ] |
| 13. | Kudo M, Hatanaka K, Maekawa K. Defect reperfusion imaging, a newly developed novel technology using sonazoid in the treatment of hepatocellular carcinoma. J Med Ultrasound. 2008;16:169-176. [Cited in This Article: ] |
| 14. | Kudo M, Hatanaka K, Maekawa K. Sonazoid-enhanced ultrasound in the diagnosis and treatment of hepatic tumors. J Med Ultrasound. 2008;16:130-139. [Cited in This Article: ] |
| 15. | Hatanaka K, Kudo M, Minami Y, Maekawa K. Sonazoid-enhanced ultrasonography for diagnosis of hepatic malignancies: comparison with contrast-enhanced CT. Oncology. 2008;75 Suppl 1:42-47. [Cited in This Article: ] |
| 16. | Xia Y, Kudo M, Minami Y, Hatanaka K, Ueshima K, Chung H, Hagiwara S, Inoue T, Ishikawa E, Kitai S. Response evaluation of transcatheter arterial chemoembolization in hepatocellular carcinomas: the usefulness of sonazoid-enhanced harmonic sonography. Oncology. 2008;75 Suppl 1:99-105. [Cited in This Article: ] |