Published online Apr 21, 2008. doi: 10.3748/wjg.14.2394
Revised: December 27, 2007
Published online: April 21, 2008
AIM: To evaluate the efficacy of radiotherapy (RT) in patients with advanced unresectable hepatocellular carcinoma (HCC).
METHODS: A total of 65 patients were treated with RT in the Korea University Medical Center. The median age of the patients was 60 years, and 86.2% were men. 18.5% and 81.5% of the patients were diagnosed as TNM stage III and IV-A, respectively. Treatment response was assessed 4 mo after initiation of RT. Tumor regression rate 1 mo after initiation of RT (TRR1m) was also assessed. Duration of survival was calculated from the initiation of RT.
RESULTS: The objective treatment response was 56.9%. The 12 mo survival rate was 34.7%. Predictive factors for survival were Child-Pugh grade, α-fetoprotein level and treatment response. An objective response was achieved more frequently in patients with TRR1m≥ 20% than in those with TRR1m < 20% (P < 0.001).
CONCLUSION: RT is effective in treating advanced HCC with a tumor response rate of 56.9%.
- Citation: Seo YS, Kim JN, Keum B, Park S, Kwon YD, Kim YS, Jeen YT, Chun HJ, Kim CY, Kim CD, Ryu HS, Um SH. Radiotherapy for 65 patients with advanced unresectable hepatocellular carcinoma. World J Gastroenterol 2008; 14(15): 2394-2400
- URL: https://www.wjgnet.com/1007-9327/full/v14/i15/2394.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2394
In Korea, hepatocellular carcinoma (HCC) accounts for 83% of primary liver cancer, which is the third most common cancer and the third leading cause of cancer-related death[12]. Although surgical resection is considered to be the treatment of choice for long-term control of HCC, this treatment is considered at diagnosis in less than 20% of HCC patients due to disease extent or a hepatic function that is inadequate for resection[3–5].
Although percutaneous ablation therapy, such as percutaneous ethanol injection or radiofrequency ablation, could be the best treatment in patients who are not suitable for resection, this treatment is limited to early-stage HCC[6]. Transarterial chemoembolization (TACE) is used for patients with unresectable HCC who are also ineligible for percutaneous ablation[6]. However, because complete tumor necrosis is rare with TACE, repeated treatments are often needed. Additionally, TACE-induced vascular injury can limit further TACE[7–10]. Finally, patients with portal vein thrombosis or extensive tumor burden are poor candidates for TACE, because their tumors are frequently associated with arterio-portal shunts and TACE-related liver damage. For these advanced HCCs, hepatic arterial infusion of chemotherapy (HAI) has yielded promising results in several recent studies[11–13], but the benefit of HAI is still controversial.
Radiotherapy (RT) for the treatment of HCC has been attempted over the last four decades, but the results have been unsatisfactory because the doses were too low to be adequately tumoricidal[14–16]. Recently, however, several studies have suggested local, high-dose RT is well tolerated and leads to a favorable treatment response in patients with unresectable HCC[17–20]. Therefore, this study was performed to evaluate the treatment responses of RT and survival in patients who underwent RT for unresectable HCC.
This study was performed with patients who underwent RT for unresectable advanced HCC without distant metastases. Between July 2003 and June 2006, 80 patients with unresectable HCC underwent local RT to the liver at the Korea University Medical Center. Fifteen of these patients were excluded due to the presence of distant metastases prior to RT.
Diagnosis of HCC was based on either the identification of hypervascular masses by two imaging studies or by one imaging study combined with a serum alpha-fetoprotein (AFP) level > 400 ng/mL. If the vascular profile by dynamic imaging was not characteristic of HCC and the AFP was less than 400 ng/mL a biopsy was performed[21]. Unresectability was determined using accepted surgical criteria[3].
The baseline characteristics of the 65 patients are presented in Table 1. Fifty-six patients (86.2%) were male and 9 were female. The median age was 60 years (range, 42-83) years. Underlying liver diseases included chronic Hepatitis B virus (HBV) infection in 49 patients (75.4%), alcoholic liver cirrhosis in 13 patients (20%) and chronic Hepatitis C virus (HCV) infection in two patients (3.1%). In one patient (1.5%), co-infection with HBV and HCV was noted. Liver cirrhosis was present in 50 patients (76.9%). According to the Child-Pugh classification, 43 patients (66.2%) were classified as grade A and 22 patients (33.8%) were classified as grade B. Patients in class C were not included. Baseline tumor size was 10.8 ± 4.7 cm (median, 9.9 cm). In 31 patients (47.7%), the tumor size was larger than 10 cm. Based on the types of HCC described by Eggel[22], the most frequent tumor type was massive (58.5%), followed by multinodular (36.9%) and single nodular (4.6%). Prior to RT, portal vein thrombosis was observed in 45 patients (69.2%); this was confirmed by CT and/or angiogram. Among these 45 patients, thrombosis was observed in the main portal vein in 20 patients (30.8%), at the first branch level in 23 (35.4%), and at the second branch level in 2 (3.1%). The hepatic vein and bile duct were involved in 8 and 6 patients, respectively. No patients showed evidence of extrahepatic metastasis prior to RT. According to the TNM staging system of the Liver Cancer Study Group of Japan[23], 53 patients (81.5%) fell into stage IV-A, and 12 (18.5%) fell into stage III.
| Characteristics | Number of patients (%) |
| Age (yr) | 60 (42-83)1 |
| < 60/≥ 60 | 35 (53.8)/30 (46.2) |
| Gender (Male/Female) | 56 (86.2)/9 (13.8) |
| Underlying liver disease (viral/alcohol) | 52 (80.0)/13 (20.0) |
| Liver cirrhosis | 50 (76.9) |
| Ascites | 24 (36.9) |
| Child-Pugh class (A/B) | 43 (66.2)/22 (33.8) |
| Albumin (g/dL)2 | 3.4 ± 0.5 |
| Bilirubin (mg/dL)2 | 1.2 ± 0.9 |
| Alkaline phosphatase (IU/L)2 | 151.8 ± 79.3 |
| Platelet (103/mL)2 | 156.3 ± 66.2 |
| Prothrombin time (INR)2 | 1.2 ± 0.2 |
| Sodium (mEq/L)2 | 137.7 ± 3.7 |
| Creatinine (mg/dL)2 | 1.0 ± 0.9 |
| Tumor size (cm)2 | 10.8 ± 4.7 |
| < 10/≥ 10 | 34 (52.3)/31 (47.7) |
| Tumor type (SN/MN/massive) | 3 (4.6)/24 (36.9)/38 (58.5) |
| Portal vein thrombosis | 45 (69.2) |
| UICC stage (III/IV-A) | 12 (18.5)/53 (81.5) |
| α-fetoprotein (IU/mL)2 | 17 454 ± 66 005 |
| > 400/ ≤ 400 | 28 (43.1)/37 (56.9) |
| Radiotherapy aim (primary/salvage) | 40 (61.5)/25 (38.5) |
RT was performed as a primary treatment in 40 of the 65 patients (61.5%) due to an overly large tumor size in 20 patients (30.8%), portal vein thrombosis in 12 patients (18.5%), IVC thrombosis in 3 patients (4.6%), bile duct invasion in 3 patients (4.6%), and a massive porto-systemic shunt around the tumor in 2 patients (3.1%). In the remaining 25 patients (38.5%), RT was performed as a salvage treatment after ineffective TACE (21 patients, 32.3%) or vascular inaccessibility to the feeding vessel of the HCC (4 patients, 6.2%). External beam RT at a target dose of 61 Gy/34 fractions was planned, using 10 MV of X-rays. The RT strategy was devised using a CT-based 2-D planning system (CT Port, Toshiba, Tokyo, Japan). To account for respiratory-based liver motion, a 1-1.5 cm margin was added in the craniocaudal direction. The full 61-Gy irradiation dose was feasible in 55 of the 65 patients (84.6%).
During and after RT, TACE was also employed in 57 patients (87.7%; 2.9 ± 1.8 sessions; median, three sessions; range, 1-8 sessions). TACE was performed with an emulsion of doxorubicin at a dose of 10-30 mg and 4-12 mL of mixed solution of lipiodol and contrast agent. TACE was usually combined with embolization using gelfoam particles, except in cases with significant portal vein thrombosis. In 16 patients with portal vein invasion (24.6%), HAI with cisplatin and 5-FU was combined with or without TACE (3.3 ± 2.4 cycles; median, 2.5 cycles; range, 1-8 cycles).
Tumor size was measured by computed tomography (CT) and was calculated as the longest diameter multiplied by the longest perpendicular diameter. CT scans were obtained before RT, 1 and 4 mo after the initiation of RT, and then every 2-3 mo. If a patient had multiple nodules, the extent of the tumor was determined by the sum of the extent of all tumors > 2 cm in diameter.
Treatment response was assessed at four months after initiation of RT. A complete response was defined as the complete disappearance of all clinical and radiographic tumor evidence. A partial response was defined as more than a 50% decrease in tumor size from baseline. Stable disease was defined as less than a 50% decrease or a 25% increase in tumor size. The objective treatment response was calculated based on the complete and partial responses. Progressive disease was defined as a greater than 25% increase in extent of the tumor from the nadir extent of the tumor.
To evaluate the efficacy of using the early tumor response to predict the treatment response, the tumor regression rate at one month after initiation of RT (TRR1m) was assessed using the following equation: TRR1m = [(baseline tumor extent - tumor extent at one month after RT)/baseline tumor extent] × 100.
Adverse events were evaluated weekly during RT and one month following the treatment. Adverse hematologic events were evaluated by measuring hemoglobin, white blood cell (WBC) and platelet counts, while hepatic adverse events were evaluated by measuring serum bilirubin, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) levels. Gastrointestinal (GI) bleeding included any bleeding from the esophagus, stomach, duodenum, or liver. All adverse events were graded according to Common Terminology Criteria for Adverse Events V3.0.
All calculations were performed using SPSS 10.0 software for Windows (SPSS, Chicago, IL). Quantitative variables were expressed as mean ± SD or medians. Differences in quantitative and qualitative variables were assessed using the Student’s t-test and chi-square test, respectively. Logistic regression analysis was performed to evaluate predictive factors for tumor response. Survival and progression-free survival were assessed from the initiation of RT according to the Kaplan-Meier method. Differences between variables were assessed using the log-rank test. The Cox regression model was used to detect associations between survival and AFP status, tumor type, the location of tumor thrombi, stage and therapeutic models. For multivariate analysis, variables with P < 0.2 at univariate analysis were entered. Differences with P < 0.05 were considered to be statistically significant.
Fifty-five of 65 patients (84.6%) completed the RT schedule. Ten patients (15.4%) could not complete RT due to HCC aggravation or deterioration of liver function after RT. Interruption of RT was more frequent in Child-Pugh class B patients (7 of 20 patients, 35%) than in class A patients (3 of 45, 6.7%; P = 0.003). Among the 55 patients who completed RT, treatment response was evaluated 4 mo after the initiation of RT. None of our patients had completely responded at this point in the response evaluation, but 37 patients (67.3%) had partially responded. Seventeen patients (30.9%) had stable disease, and 1 (1.8%) had progressive disease. Therefore, the objective treatment response at four months was 67.3%. However, if we label the 10 patients who did not complete RT as non-responders, then the partial response and stable disease rates decreased to 56.9% (37 of 65 patients) and 26.2% (17 of 65 patients), respectively.
Table 2 presents baseline characteristics according to treatment response. Logistic regression analysis was performed to evaluate predictive factors for an objective treatment response. Child-Pugh grade was the only independent predictive factor for an objective treatment response (Child-Pugh grade A vs B; OR, 5.167; 95% CI, 1.643-16.250; P = 0.005). Among the 45 patients with Child-Pugh grade A, 31 patients (68.9%) showed a partial response, as did 6 of the 20 grade B patients (30%, P = 0.003).
| Pts without OTR (n = 28) | Pts with OTR (n = 37) | P value | |
| Age (yr) | 61 ± 10 | 58 ± 8 | 0.271 |
| Gender (M:F) | 22:6 | 34:3 | 0.124 |
| Hepatitis B | 21 (75%) | 28 (75.7%) | 0.950 |
| Hepatitis C | 2 (7.1%) | 1 (2.7%) | 0.573 |
| Alcohol abuse | 5 (17.9%) | 8 (21.6%) | 0.707 |
| WBC (/mm3) | 5450 ± 1671 | 6079 ± 2229 | 0.216 |
| Hemoglobin (g/dL) | 11.4 ± 2.2 | 12.0 ± 1.8 | 0.207 |
| Platelet (× 103/mm3) | 160 ± 73 | 154 ± 608 | 0.733 |
| AST (IU/L) | 111 ± 93 | 71 ± 53 | 0.032 |
| ALT (IU/L) | 62 ± 51 | 69 ± 90 | 0.686 |
| ALP (IU/L) | 175 ± 89 | 134 ± 67 | 0.038 |
| Bilirubin (mg/dL) | 1.47 ± 1.27 | 0.91 ± 0.49 | 0.035 |
| Albumin (g/dL) | 3.3 ± 0.4 | 3.5 ± 0.5 | 0.103 |
| Prothrombin time, INR | 1.16 ± 0.22 | 1.15 ± 0.14 | 0.796 |
| Creatinine (mg/dL) | 1.17 ± 1.31 | 0.92 ± 0.25 | 0.329 |
| Liver cirrhosis | 21 (75%) | 29 (78.4%) | 0.749 |
| Child-Pugh grade A | 14 (50%) | 31 (83.8%) | 0.003 |
| Alpha-fetoprotein (ng/dL) | 31 474 ± 98 160 | 6844 ± 15 814 | 0.199 |
| ≥ 400 ng/dL | 15 (53.6%) | 22 (59.5%) | 0.635 |
| Tumor size (mm) | 118 ± 37 | 99 ± 52 | 0.112 |
| ≥ 10 cm | 18 (64.3%) | 14 (37.8%) | 0.035 |
| Multiple tumor | 24 (85.7%) | 30 (81.1%) | 0.622 |
| Massive type | 18 (64.3%) | 20 (54.1%) | 0.407 |
| Main portal vein thrombosis | 10 (35.7%) | 10 (27%) | 0.452 |
| Tumor stage IV | 23 (82.1%) | 30 (81.1%) | 0.913 |
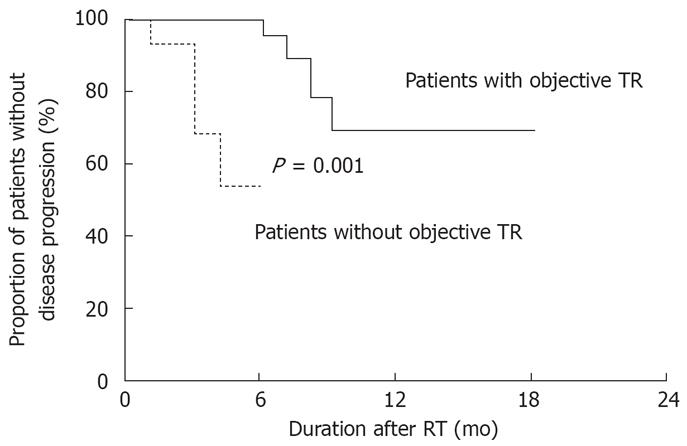
During follow-up, 4 of the 37 patients showing a partial response (10.8%) and 3 of the 25 patients showing stable disease (12%) had progressive disease after a median of 6 (range, 3-9) mo. Among the 65 patients, time to progressive disease was 5 ± 3 mo after initiation of RT (median, 4 mo). Duration without progressive disease was longer in patients who met the objective treatment response (14.8 ± 1.4 mo) than in patients who did not (4.6 ± 0.4 mo, P < 0.001; Figure 1).
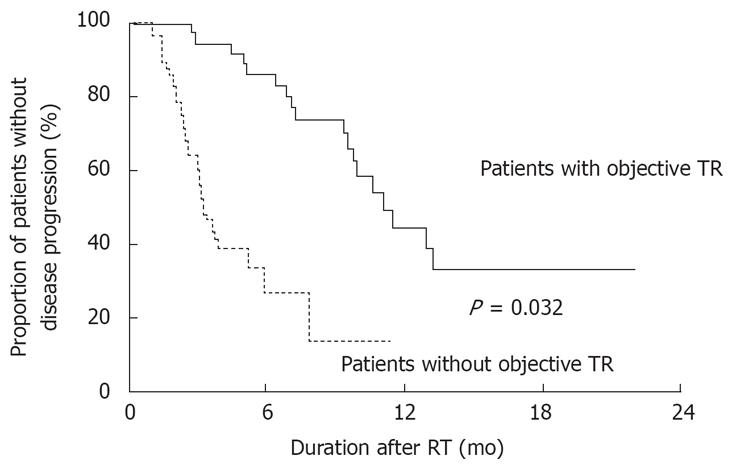
All enrolled patients were followed for 8 ± 6 (median, 6; range, 1-30) mo. During this period, 37 patients died. Fourteen patients died of hepatic failure, 13 died of HCC aggravation, 5 died of gastrointestinal bleeding, 2 died of tumor rupture, and 2 died of sepsis. The cumulative survival rates at 6, 12 and 18 mo were 61.5%, 34.7% and 27.0%, respectively. Patients who showed an objective treatment response (median survival, 346 d) survived longer than those who did not (median survival, 212 d; P = 0.032; Figure 2).
When multivariate Cox-regression analysis was performed with baseline characteristics, large tumor size (≥ 10 cm vs < 10 cm; OR, 2.416; 95% CI, 1.213-4.811; P = 0.012), Child-Pugh grade B vs A (OR, 4.094; 95% CI, 1.977-8.480; P < 0.001) and the presence of tumor thrombi in the main portal vein (OR, 2.315; 95% CI, 1.156-4.634; P = 0.018) were independent predictive factors for mortality. However, when multivariate analysis was performed after inclusion of the objective treatment response, Child-Pugh grade B vs A (OR, 3.706; 95% CI, 1.718-7.996; P = 0.001), high serum AFP level (OR, 2.459; 95% CI, 1.187-5.094; P = 0.015) and a failure to meet the objective treatment response (OR, 5.619; 95% CI, 2.475-12.760; P < 0.001) were independent prognostic factors for mortality (Table 3).
| P value | β | Odd ratio | 95% CI | ||
| Tumor size | 0 ≤ 10 cm; | 0.012 | 0.954 | 2.597 | 1.232-5.473 |
| 1 ≥ 10 cm | |||||
| Child-Pugh grade | 0 = Grade A; | 0.001 | 1.336 | 3.802 | 1.687-8.568 |
| 1 = Grade B | |||||
| Combined with TACE | 0 = Yes; | 0.001 | 1.671 | 5.315 | 2.015-14.018 |
| 1 = No | |||||
| Objective treatment response | 0 = Yes; | 0.006 | 1.194 | 3.300 | 1.414-7.699 |
| 1 = No |
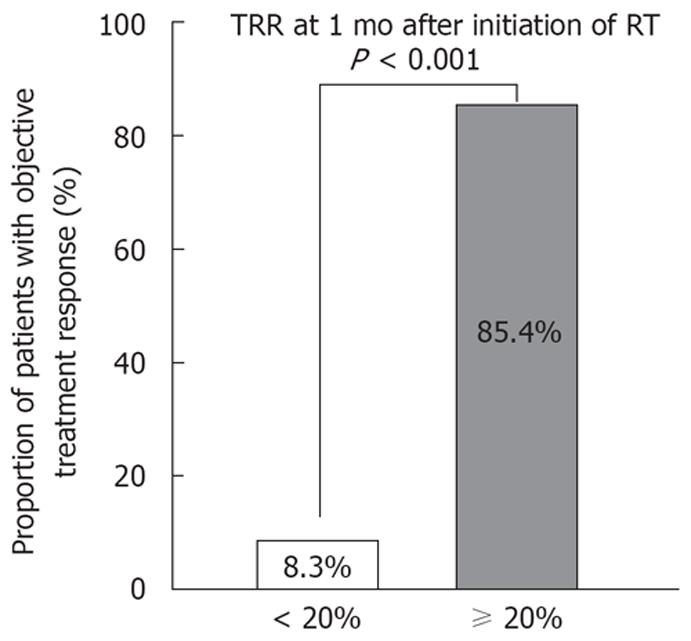
TRR1m was assessed in all 65 patients. TRR1m was more than 20% in 41 patients (63.1%). Of the 41 patients with a TRR1m≥ 20%, 35 patients (85.4%) showed a partial response, while only 2 (8.3%) of the 24 patients with TRR1m < 20% showed a partial response (P < 0.001; Figure 3). When logistic regression analysis was performed after inclusion of TRR1m among the variables used to predict objective treatment response, Child-Pugh grade A (OR, 0.121; 95% CI, 0.019-0.784; P = 0.027) and TRR1m≥ 20% (OR, 158.302; 95% CI, 14.032-1785.827; P < 0.001) were independent predictive factors.
Adverse events during and one month after RT are summarized in Table 4. Adverse hematologic events were identified in 78.5% of patients. Although most of them were mild and transient, grade 3 or 4 adverse events were noted in six patients (9.2%). Grade 3 or 4 hematologic adverse events were more frequent in patients who underwent RT combined with HAI (4 of 16 patients, 25%) than in patients with RT alone (2 of 49, 4.1%; P = 0.012). Adverse hepatic events were identified in 51.8% of the patients; the most common were hypoalbuminemia (33.8%) and hyperbilirubinemia (24.6%). Grade 3/4 adverse hepatic events developed in four patients (6.2%), with hyperbilirubinemia or elevation of AST in 1 (1.5%). Grade 3/4 adverse hepatic events were more frequent in patients with TRR1m < 20% (4 of 24 patients, 16.7%) than in those with TRR1m≥ 20% (1 of 41, 2.4%; P = 0.038). GI bleeding developed in five patients. The status of two patients with peptic ulcer disease and one patient with variceal bleeding was improved with medical or endoscopic treatment. However, two patients with hemobilia or HCC rupture expired after these events.
| Grade | ||||||
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Hematologic | 14 (21.5) | 33 (50.8) | 12 (18.5) | 5 (7.7) | 1 (1.5) | - |
| Hepatic | 32 (49.2) | 20 (30.8) | 8 (12.3) | 4 (6.2) | 1 (1.5) | - |
| GI hemorrhage | 1 (1.5) | 2 (3.1) | 2 (3.1) | |||
Recently, a number of reports have documented the effect of local RT on HCC[17–20]. Although fractionation schemes were not identical to each other, local, high-dose RT alone or in combination with another modality such as TACE[1924], systemic chemotherapy[25] or intra-arterial chemotherapy[2627] has achieved a substantial objective response. In this study, RT was performed with or without other treatment modalities and the objective response rate was 56.9%, which was somewhat lower than previous reported[2428–30]. However, when 10 patients (15.4%) who did not complete the whole RT schedule were excluded, the objective treatment response rate increased to 67.3%. We have no idea how the patients who could not complete RT were treated during these previous studies, because this was not reported. It is possible that all patients completed RT in the previous studies. However, a significant proportion of patients could not complete the RT schedule in the present study. Therefore, selection of appropriate patients for RT may be very important before RT initiation.
Child-Pugh grade was the only significant predictive factor for treatment response. This seems to be associated with the higher proportion of Child-Pugh grade B patients (35%) who could not complete the RT schedule compared with those with grade A (6.7%; P = 0.003). This speculation is supported by the fact that no variable was significantly associated with treatment response when the logistic analysis was performed on the 55 patients who completed RT (data was not shown). These results suggest that a circumspective decision was required in considering RT for patients with Child-Pugh grade B. Previously, tumor size was the one significant factor affecting treatment response[29]. Similarly, a treatment response was more frequently seen in patients with a smaller HCC (23 of 32 patients, 69.7%) than in patients with a larger HCC (14 of 32 patients, 43.8%; P = 0.035). However, when multivariate analysis was performed, the significance disappeared.
Our results suggest RT may improve prognosis in patients who achieved an objective treatment response. RT appears to be associated with prolonged survival as well as prolonged suppression of HCC progression in patients who show an objective treatment response. After the effects of other prognostic factors were corrected for, patients who achieved objective treatment responses survived longer than those who did not, as determined by multivariate analysis. In addition, time to progression was significantly longer in patients who met the objective treatment response than in patients who did not. However, several limitations should be discussed. First, 10 patients who could not complete the RT schedule were included in this analysis. However, even though these 10 patients were later excluded, patient survival still differed according to treatment response (P = 0.002; data not shown). Second, most patients were treated with not only radiotherapy, but also with TACE or HAI, and these combined treatments may affect patients’ survival. To ideally assess the effect of RT on patient prognosis, RT should be the only treatment modality. However, considering the limitations of dose and field of RT, it seems unwise to use RT as the only treatment modality for advanced HCC.
In this study, the one-year survival rate was 34.7%, which was lower than in previous studies[2428–31]. It may be the patients enrolled in this study had more advanced disease than those in previous studies[2428–31]. In the present study, tumors were larger than 10 cm in 47.7% of patients, 69.2% of the cases had thrombi in portal vein, and 81.5% of the patients had stage IV-A disease. In addition, 10 patients (15.4%) who could not complete RT schedule were included in this study; none of these patients survived more than four months after initiation of RT. By contrast, most of the previous studies included patients who completed the RT schedule[2428–31], which may have led to the observed discrepancies with the present study.
In recent studies, PVT was the one prognostic factor for survival[3032]. Similarly, in this study, the presence of tumor thrombi in main portal vein as well as Child-Pugh grade and tumor size were independent prognostic factors for survival when multivariate analysis was performed with variables of baseline characteristics. However, when treatment response was included in the analysis, Child-Pugh grade, AFP level and treatment response were associated with survival. This result suggests that even if tumor thrombi are present in main portal vein before RT, RT may still improve survival when an objective treatment response is achieved.
In all of our patients, CT was performed at one month after initiation of RT. Tumor response at one month after initiation of RT was a useful predictor for RT response. In addition, grade 3 or 4 adverse hepatic events were more frequent in patients with TRR1m < 20%. These results suggest if the mass does not decrease to 20% from baseline after one month of RT, interruption of RT can be considered due to the likelihood of a low objective treatment response rate and a high rate of severe adverse hepatic events.
In conclusion, RT was effective for the treatment of HCC with an objective tumor response rate of 56.9%; moreover, patients who met the objective treatment response survived longer than those who did not. Tumor regression at one month after the initiation of RT may be a useful predictor for RT response as well as severe adverse hepatic events.
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related death in the world, especially in Asia. In a large proportion of patients, HCC is diagnosed at an advanced stage and, in this stage, widely performed treatment modalities including surgical resection, local ablation therapy and transarterial chemoembolization are not indicated. Recently, several reports have suggested high-dose radiotherapy (RT) could be an effective treatment option for advanced HCC.
Most previous studies have included only patients who completed the RT schedule. However, according to our experience, some proportion of patients could not complete the whole RT schedule and their prognosis was usually very poor. Therefore, this might lead to a selection bias when analyzing the treatment response and survival of patients. In this study, all patients with HCC who were treated with RT for more than 1 mo during the study period were included. In addition, we evaluated the prognostic significance of early tumor response by follow-up CT at 1 month after the initiation of RT.
RT was effective in patients with advanced HCC with an objective tumor response rate of 56.9%. Early tumor response rate at 1 month after the initiation of RT was shown to be a good prognostic indicator for RT response. In addition, severe adverse events were more frequent in patients with poor early tumor response rates.
RT could be considered as a treatment option for patients with advanced HCC. If the tumor does not decrease to 20% from baseline after one month of RT, interruption of RT can be considered due to the likelihood of a low objective treatment response rate and a high rate of severe adverse hepatic events.
This is an interesting article, which may offer new insights in the treatment of advanced unresectable HCC. The paper is well organized and the results are clearly described and commented.
| 1. | Statistics of cancer/incidence of cancer and cancer-related mortality in National Cancer Information Center. Available from: URL: http://www.cancer.go.kr. [Cited in This Article: ] |
| 2. | Cause of mortality in Korean Statistical Information Service. Available from: URL: http://www.kosis.kr. [Cited in This Article: ] |
| 3. | Chen MF, Hwang TL, Jeng LB, Jan YY, Wang CS, Chou FF. Hepatic resection in 120 patients with hepatocellular carcinoma. Arch Surg. 1989;124:1025-1028. [Cited in This Article: ] |
| 4. | Tsuzuki T, Sugioka A, Ueda M, Iida S, Kanai T, Yoshii H, Nakayasu K. Hepatic resection for hepatocellular carcinoma. Surgery. 1990;107:511-520. [Cited in This Article: ] |
| 5. | Nagorney DM, van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery. 1989;106:740-748; discussion 748-749. [Cited in This Article: ] |
| 6. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [Cited in This Article: ] |
| 7. | Sasaki Y, Imaoka S, Kasugai H, Fujita M, Kawamoto S, Ishiguro S, Kojima J, Ishikawa O, Ohigashi H, Furukawa H. A new approach to chemoembolization therapy for hepatoma using ethiodized oil, cisplatin, and gelatin sponge. Cancer. 1987;60:1194-1203. [Cited in This Article: ] |
| 8. | Yu YQ, Xu DB, Zhou XD, Lu JZ, Tang ZY, Mack P. Experience with liver resection after hepatic arterial chemoembolization for hepatocellular carcinoma. Cancer. 1993;71:62-65. [Cited in This Article: ] |
| 9. | Ohto M, Yoshikawa M, Saisho H, Ebara M, Sugiura N. Nonsurgical treatment of hepatocellular carcinoma in cirrhotic patients. World J Surg. 1995;19:42-46. [Cited in This Article: ] |
| 10. | Ikeda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer. 1991;68:2150-2154. [Cited in This Article: ] |
| 11. | Wellwood JM, Cady B, Oberfield RA. Treatment of primary liver cancer: response to regional chemotherapy. Clin Oncol. 1979;5:25-31. [Cited in This Article: ] |
| 12. | Atiq OT, Kemeny N, Niedzwiecki D, Botet J. Treatment of unresectable primary liver cancer with intrahepatic fluorodeoxyuridine and mitomycin C through an implantable pump. Cancer. 1992;69:920-924. [Cited in This Article: ] |
| 13. | Patt YZ, Charnsangavej C, Yoffe B, Smith R, Lawrence D, Chuang V, Carrasco H, Roh M, Chase J, Fischer H. Hepatic arterial infusion of floxuridine, leucovorin, doxorubicin, and cisplatin for hepatocellular carcinoma: effects of hepatitis B and C viral infection on drug toxicity and patient survival. J Clin Oncol. 1994;12:1204-1211. [Cited in This Article: ] |
| 14. | INGOLD JA, REED GB, KAPLAN HS, BAGSHAW MA. RADIATION HEPATITIS. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200-208. [Cited in This Article: ] |
| 15. | Austin-Seymour MM, Chen GT, Castro JR, Saunders WM, Pitluck S, Woodruff KH, Kessler M. Dose volume histogram analysis of liver radiation tolerance. Int J Radiat Oncol Biol Phys. 1986;12:31-35. [Cited in This Article: ] |
| 16. | Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237-1248. [Cited in This Article: ] |
| 17. | Matsuzaki Y. Powerful radiotherapy for hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:941-945. [Cited in This Article: ] |
| 18. | Tokuuye K, Sumi M, Kagami Y, Murayama S, Kawashima M, Ikeda H, Ueno H, Okusaka T, Okada S. Radiotherapy for hepatocellular carcinoma. Strahlenther Onkol. 2000;176:406-410. [Cited in This Article: ] |
| 19. | Cheng JC, Chuang VP, Cheng SH, Huang AT, Lin YM, Cheng TI, Yang PS, You DL, Jian JJ, Tsai SY. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2000;47:435-442. [Cited in This Article: ] |
| 20. | Qian J, Feng GS, Vogl T. Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol. 2003;9:1885-1891. [Cited in This Article: ] |
| 21. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [Cited in This Article: ] |
| 22. | Eggel H. Ueber das primare carcinom der leber. Beitr Pathol Anat. 1901;30:506-604. [Cited in This Article: ] |
| 23. | Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer, 3rd ed, Tokyo: Kanehara Co Ltd. 1992;. [Cited in This Article: ] |
| 24. | Seong J, Keum KC, Han KH, Lee DY, Lee JT, Chon CY, Moon YM, Suh CO, Kim GE. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:393-397. [Cited in This Article: ] |
| 25. | Abrams RA, Cardinale RM, Enger C, Haulk TL, Hurwitz H, Osterman F, Sitzmann JV. Influence of prognostic groupings and treatment results in the management of unresectable hepatoma: experience with Cisplatinum-based chemoradiotherapy in 76 patients. Int J Radiat Oncol Biol Phys. 1997;39:1077-1085. [Cited in This Article: ] |
| 26. | Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, Lawrence TS. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210-2218. [Cited in This Article: ] |
| 27. | Robertson JM, Lawrence TS, Dworzanin LM, Andrews JC, Walker S, Kessler ML, DuRoss DJ, Ensminger WD. Treatment of primary hepatobiliary cancers with conformal radiation therapy and regional chemotherapy. J Clin Oncol. 1993;11:1286-1293. [Cited in This Article: ] |
| 28. | Seong J, Park HC, Han KH, Lee DY, Lee JT, Chon CY, Moon YM, Suh CO. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000;47:1331-1335. [Cited in This Article: ] |
| 29. | Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, Yoo BC, Lee JE, Kang MK, Park YJ. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143-1150. [Cited in This Article: ] |
| 30. | Seong J, Park HC, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55:329-336. [Cited in This Article: ] |
| 31. | Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150-155. [Cited in This Article: ] |
| 32. | Leung TK, Lee CM, Shen LK, Chen HC, Kuo YC, Chiou JF. Post-radiation survival time in hepatocellular carcinoma based on predictors for CT-determined, transarterial embolization and various other parameters. World J Gastroenterol. 2005;11:1697-1699. [Cited in This Article: ] |