Abstract
Herein, we have studied the exciton dynamics of a novel fused ring π-conjugated molecule (YS3) in solution and film states by spectroscopic measurements. This molecule incorporates dithienonaphthobisthiadiazole as a core unit that is a two-dimensionally π-extended fused ring. As a result, we found a long exciton lifetime in YS3 films originating from reduced radiative and nonradiative transitions. This is partly because radiative deactivation is effectively suppressed because of the dipole-forbidden transition in H-aggregates and partly because rotational deactivation is effectively suppressed in the crystalline film state.
Export citation and abstract BibTeX RIS
1. Introduction
Nonfullerene acceptors (NFAs) based on fused ring π-conjugated cores have been recently employed as an alternative to fullerene derivatives such as [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) for polymer solar cells. 1–9) In contrast to fullerene derivatives, NFAs have an intense absorption band from the visible to the near-IR region and hence have boosted the power conversion efficiency (PCE) of polymer solar cells. Very recently, the PCE has exceeded 19% even for single-junction cells. 10–16) Thus, it is highly important to develop novel NFAs based on fused ring π-conjugated cores for further improvement in the PCE.
In addition to their efficient light-harvesting properties, especially in the near-IR region, NFAs have another advantage of exciton diffusion lengths longer than π-conjugated polymers. As reported previously, it is as long as 20–47 nm for NFAs while it is as short as 5–20 nm for typical π-conjugated polymers. 17) This is partly because of the longer lifetimes of NFA singlet excitons. For example, recent studies have shown that one of the most efficient NFA (Y6) exhibits an exciton lifetime of longer than 1 ns. 18,19) As a result, Y6-based polymer solar cells gave a short-circuit current density (JSC) of more than 25 mA cm−2 and hence a PCE of more than 17%. 20–22)
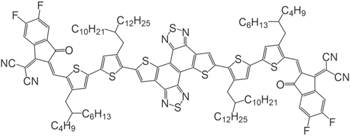
Herein, we have synthesized a novel π-conjugated molecule based on a fused ring, 2,2'-((2Z,2'Z)((dithieno[3',2':3,4;3',2':7,8]naphtho[1,2-c:5,6-c']bis([1,2,5]thiadiazole)-5,11-diylbis(4-(2-butyloctyl)-4'-(2-decyltetradecyl)-[2,2'-bithiophene]-5',5-diyl))bis(methaneylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (YS3), as an NFA candidate (Fig. 1). The central core of YS3 is based on dithienonaphthobisthiadiazole attached with two alkylthiophene units at both ends, in which the alkyl groups are designed not to cause sterical twist of the molecule. Such a large π-conjugation in the fused ring core would be beneficial for a longer exciton lifetime as reported previously. 23) We therefore study exciton dynamics of this molecule in solution and in film states.
Fig. 1. Chemical structure of YS3.
Download figure:
Standard image High-resolution image2. Experimental methods
2.1. Synthesis
YS3 was synthesized according to Scheme S1 and was characterized by NMR measurements and high-resolution mass spectroscopy (HRMS). YS3 was soluble in common organic solvents such as chlorobenzene at 110 °C. The structure of YS3 was identified with 1H NMR and HRMS (Fig. S1).
2.2. Sample preparation
For steady-state absorption and photoluminescence (PL) spectra, time-resolved PL decay and spectra, and PL quantum yield (PLQY) measurements, chlorobenzene solution and neat films of YS3 molecules were prepared. For spectroscopic measurements, the YS3 chlorobenzene solution was prepared to be 1.2 μM and purged by Ar bubbling for 15 min before the measurements. For film preparation, YS3 was dissolved in chlorobenzene with a weight concentration of 2.0 mg ml−1 and heated at 110 °C for about 2 h. The YS3 neat films were fabricated on a quartz substrate (20 × 20 mm2) by spin-coating from the chlorobenzene solution at a spin rate of 1000 rpm for 30 s in a glovebox (Korea Kiyon, KK-011AS). Finally, the neat films were encapsulated with another glass plate by using epoxy resin to prevent oxygen exposure to the film.
2.3. Measurements
Absorption and PL spectra are measured with a spectrophotometer (Hitachi, U-4100) and a fluorescence spectrophotometer (Horiba Jobin Yvon, NanoLog), respectively. Time-resolved PL decay and spectra were measured with a time-correlated single-photon-counting system (Horiba, FluoroCube). The PLQY of solution samples was measured with an absolute PLQY spectrometer (Hamamatsu Photonics, Quantaurus-QY Plus C13534-01), and that of film samples was measured with an absolute PL/EL quantum yield spectrometer (Bunkoukeiki, BEL-300). The excitation wavelength was set at 560 nm for solution samples and at 530 nm for film samples to avoid scattering of the excitation light. Grazing incidence wide-angle X-ray diffraction (GIXD) measurement of the thin film was performed with a reported procedure in the beamline BL46XU at SPring-8. 24) The coherence length (LC) was estimated from the simplified Scherrer's Eq. (1), where FWHM is the full-width at half-maximum of the π–π stacking diffraction peak
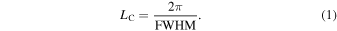
2.4. DFT calculation
Geometry optimization and electronic structure were calculated by using density functional theory (DFT) at the B3LYP/31-G(d) level. Calculation was performed by using the Gaussian 09 program. 25)
3. Results and discussion
3.1. Absorption and PL spectra
The absorption and PL spectra are shown in Fig. 2. As shown in the figure, YS3 in solution exhibits a broad absorption band at around 600 nm while the neat film exhibits sharp vibrational bands originating from 0–0 and 0–1 transitions at around 740 and 680 nm, respectively. On the basis of the 0–0 bands, the Stokes shift was estimated to be 0.34 eV for the solution and to be 0.11 eV for the neat film. The red-shifted absorption suggests longer effective conjugation length in thin films than in solution. This is probably due to the interaction between the carbonyl group of the terminal indanone group and the sulfur of the adjacent thiophenyl group, which would induce a planar structure of π-conjugation. Interestingly, as shown in Fig. 2(b), the intensity of the 0–0 band was much larger than that of the 0–1 band in the absorption spectrum while the intensity of the 0–0 band was much smaller than that of the 0–1 band in the PL spectrum. This finding suggests that there are both J- and H-aggregates in the film state. 26) The PL originates mainly from more stable H-aggregates.
Fig. 2. Absorption (black line) and PL spectra (blue line) of YS3 (a) in solution and (b) in film states excited at 640 nm.
Download figure:
Standard image High-resolution image3.2. DFT calculation
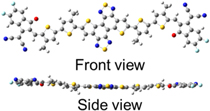
The DFT calculation was performed for the model structure at the B3LYP/31 G. The long side chains were replaced with a methyl group to save computational cost. The π-conjugation of this molecule was almost flat in the optimized structure as shown in Fig. 3. As mentioned before, this is due to the planar structure of dithienonaphthobisthiadiazole, placement of the alkyl groups, and S···O interaction between the alkylthiophenes and the electron withdrawing end groups. 27,28)
Fig. 3. Molecular structures of a model compound obtained by DFT calculation: (upper) the front view and (bottom) the side view.
Download figure:
Standard image High-resolution image3.3. Thin-film structures
Figure 4 shows the two-dimensional (2D) GIXD pattern of the YS3 neat film. Diffractions that most likely correspond to the lamellar and π–π stacking structures were observed in the ∼qz and qxy axes, respectively. This indicates the edge-on orientation or possibly end-on orientation of the molecules with respect to the substrate. The lamellar distance dL and the π–π stacking distance dπ–π were evaluated to be 20.6 Å and 3.65 Å, respectively. The coherence length LC was evaluated to be 43.4 Å for the π–π stacking structure. Such a crystalline order would originate in the highly coplanar molecular structure.
Fig. 4. 2D GIXD pattern of the YS3 neat film.
Download figure:
Standard image High-resolution image3.4. Exciton dynamics
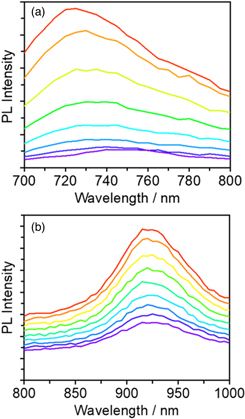
Exciton lifetimes were evaluated by time-resolved PL decay and spectra. In solution, as shown in Fig. 5(a), the PL intensity rapidly decayed in less than 1 ns. This decay was fitted with a three-exponential function. The average lifetime was estimated to be 0.14 ns. On the other hand, as shown in Fig. 6(a), the time-resolved PL spectra show that the PL peak was gradually red shifted from 730 to 750 nm. The peak shift is probably due to structural relaxation in the excited state because YS3 has free-rotational single bonds. This is consistent with the larger Stokes shift observed for the solution samples, suggesting a large difference in molecular structures between the ground and excited states. At a later time stage, a new PL band was observed at around 750 nm, which is almost the same as that observed for neat films. Thus, it may be indicative of the formation of H-aggregated excimer-like sites even in solution.
Fig. 5. PL decay of YS3 molecule (a) in solution and (b) in neat film excited at 640 nm. Black squares, red circles, and blue line represent instrument response function, PL signals, and fitting curves by exponential functions, respectively.
Download figure:
Standard image High-resolution imageFig. 6. Time-resolved emission spectra of YS3 molecule (a) in solution measured at 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, and 0.7 ns from top to bottom and (b) in neat film measured at 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, and 2 ns from top to bottom. The excitation wavelength was set at 640 nm.
Download figure:
Standard image High-resolution imageFor neat films, as shown in Fig. 5(b), the PL intensity decayed slowly with a time constant of longer than 1 ns. Such a long exciton lifetime would be partially because of the large core unit, as reported previously. 23) As summarized in Table I, this time constant was one order of magnitude larger than that for the solution. In contrast to the solution, as shown in Fig. 6(b), no peak shift was observed in the time-resolved PL spectra of the neat film. The PL spectrum at 0 ns was the same as that observed for the steady-state PL spectrum originating from H-aggregates. Thus, this slow dynamics is ascribed to singlet excitons in H-aggregates in the neat films.
Table I. PL decay constants for YS3 excitons.
| Sample | τ1/ns | τ2/ns | τ3/ns | τave/ns |
|---|---|---|---|---|
| solution | 0.098 (0.536) | 0.28 (0.383) | 1.22 (0.071) | 0.14 |
| neat film | 1.70 (0.962) | 3.39 (0.038) | 1.76 |
3.5. Radiative and nonradiative transition
Finally, we discuss radiative and nonradiative transition rates in the solution and film states. 29) Table II summarizes radiative and nonradiative transition rates evaluated from the PL lifetime and PLQY. As shown in the table, the radiative transition rate in chlorobenzene solution was more than two orders of magnitude larger than that in the film. This is probably because the PL of the neat films is ascribed to forbidden transition due to H-aggregates 30) where π–π stacking and lamella structures are formed because of the highly coplanar structure in the film state due to S···O interaction between the sulfur of the core unit and the oxygen of the end group. There are some examples of closely packed structures by S···O interaction. 27,28) On the other hand, as shown in the table, the nonradiative transition rate in solution was 10 times larger than that in neat films. This is partly because several single bonds can freely rotate in solution but not in a crystalline film. As mentioned before, YS3 is highly crystalline because the lamella diffraction patterns are observed up to the third order and the coherence length LC was as large as 43.4 Å in the π–π stacking direction. As reported previously, 31) Y6 and Y6-C2 neat films have shown clear lamella patterns. The coherence length LC in the π–π direction was evaluated to be 34.9 Å for Y6 and 38.2 Å for Y6-C2 neat films. In addition, a long exciton lifetime of >1 ns has been reported for Y6 neat films. 18,19) Thus, we conclude that such high crystallinity of YS3 would be also one of the key parameters to suppress nonradiative transition and therefore enhance exciton lifetime effectively.
Table II. Kinetic parameters for YS3 excitons.
| Sample | knr/108 s−1 | kr/108 s−1 | PLQY |
|---|---|---|---|
| solution | 68 | 3.2 | 0.045 |
| neat film | 5.6 | 0.068 | 0.012 |
4. Conclusions
We studied the exciton dynamics of a novel molecule based on a 2D π-extended fused ring dithienonaphthobisthiadiazole, named YS3. This molecule exhibited longer exciton lifetime in neat films than in chlorobenzene solution. This is partly because rotational deactivation is effectively suppressed in crystalline film states and partly because PL is due to dipole-forbidden transition in H-aggregates.
Acknowledgments
This study was partly supported by KAKENHI from the Japan Society for the Promotion of Science (Grant Nos. JP21H04692, JP22K19062) and the JST MIRAI Program from the Japan Science and Technology Agency (JPMJMI20E2). The authors thank Prof. K. Tanaka at Kyoto University for support in the absolute PLQY measurement. The 2D-GIXD experiments were performed at BL46XU of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Nos. 2021A1614 and 2021A1687). The authors thank Dr. T. Koganezawa (JASRI) for support in the 2D-GIXD measurements.
Supplementary data (0.4 MB PDF)