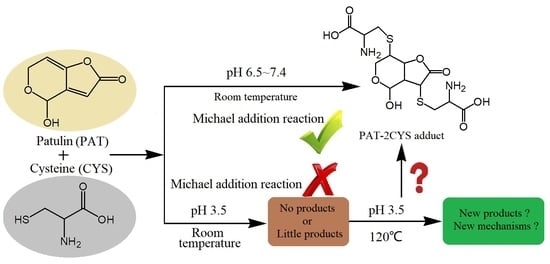
Possible Reaction Mechanisms Involved in Degradation of Patulin by Heat-Assisted Cysteine under Highly Acidic Conditions
Abstract
:1. Introduction
2. Results
2.1. Degradation of PAT by Heat-Assisted Cysteine under Highly Acidic Conditions
2.2. Fragment Patterns of PAT Based on the LC-Q-TOF-MS Analysis
2.3. Identification of PAT Degradation Products Based on the LC-Q-TOF-MS Analysis
2.3.1. Degradation Product A (DP A)
2.3.2. Degradation Product B (DP B)
2.3.3. Degradation Product C (DP C)
2.3.4. Degradation Product D (DP D)
2.3.5. Degradation Product E (DP E)
2.3.6. Degradation Product F (DP F)
2.3.7. Degradation Product G (DP G)
2.3.8. Degradation Product H (DP H)
2.4. Degradation Mechanism of PAT by Heat-Assisted Cysteine under Highly Acidic Conditions
3. Discussion
3.1. Degradation Efficiency of PAT with and without Cysteine
3.2. Identification of PAT Degradation Products
3.3. Degradation Mechanisms of PAT
3.4. Toxicity Prediction of PAT and Its Degradation Products
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Preparation of Simulated Apple Juice Solution (pH 3.5)
5.3. Preparation of PAT and Cysteine Solutions with pH 3.5
5.4. Determination of PAT in Simulated Apple Juice Solution
5.5. Degradation of PAT by Heat-Assisted Cysteine under Highly Acidic Conditions (pH 3.5)
5.6. Structure Analysis of PAT Degradation Products and Possible Reaction Mechanisms
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdoğan, A.; Ghimire, D.; Gürses, M.; Çetin, B.; Baran, A. Patulin contamination in fruit juices and its control measures. Eur. J. Sci. Technol. 2018, 14, 39–48. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, Z.; Yuan, Y.; Yue, T. Survey of patulin in apple juice concentrates in Shaanxi (China) and its dietary intake. Food Control 2013, 34, 570–573. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Spadaro, D.; Lore, A.; Gullino, M.L.; Garibaldi, A. Potential of patulin production by Penicillium expansum strains on various fruits. Mycotoxin Res. 2010, 26, 257–265. [Google Scholar] [CrossRef]
- Zheng, X.F.; Wei, W.N.; Zhou, W.Y.; Li, H.X.; Rao, S.Q.; Gao, L.; Yang, Z. Prevention and detoxification of patulin in apple and its products: A review. Food Res. Int. 2021, 140, 110034. [Google Scholar] [CrossRef] [PubMed]
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, A.; Ouhibi, S.; Ghali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef]
- EU. Assessment of Dietary Intake of Patulin by the Population of EU Member States. 2002. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_catalogue_PAT_3.2.8_en.pdf (accessed on 7 October 2022).
- WHO. Children’s Health and the Environment. 2005. Available online: http://apps.who.int/iris/bitstream/handle/10665/43162/9241562927_eng.pdf?sequence=1&isAllowed=y&ua=1 (accessed on 7 October 2022).
- Diao, E.J.; Hou, H.X.; Hu, W.C.; Dong, H.Z.; Li, X.Y. Removing and detoxifying methods of patulin: A review. Trends Food Sci. Technol. 2018, 81, 139–145. [Google Scholar] [CrossRef]
- De Souza Sant’Ana, A.; Rosenthal, A.; De Massaguer, P.R. The fate of patulin in apple juice processing: A review. Food Res. Int. 2008, 41, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Gökmen, V.; Artlk, N.; Acar, J.; Kahraman, N.; Poyrazoğlu, E. Effects of various clarification treatments on patulin, phenolic compound and organic acid compositions of apple juice. Eur. Food Res. Technol. 2001, 213, 194–199. [Google Scholar] [CrossRef]
- Diao, E.J.; Ma, K.; Qian, S.Q.; Zhang, H.; Xie, P.; Mao, R.F.; Song, H.W. Removal of patulin by thiol-compounds: A review. Toxicon 2022, 205, 31–37. [Google Scholar] [CrossRef]
- Wall, S.B.; Oh, J.Y.; Diers, A.R.; Landar, A. Oxidative modification of proteins: An emerging mechanism of cell signaling. Front. Physiol. 2012, 3, 369. [Google Scholar] [CrossRef] [Green Version]
- Fliege, R.; Metzler, M. Electrophilic properties of patulin. N-acetylcysteine and glutathione adducts. Chem. Res. Toxicol. 2000, 13, 373–381. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Sanchis, V.; Viñas, I.; Martín-Belloso, O.; Soliva-Fortuny, R. Formation of patulin-glutathione conjugates induced by pulsed light: A tentative strategy for PAT degradation in apple juices. Food Chem. 2020, 315, 126283. [Google Scholar] [CrossRef]
- Sadok, I.; Szmagara, A.; Staniszewska, M.M. The validated and sensitive HPLC-DAD methods for determination of patulin in strawberries. Food Chem. 2017, 245, 364–370. [Google Scholar] [CrossRef]
- Sadok, I.; Stachniuk, A.; Staniszewska, M.M. Developments in monitoring of patulin in fruits using liquid chromatography: An overview. Food Anal. Methods 2019, 12, 76–93. [Google Scholar] [CrossRef]
- Ma, K.; Diao, E.J.; Zhang, H.; Qian, S.Q.; Xie, P.; Mao, R.F.; Song, H.W.; Zhang, L.M. Factors influencing the removal of patulin by cysteine. Toxicon 2021, 203, 51–57. [Google Scholar] [CrossRef]
- Diao, E.J.; Ma, K.; Zhang, H.; Xie, P.; Qian, S.Q.; Song, H.W.; Mao, R.F.; Zhang, L.M. Thermal stability and degradation kinetics of patulin in highly acidic conditions: Impact of cysteine. Toxins 2021, 13, 662. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, Z.; Boucetta, H.; Liu, J.; Song, M.; Hang, T.; Lu, Y. Identification of degradation products in flumazenil using LC-Q-TOF/MS and NMR: Degradation pathway elucidation. J. Pharm. Biomed. Anal. 2022, 215, 114764. [Google Scholar] [CrossRef]
- Pokar, D.; Sahu, A.K.; Sengupta, P. LC-Q-TOF-MS driven identification of potential degradation impurities of venetoclax, mechanistic explanation on degradation pathway and establishment of a quantitative analytical assay method. J. Anal. Sci. Technol. 2020, 11, 54. [Google Scholar] [CrossRef]
- Mikolajczak, B.; Fornal, E.; Montowska, M. LC–Q–TOF–MS/MS identification of specific non-meat proteins and peptides in beef burgers. Molecules 2019, 24, 18. [Google Scholar] [CrossRef]
- Zhou, N.; Qian, Q.; Qi, P.; Zhao, J.; Wang, C.; Wang, Q. Identification of degradation products and process impurities from Tterbutaline sulfate by UHPLC-Q-TOF-MS/MS and in silico toxicity prediction. Chromatographia 2017, 80, 793–804. [Google Scholar] [CrossRef]
- Collin, S.; Bodart, E.; Badot, C.; Bouseta, A.; Nizet, S. Identification of the main degradation products of patulin generated through heat detoxification treatments. J. Inst. Brew. 2008, 114, 167–171. [Google Scholar] [CrossRef]
- Lindroth, S.; Von Wright, A. Detoxification of patulin by adduct formation with cysteine. J. Environ. Pathol. Toxicol. Oncol. 1990, 10, 254–259. [Google Scholar]
- Krivobok, S.; Seigle-Murandi, F.; Sreiman, R.; Benoit-Guyod, J.L.; Bartoli, M.H. Antitumoral activity of patulin and patulin-cysteine adducts. Pharmazie 1994, 49, 277–279. [Google Scholar] [PubMed]
- Liu, M.; Wang, J.; Wang, X.; Zhu, W.; Yao, X.; Su, L.; Sun, J.; Yue, T.; Wang, J. Highly efficient and cost-effective removal of patulin from apple juice by surface engineering of diatomite with sulfur-functionalized graphene oxide. Food Chem. 2019, 300, 125111. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Yang, Q.; Hu, N.; Zhang, W.; Zhu, W.; Wang, R.; Suo, Y.; Wang, J. Patulin removal from apple juice using a novel cysteine-functionalized metal-organic framework adsorbent. Food Chem. 2019, 270, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, Y.; Wei, J.; Gu, Y.; Yue, T.; Yuan, Y. Thiol-functionalized inactivated yeast embedded in agar aerogel for highly efficient adsorption of patulin in apple juice. J. Hazard. Mater. 2020, 388, 121802. [Google Scholar] [CrossRef] [PubMed]
- Wallen, L.L.; Lyons, A.J.; Pridham, T.G. Antimicrobial activity of patulin derivatives: A preliminary report. J. Antibiot. 1980, 33, 767–769. [Google Scholar] [CrossRef] [PubMed]






| No. | Formula | Retention Time (min) | Observed Mass (m/z) b | Calculated Mass (m/z) b | Error (ppm) | DBE c | Score d (%) | Major Fragment Ions |
|---|---|---|---|---|---|---|---|---|
| PAT | C7H6O4 | 2.745 | 153.0197 | 153.0193 | –2.39 | 5 | 99.22 | 109.0260, 111.0078, 125.0215 |
| DP A a | C10H11NO5S | 1.212 | 256.0283 | 256.0285 | 0.84 | 6 | 99.81 | 136.0399, 150.0538, 162.0557 |
| DP B a | C9H11NO3S | 1.845 | 212.0388 | 212.0387 | –0.53 | 5 | 100.00 | 112.0402,126.0560, 138.0560, 154.0305, 172.0422 |
| DP C a | C13H18N2O7S2 | 1.928 | 377.0475 | 377.0483 | 2.03 | 6 | 98.38 | 168.0313, 182.0254, 273.0051, 290.0826 |
| DP D a | C12H18N2O6S2 | 1.520 | 349.0530 | 349.0534 | 1.00 | 5 | 99.63 | 120.0121, 150.0556, 305.1421 |
| DP E a | C9H13NO5S | 1.622 | 246.0439 | 246.0442 | 1.08 | 4 | 99.70 | 123.0318, 138.0557, 150.0556 |
| DP F a | C12H16N2O5S2 | 2.152 | 331.0424 | 331.0428 | 1.16 | 6 | 99.52 | 166.0328, 200.0204 |
| DP G a | C12H14N2O4S2 | 1.691 | 313.0319 | 313.0322 | 1.03 | 7 | 99.65 | 193.0804, 282.0426 |
| DP H a | C8H12NO4S | 1.553 | 217.0441 | 217.0441 | 0.03 | 4 | 100.00 | 117.0453, 131.0359, 174.0264, 204.0091 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, E.; Ma, K.; Li, M.; Zhang, H.; Xie, P.; Qian, S.; Song, H.; Mao, R.; Zhang, L. Possible Reaction Mechanisms Involved in Degradation of Patulin by Heat-Assisted Cysteine under Highly Acidic Conditions. Toxins 2022, 14, 695. https://doi.org/10.3390/toxins14100695
Diao E, Ma K, Li M, Zhang H, Xie P, Qian S, Song H, Mao R, Zhang L. Possible Reaction Mechanisms Involved in Degradation of Patulin by Heat-Assisted Cysteine under Highly Acidic Conditions. Toxins. 2022; 14(10):695. https://doi.org/10.3390/toxins14100695
Chicago/Turabian StyleDiao, Enjie, Kun Ma, Minghua Li, Hui Zhang, Peng Xie, Shiquan Qian, Huwei Song, Ruifeng Mao, and Liming Zhang. 2022. "Possible Reaction Mechanisms Involved in Degradation of Patulin by Heat-Assisted Cysteine under Highly Acidic Conditions" Toxins 14, no. 10: 695. https://doi.org/10.3390/toxins14100695