Published online Jul 28, 2008. doi: 10.3748/wjg.14.4512
Revised: May 23, 2008
Accepted: May 30, 2008
Published online: July 28, 2008
AIM: To evaluate the effects of chlorella crude extract (CCE) on intestinal adaptation in rats subjected to short bowel syndrome (SBS).
METHODS: Wistar rats weighing 230-260 g were used in the study. After anesthesia a 75% small bowel resection was performed. Rats were randomized and divided into groups. Control group (n = 10): where 5% dextrose was given through a gastrostomy tube, Enteral nutrition (EN) group (n = 10): Isocaloric and isonitrogen EN (Alitraq, Abbott, USA), study group (n = 10): CCE was administrated through a gastrostomy tube. Rats were sacrificed on the fifteenth postoperative day and blood and tissue samples were taken. Histopathologic evaluation, intestinal mucosal protein and DNA levels, intestinal proliferation and apoptosis were determined in intestinal tissues, and total protein, albumin and citrulline levels in blood were studied.
RESULTS: In rats receiving CCE, villus lengthening, crypt depth, mucosal DNA and protein levels, intestinal proliferation, and serum citrulline, protein and albumin levels were found to be significantly higher than those in control group. Apoptosis in CCE treated rats was significantly reduced when compared to EN group rats.
CONCLUSION: CCE has beneficial effects on intestinal adaptation in experimental SBS.
- Citation: Kerem M, Salman B, Pasaoglu H, Bedirli A, Alper M, Katircioglu H, Atici T, Perçin EF, Ofluoglu E. Effects of microalgae chlorella species crude extracts on intestinal adaptation in experimental short bowel syndrome. World J Gastroenterol 2008; 14(28): 4512-4517
- URL: https://www.wjgnet.com/1007-9327/full/v14/i28/4512.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4512
Short bowel syndrome (SBS) is a clinical condition characterized by diarrhea, dehydration, electrolyte imbalance, malabsorption, and progressive malnutrition related to a wide resection of the small intestine[1–3]. In the pediatric population, necrotizing enterocolitis, gastroschisis, omphalocele, intestinal atresia and Hirschsprung disease, and in the adults mesenteric vascular occlusion, inflammatory bowel disease (IBD) and malignancies, are the most common reasons for performing extensive resection of the intestine[2]. Retaining intestinal autonomy depends on the length of remaining intestine and the adaptive capacity of the intestinal remnant. Some compensatory changes occur after massive intestinal resection in order to maintain adequate digestion. Restoration of the absorptive surface area and functional capacity result in morphologic and functional improvement. Structural adaptation after intestinal resection involves intestinal dilatation and elongation, villus lengthening, and increasing crypt cell proliferation. These changes result in a marked increase in the intestinal absorptive surface area[4]. The functional adaptation mechanism in the remnant intestine is still not entirely understood. Regulation of intestinal adaptation is an extremely complicated process influenced by many factors[5]. Some of these factors are nutrients, gastrointestinal secretions, hormones, and a variety of polypeptides stimulating growth capability[6]. The most important therapeutic objectives in the management of SBS are maintenance of the patient’s calorie intake and nutritional status. Optimal intestinal rehabilitation should enhance the intestinal adaptation and shorten the period of intestinal recovery[78]; Yet, today no such optimal therapy exists. However, some enteral nutrition (EN) products are used for energy supports in order to reduce demand for total parenteral nutrition (TPN). The prospective, randomised and double blind clinical study of Byrne and colleagues[9] showed that glutamine, growth factor and optimum diet could reduce the length of TPN support. O’Dwyer and colleagues[10] emphasized that glutamine enriched TPN significantly improved the mucosal recovery and adaptation. New treatment alternatives to the current ones are still under research in experimental and clinical studies.
Chlorella is a species of green algae that grows in fresh water. The name Chlorella is taken from the Greek word meaning “small, fresh green”, it contains the highest level of chlorophyll in the world when compared with all other nutrients. It has been consumed as a food source for centuries mainly in Japan and other Far East countries, and suggestion of its healing properties has enhanced consumption[1112]. Biotechnological processing for single cell protein production is the most emphasized area of chlorella studies. Because of high protein ingredients, chlorella was considered to be a protein source in the beginning, but later it was seen as a “functional nutrient” first in Japan then Europe and America, and today it is accepted that chlorella is rich in nutritional ingredients[1112]. Active ingredients of chlorella are: 61.6% protein, 12.5% fat, 13.7% carbohydrate, trace elements (Al, Ze, P, Ca, Mg, Mn, Ni, Se), vitamins (carotene, beta-carotene, thiamine, B1, B2, B6, C, D, E, K), nucleic acids (RNA and DNA), and various enzymes[1213]. In our previous research, we showed that feeding with chlorella crude extract (CCE) has beneficial effects on malnourished rats which had undergone colon anastomosis[13].
In the current study, we aimed to evaluate the efficacy of chlorella extract which is rich in amino acids, beta carotene, and trace elements on intestinal adaptation in SBS.
All procedures were conducted according to recommendations of the Animal Research Committee at Gazi University in Ankara, Turkey. Wistar rats weighing 230-260 g were used in the study. The rats were maintained at 23°C in a 12 h light dark cycle, with free access to water and standard rat chow for a week. Eight hours prior to the start of experiments, rats were deprived of food while drinking water was available ad libitum. Animals were anesthetized by an intramuscular injection of 40 mg/kg ketamine (Ketalar®, Parke Davis, Eczacibasi, Istanbul, Turkey) and 5 mg/kg xylazine (Rompum®, Bayer AG, Leverkusen, Germany). Rats underwent central venous catheterization by inserting the catheter into the right external jugular vein, and were operated on under sterile conditions. A gastrostomy tube was placed for enteral feeding before the 75% small bowel resection. A 3 cm midline laparotomy was performed and intestinal transections were done 15 cm above the ileocecal junction and 5 cm from the duodenojejunal transition. Interrupted sutures of 7-0 PDS (Ethicon®, USA) were used for end to end bowel anastomosis. The venous catheter and the gastrostomy tube were tunneled subcutaneously through the dorsal cervical area, and attached with special apparatus (Swivel 56-1308; Harvard Apparatus, USA) which has a port and equipped with a spring system just beneath the skin. Before the closure of the abdominal cavity, 3 mL saline was administered intraperitoneally for the fluid resuscitation.
During the postoperative 3 d, rats received 60 kcal non-protein energy and 0.414 g nitrogen total paranteral nutrition (TPN). After postoperative day 4, rats were randomized and divided into the groups below: (1) Sham group: Laparotomy was performed; (2) Control group: 5% dextrose 12 mL/24 h was given to the rats by the gastrostomy tube with the infusion pump; (3) EN group: isocaloric (60 kcal/d) and isonitrogen (0.686 g/d) Alitraq (Abbott, USA) given to the rats through the gastrostomy tube with the infusion pump. Since, Alitraq is the EN product which has the highest amount of glutamine, and research clearly shows that glutamine has beneficial effect in SBS, Alitraq was used for enteral feeding in this study; (4) Study group: rats received CCE (60 kcal/d) through the gastrostomy tube by the infusion pump. In the sham, both EN and study group rats were not allowed to eat solid food, but were free to drink water.
Rats were anesthetized by intraperitoneal injection of 50 mg/kg sodium pentothal before laparotomy. The length of the small bowel was measured from the Treitz to the caecum. After withdrawal of blood samples from vena cava, rats were sacrificed by bleeding. Intestinal resection was quickly performed, and specimens were washed with cold saline. Histopathologic samples were taken from both the jejunal and ileal side of the anastomosis, and the rest of it was weighed.
Culturing and growth conditions: Collection and isolation of microalgae were made in compliance with Rippka et al[14]. Microalgae were obtained from GUMACC (Gazi University Microalgae Culture Collection) Chlorella sp. C1 were expanded in number by culture in BG11 nutrition medium (blue-green medium 11) at less than 3000 lux light intensity, under illumination for 16 h and under darkness for 8 h. Algae were harvested after approximately a 15-d production period.
Preparation of the extracts: Algal mass from an axenic exponential culture of the microalgae strains grown in BG11 were separated from the culture medium by centrifugation and pellets were dried at 60°C for 24 h. Methanol extracts were prepared according to the methods of Khan et al[15] and Vlachos et al[16], from dry algal mass (ratio 1:15 g/mL) extracted throughout 24 h. After separating the extraction phase, all of the extracts were preserved at 4°C. The chlorella sp. extract was resuspended at 1 g/mL in 0.9% sterile saline.
Ingredients of algae extract and its use: The dose of algae extract used was 50 g/kg BW/d[131516]. The suspension was given via oral gavage three times a day in equal doses (each dose was less than 5 mL).
Serum protein, albumin and blood sugar levels were analyzed from blood samples. The mucosal layers of the intestinal samples were brushed with slides and then weighed. After a homogenization process, mucosal DNA and protein levels were evaluated by the techniques of Chomczynski as described previously[17].
Serum citrulline levels were measured by the tandem mass spectrophotometric technique with isotope embedded amino acid standards. The results were expressed as mmol/L.
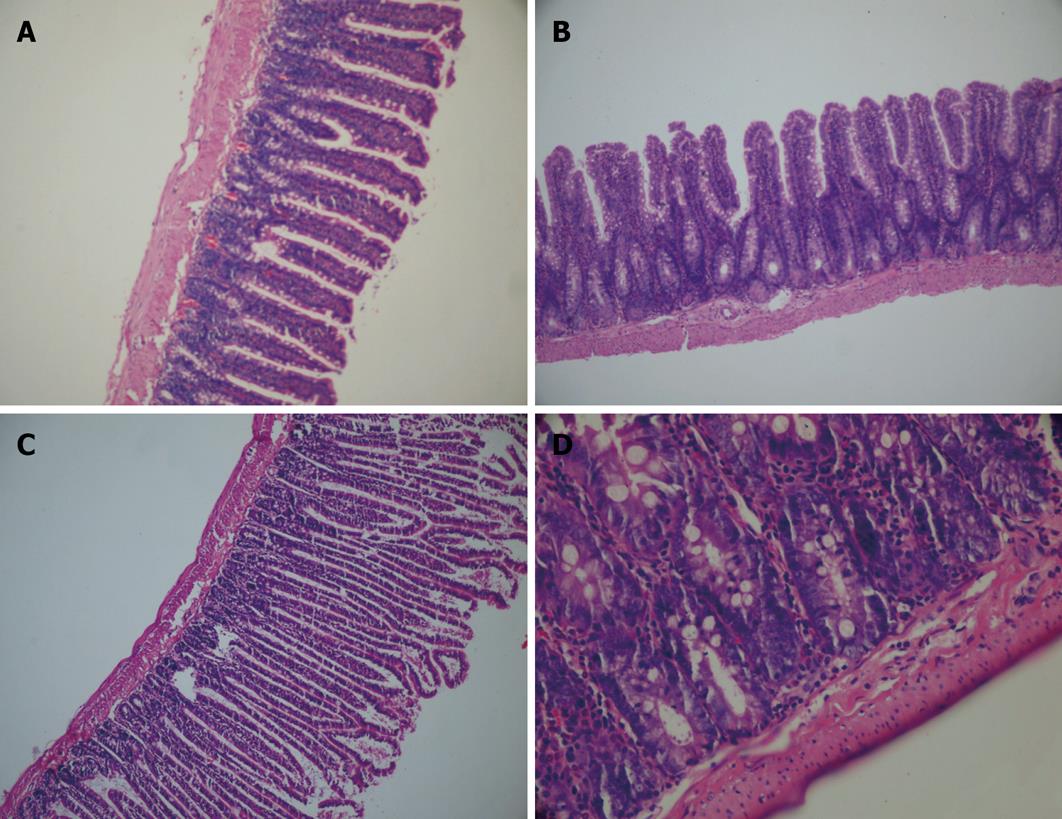
Jejunal and ileal tissue samples were fixed in a 10% solution of formaldehyde, and embedded in paraffin wax from which 3-&mgr;m-thick sections were mounted on slides. The sections were stained with hematoxylin-eosin (HE), and mucosal widening, villus length and crypt depth were evaluated.
Crypt cell proliferation was determined using 5-bromodeoxyuridine (BrdU). Twelve hours before sacrifice, 100 mg/kg BrdU was given intraperitoneally to the rats. Sections were stained with anti-BrdU antibodies. Every 10 crypts stained with positive BrdU were calculated as a proliferation index. The TUNEL technique was used for the determination of apoptotic cells.
All values were expressed as mean ± SE and the results were compared by analysis of variance (one-way ANOVA) and Scheffe’s post hoc analysis. P < 0.05 was considered statistically significant. Statistical evaluation was carried out using SPSS 11.5 software (SPSS, Chicago, IL, USA).
Three rats died during the creation of the SBS, and they were replaced with new ones.
All rats subjected to SBS lost significant weight when compared with their orginal weight before the experiment. In the EN and study (CCE) groups, rats lost less weight than the control group (P < 0.05). When they were compared with each other, it was observed that in the CRE group rats lost significantly less weight than the EN group (P < 0.001, Table 1).
| Sham | Control | EN | CCE | ANOVA | Sheffe’s | |
| Weight loss (g) | 0 ± 0 | 52 ± 8 | 34 ± 6 | 24 ± 5 | < 0.001 | a, c, e |
| Width of jejunum (cm) | 0.84 ± 0.1 | 0.48 ± 0.4 | 0.62 ± 0.3 | 0.78 ± 0.1 | < 0.05 | c, e, g |
| Length of jejunum villi (mm) | 0.74 ± 0.22 | 0.38 ± 0.18 | 0.54 ± 0.12 | 0.76 ± 0.11 | < 0.05 | c, e, g |
| Depth of jejunum crypt (mm) | 0.51 ± 0.13 | 0.32 ± 0.13 | 0.52 ± 0.10 | 0.68 ± 0.08 | < 0.05 | c, e, g |
| Number of jejunal mitosis (n) | 11.2 ± 2.1 | 4.2 ± 2.1 | 13.4 ± 4.3 | 22.8 ± 5.2 | < 0.001 | c, e, g |
| Width of ileum (cm) | 0.84 ± 0.1 | 0.48 ± 0.4 | 0.62 ± 0.3 | 0.78 ± 0.1 | < 0.05 | c, e, g |
| Length of ileum villi (mm) | 0.74 ± 0.22 | 0.38 ± 0.18 | 0.54 ± 0.12 | 0.56 ± 0.11 | < 0.05 | a, c |
| Depth of ileum crypt (mm) | 0.61 ± 0.10 | 0.36 ± 0.13 | 0.58 ± 0.19 | 0.64 ± 0.08 | < 0.05 | c, e, g |
| Number of ileum mitosis (n) | 11.2 ± 2.1 | 4.2 ± 2.1 | 13.4 ± 4.3 | 22.8 ± 5.2 | < 0.001 | c, e, g |
| Total protein (mg/dL) | 6.8 ± 1.8 | 4.3 ± 1.3 | 6.1 ± 1.4 | 6.6 ± 2.0 | < 0.05 | c, g |
| Albumin (mg /dL) | 2.2 ± 0.2 | 1.1 ± 0.3 | 1.9 ± 0.1 | 2.0 ± 0.3 | < 0.05 | c, g |
| Serum citrulline (micromol/L) | 72.2 ± 11.2 | 34.2 ± 6.2 | 52.3 ± 7.9 | 68.8 ± 9.8 | < 0.001 | c, g |
| Mucosal DNA (ng/&mgr;L) | 622 ± 48 | 318 ± 32 | 716 ± 61 | 898 ± 182 | < 0.001 | c, g |
| Mucosal protein (mg/mL) | 16.2 ± 3.8 | 6.2 ± 2.6 | 12.9 ± 3.8 | 15.3 ± 4.5 | < 0.001 | c, g |
| Cell proliferation index Jejunum | 310 ± 27 | 550 ± 40 | 710 ± 50 | 850 ± 55 | < 0.001 | a, c, g |
| (BrdU (+) cells/ 10 crypts) Ileum | 280 ± 32 | 510 ± 35 | 730 ± 45 | 970 ± 65 | < 0.001 | a, c, g |
| Apoptosis index Jejunum | 12 ± 2.1 | 25 ± 3.2 | 18 ± 3.1 | 14 ± 2.1 | < 0.001 | a, c, g |
| (Apoptotic cell/1000 cell) Ileum | 13 ± 2.3 | 29 ± 2.5 | 21 ± 4.1 | 16 ± 3.7 | < 0.001 | a, c, g |
In the control group, mucosal widening, villous length, crypt depth and amount of mitosis markedly decreased when compared to sham, EN and CCE groups (P < 0.05). Jejunal and ileal mitosis number in the EN and CCE groups were significantly higher than those at the control group (P < 0.05). The amount of mitosis in both segments of intestine in the CCE group was markedly higher than those in the EN group. Villus length and mucosal widening were not significantly different between the EN and CCE groups, whereas crypt depth was remarkably increased in the CCE group over the others (P < 0.05, Table 1, Figure 1).
Mucosal DNA levels significantly decreased in the control group when compared to the other groups. The mucosal DNA levels for EN and CCE groups were found to be remarkably higher than the sham and control groups. Moreover, the same parameters significantly increased in the CCE group when compared to the EN group (P < 0.05, Table 1).
Ileal mucosal protein levels were significantly higher in the CCE group than all other groups (P < 0.05). The mucosal protein levels of the control group were markedly reduced when compared the other groups (P < 0.05). There was no difference between the EN and CCE groups (P < 0.05, Table 1).
The jejunal and ileal cell proliferation indexes were significantly higher in the CRE group than all other groups (P < 0.05). The cell proliferation indexes in the control group were increased when compared to the sham group while they were significantly lower than the EN and CCE groups (P < 0.05, Table 1).
Apoptosis in the control group was markedly increased compared to other groups. The apoptotic index in subjects fed with CCE was significantly lower than those in the EN and control groups (P < 0.05), and it was found to be insignificantly higher than the apoptotic indexes of the sham group (P < 0.05, Table 1).
Loss of small bowel function caused by extended intestinal resection results in malabsorption and fluid and electrolyte imbalances[1]. In the early postoperative period the first priority of SBS treatment is adequate resuscitation of volume and electrolyte disturbances. When these parameters are stabilized, parenteral nutrition can be started[1–3]. Although parenteral nutrition causes a significant decrease in the mortality rates of SBS, the time required for optimal TPN therapy is too long and has many disadvantages and severe complications[4–7]. Searching for new treatment methods for increasing bowel adaptation mechanisms in order to reduce complications of SBS is an area which many recent studies concentrated on. The hormones; bombesin, growth factors, insulin like growth factors, ghrelin, leptin, and EN products; glutamine, fish oil (omega-3 fatty acids), immune nutrients and fibers, have been investigated, and found to have beneficial effects on bowel adaptation mechanism in SBS[8–1018]. We designed our research on the use of species of chlorella algae for healing effects because of its high protein, nucleic acid, antioxidant and fiber content[1112]. In this study, it was observed that enteral feeding with CCE has beneficial effects on intestinal adaptation in rats with SBS. Lengthening of the intestinal villus, increasing crypt depth, intestinal proliferation, mucosal protein, DNA, serum citrulline, protein and albumin levels, and decrease of apoptosis were found in the study. This is the first study to demonstrate the healing effects of chlorella on SBS.
When we look at the current literature, we can find few trials of different algae species[1920]. Tokida ameliorated murine chronic colitis through down-regulation of interleukin-6 production on colonic epithelial cells. Sakai et al[21] have also described that Sargassum horneri, a marine brown algae, increases Cl- absorption in isolated rat colon by activation of leukotrienes. In another study, it was shown that algae extract can reduce inflammation and ultrastructural changes in rats colitis induced by acetic acid[20]. Gonzalez et al[22], demonstrated that intestinal myeloperoxidase enzyme levels remarkably decrease in response to algae extract. Dvir and colleagues[23] suggested that red algae can regulate intestinal physiology and lipid metabolism, it can also be used as a functional nutrient. The same investigators claimed that algae-derived polysaccharides can increase jejunal muscular hypertrophy, and algae fiber can lengthen both colon and small bowel and increase colonic transit time by 44% compared to control group. Significant increases in mucosal villous lengthening and crypt depth due to the effects of micro-algae were observed in the same study. Although it is very rare, we can see the use of algae for SBS in the literature.
To our knowledge, if fluid resuscitation is not subsequently provided following the surgery, mortality can be significantly high in animal models of SBS[1]. For this reason, we inserted an intravenous line in rats, before starting TPN, and we resuscitated them with iv fluid for a short period of time. After the third postoperative day, rats received dextrose in addition to EN and CCE. They were weighed daily until the end of the experiment. All rats subjected to SBS lost significant weight. However, weight lost in rats fed with EN and CCE was markedly less than the control group. Though both EN and CCE resulted in weight gain in rats, the CCE group rats gained more weight than the EN group. Both nutritional solutions have almost the same energy distribution, but CCE has higher nucleotide content, and better absorptive and adaptive capacity than the EN, so rats gained more weight in the CCE group than those in other groups. In our previous study, we observed that CCE increases weight gain in malnourished rats.
Recent articles feature the amino acid citrulline, the best marker of intestinal absorptive capacity in SBS due to massive intestinal resection[3–7]. The clinical study of Rhoads et al[24], found that there was a correlation between serum citrulline levels and enteral tolerance, and levels of serum citrulline could be used as a predictive test. In our study, fifteenth day serum citrulline levels were significantly lower than those in other groups. However, the CCE group serum citrulline levels were markedly higher than those in the control and EN groups. Another result of absorptive and adaptive responses was the significant increase in serum protein and albumin levels in CCE fed rats when compared to control rats. High amino acid and nucleotide levels in CCE could be responsible for these effects.
Mucosal DNA and BrdU proliferation index for evaluating intestinal proliferation rate were found significantly reduced in SBS rats when compared to control group rats. Mucosal DNA and intestinal proliferation index were markedly higher in CCE and EN groups than those in control group. These parameters also increased in the CCE group compared to the EN group. Excessive amount of nucleic acid, protein, vitamin and other substances in CCE may be responsible for these outcomes.
In conclusion, enteral administration of CCE increases intestinal adaptation and proliferation in experimental SBS. The current study provides preliminary data for future research. More studies are needed to investigate the use of algae species growing in water as a clinical nutrition product.
Chlorella is a species of green algae that grows in fresh water. The name chlorella is taken from the Greek word meaning “small, fresh green”, it contains the highest level of chlorophyll in the world when compared with the all other nutrients. It has been consumed as a food source for centuries mainly in Japan and other Far East countries, and suggestion of its healing properties has enhanced consumption. However, there is little data about the use of chlorella in disease, which is investigated in this experimental study.
Short bowel syndrome (SBS) which affects both children and adults is a generally seen disease. The basis of treatment for this disease is enteral and intravenous nutrition. Several enteral nutrition (EN) products have been used for SBS. The aim of this study is to evaluate the effect of chlorella in SBS in rats.
In this study, positive effects of orally given chlorella were seen. This is the first study on this subject. It was seen that chlorella increased intestinal adaptation in SBS.
This experimental study will guide new experimental and clinical studies. Chlorella is an algae which is widely found in both salt and fresh water. As a usage for EN, it can be used for acute pancreatitis, inflammatory bowel diseases, colitis studies and clinical studies.
In this experimental study about SBS, orally given CCE showed a positive effect on parameters of intestinal adaptation. Since it is the first study that shows positive effects of algae in SBS, this is an interesting study.
| 1. | Collins JB 3rd, Georgeson KE, Vicente Y, Hardin WD Jr. Comparison of open and laparoscopic gastrostomy and fundoplication in 120 patients. J Pediatr Surg. 1995;30:1065-1070; discussion 1070-1071. [Cited in This Article: ] |
| 2. | Vanderhoof JA, Langnas AN. Short-bowel syndrome in children and adults. Gastroenterology. 1997;113:1767-1778. [Cited in This Article: ] |
| 3. | Wilmore DW, Robinson MK. Short bowel syndrome. World J Surg. 2000;24:1486-1492. [Cited in This Article: ] |
| 4. | Kvietys PR. Intestinal physiology relevant to short-bowel syndrome. Eur J Pediatr Surg. 1999;9:196-199. [Cited in This Article: ] |
| 5. | Weale AR, Edwards AG, Bailey M, Lear PA. Intestinal adaptation after massive intestinal resection. Postgrad Med J. 2005;81:178-184. [Cited in This Article: ] |
| 6. | Riecken EO, Stallmach A, Zeitz M, Schulzke JD, Menge H, Gregor M. Growth and transformation of the small intestinal mucosa--importance of connective tissue, gut associated lymphoid tissue and gastrointestinal regulatory peptides. Gut. 1989;30:1630-1640. [Cited in This Article: ] |
| 7. | Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117:1043-1050. [Cited in This Article: ] |
| 8. | Jackson CS, Buchman AL. The nutritional management of short bowel syndrome. Nutr Clin Care. 2004;7:114-121. [Cited in This Article: ] |
| 9. | Byrne TA, Wilmore DW, Iyer K, Dibaise J, Clancy K, Robinson MK, Chang P, Gertner JM, Lautz D. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome: a prospective, randomized, placebo-controlled, double-blind clinical trial. Ann Surg. 2005;242:655-661. [Cited in This Article: ] |
| 10. | O'Dwyer ST, Smith RJ, Hwang TL, Wilmore DW. Maintenance of small bowel mucosa with glutamine-enriched parenteral nutrition. JPEN J Parenter Enteral Nutr. 1989;13:579-585. [Cited in This Article: ] |
| 11. | Lahaye M. Marine algae as sources of fibers: Determination of soluble and insoluble dietary fiber contents in some sea vegetables. J Sci Food Agric. 1991;54:587-594. [Cited in This Article: ] |
| 12. | Michel C, Benard C, Lahaye , M , Formaglio D, Kaeffer B, Quemener B, Berot S, Yvin JC, Blottiere HM. Algal oligosaccharides as functional foods: in vitro study of their cellular and fermentative effects; Les oligosides algaux comme aliments fonctionnels: etude in vitro de leurs effets cellulaires et fermentaires. Sciences des aliments. 1999;19:311-332. [Cited in This Article: ] |
| 13. | Salman B, Kerem M, Bedirli A, Katircioglu H, Ofluoglu E, Akin O, Onbasilar I, Ozsoy S, Haziroglu R. Effects of Cholerella sp. microalgae extract on colonic anastomosis in rats with protein-energy malnutrition. Colorectal Dis. 2008;10:469-478. [Cited in This Article: ] |
| 14. | Rippka R. Isolation and purification of cyanobacteria. Methods Enzymol. 1988;167:3-27. [Cited in This Article: ] |
| 15. | Khan NH, Rahman M, Nur-e-Kamal MS. Antibacterial activity of Euphorbia thymifolia Linn. Indian J Med Res. 1988;87:395-397. [Cited in This Article: ] |
| 16. | Vlachos V, Critchley AT, von Holy A. Establishment of a protocol for testing antimicrobial activity in southern African macroalgae. Microbios. 1996;88:115-123. [Cited in This Article: ] |
| 17. | Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532-534, 536-537. [Cited in This Article: ] |
| 18. | Booth IW. Enteral nutrition as primary therapy in short bowel syndrome. Gut. 1994;35:S69-S72. [Cited in This Article: ] |
| 19. | Matsumoto S, Nagaoka M, Hara T, Kimura-Takagi I, Mistuyama K, Ueyama S. Fucoidan derived from Cladosiphon okamuranus Tokida ameliorates murine chronic colitis through the down-regulation of interleukin-6 production on colonic epithelial cells. Clin Exp Immunol. 2004;136:432-439. [Cited in This Article: ] |
| 20. | Berge JP, Debiton E, Dumay J, Durand P, Barthomeuf C. In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J Agric Food Chem. 2002;50:6227-6232. [Cited in This Article: ] |
| 21. | Sakai H, Uchiumi T, Lee JB, Ohira Y, Ohkura J, Suzuki T, Hayashi T, Takeguchi N. Leukotrienes-mediated effects of water extracts from Sargassum horneri, a marine brown alga, on Cl- absorption in isolated rat colon. Jpn J Physiol. 2004;54:71-77. [Cited in This Article: ] |
| 22. | Gonzalez R, Rodriguez S, Romay C, Ancheta O, Gonzalez A, Armesto J, Remirez D, Merino N. Anti-inflammatory activity of phycocyanin extract in acetic acid-induced colitis in rats. Pharmacol Res. 1999;39:55-59. [Cited in This Article: ] |