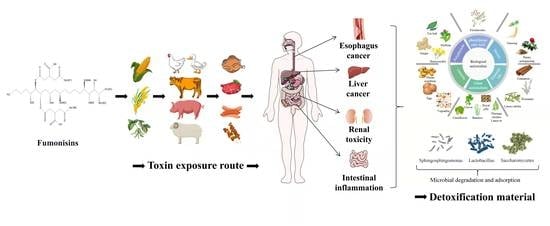
Toxic Mechanism and Biological Detoxification of Fumonisins
Abstract
:1. Introduction
2. Toxic Mechanism of Fumonisins
2.1. Effect of Fumonisins on Sphingolipid Synthesis
2.2. Fumonism Induces Oxidative Stress
3. Detoxification of Fumonisins Using Biological Antioxidants
3.1. Polyphenols
3.2. Sterols
3.3. Phenylpropionic Acids
3.4. Vitamins
3.5. Essential Oil
3.6. Other Antioxidants
4. Antagonistic Microorganisms and Degradation Mechanism of Fumonisins
4.1. Microbial Removal of Fumonisins and Its Mechanism
4.2. Microbial Adsorption of Fumonisins
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FB1 | fumonisins B1 |
| FB2 | fumonisins B2 |
| BW | body weight |
| So | sphingosine |
| Sa | sphinganine |
| So-1-p | sphingosine-1-phosphate |
| Sa-1-p | sphinganin-1-phosphate |
| SPT | serine palmitoyltransfe |
| 3-KeSa | 3-keto-sphinganine |
| 3-KR | 3-keto reductase |
| dhCerS | dihydroceramide synthetase |
| CerS | ceramide synthetase |
| Cer | ceramide |
| Ser | serine |
| dhDES | dihydroceramide dehydrogenase |
| C1P | ceramide-1-phosphate |
| CERK | ceramide kinase |
| C1PP | C1P phosphatase |
| CDases | ceramidase |
| SoKs | sphingosine kinase |
| So-1-pP | sphingosine-1-phosphate phosphatase |
| PE | phosphoethanolamine |
| HE | hexadecanal |
| So-1-pL | So-1-p lyase |
| SMases | sphingomyelinase |
| SMSs | sphingomyelin synthase |
| GlySM | Glycosphingolipids |
| GBA | glucerebrosidase |
| GCS | galactosyl-ceramide synthase |
| GSH | glutathione |
| GPx | glutathione peroxidase |
| SOD | superoxide dismutase |
| GR | glutathione reductase |
| CAT | catalase |
| MDA | malondialdehyde |
| MAPK | mitogen-activated protein kinase |
| PKC | protein kinase C |
| JNK | c-Jun N-terminal kinase |
| IRE1-α | Inositol-requiring enzyme-1-α |
| AMPK | AMP-dependent protein kinase |
| mTOR | mammalian target of rapamycin |
| TNF-α | tumor necrosis factor alpha |
| CytoC | cytochrome C |
| SIL | silymarin |
| PGE | protective effect of ginseng extract |
| REO | Rosmarinus officinalis L. essential oil |
| GML | glycerol monolaurate |
| CHL | chlorophyllin |
| IGS | indoleglucosinolates |
| MLM | moringa leaf meal |
| RJ | royal jelly |
| HFB1 | hydrolyzed FB1 |
| PHFB1 | partially hydrolysed FB1 |
| PBS | phosphate buffer saltwater |
References
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. IJERPH 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins Affecting Animals, Foods, Humans, and Plants: Types, Occurrence, Toxicities, Action Mechanisms, Prevention, and Detoxification Strategies—A Revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L.; Wang, J.-S. Co-Contamination of Aflatoxin B1 and Fumonisin B1 in Food and Human Dietary Exposure in Three Areas of China. Food Addit. Contam. Part A 2011, 28, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Miller, J.D. A Concise History of Mycotoxin Research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- Vanara, F.; Scarpino, V.; Blandino, M. Fumonisin Distribution in Maize Dry-Milling Products and By-Products: Impact of Two Industrial Degermination Systems. Toxins 2018, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Song, G.; Lim, W. Effects of Mycotoxin-Contaminated Feed on Farm Animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Gelineau-van Waes, J.; Voss, K.A.; Stevens, V.L.; Speer, M.C.; Riley, R.T. Chapter 5 Maternal Fumonisin Exposure as a Risk Factor for Neural Tube Defects. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2009; Volume 56, pp. 145–181. ISBN 978-0-12-374439-5. [Google Scholar]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Tao, B.; Pang, M.; Liu, Y.; Dong, J. Natural Occurrence of Fumonisins B1 and B2 in Maize from Three Main Maize-Producing Provinces in China. Food Control 2015, 50, 838–842. [Google Scholar] [CrossRef]
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of Fumonisin Analogs by Fusarium Species. Appl Env. Microbiol 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, Z.; Cheng, Y.; Gao, C.; Guo, L.; Wang, T.; Xu, J. Sphinganine-Analog Mycotoxins (SAMs): Chemical Structures, Bioactivities, and Genetic Controls. J. Fungi 2020, 6, 312. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Fumonisins. In Safety Evaluation of Certain Mycotoxins in Food; WHO Food Additives Series 47; FAO Food and Nutrition Paper 74; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- European Commission Regulation No. 1881/2006, setting maximum levels for certain contaminants in foodstuffs, 19 December 2006. Off. J. Eur. Union 2006, L364, 5–24.
- European Commission Regulation No. 1126/2007, setting maximum levels for certain contaminants in foodstuffs, 28 September 2007. Off. J. Eur. Union 2007, L255, 14–17.
- International Agency for Research on Cancer. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; This Publication Represents the Views and Expert Opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Which Met in Lyon, 12–19 February 2002; IARC monographs on the evaluation of carcinogenic risks to humans; IARC: Lyon, France, 2002; ISBN 978-92-832-1282-9. [Google Scholar]
- Bennett, J.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Hassan, Y.; Lepp, D.; Shao, S.; Zhou, T. Strategies and Methodologies for Developing Microbial Detoxification Systems to Mitigate Mycotoxins. Toxins 2017, 9, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngolong Ngea, G.L.; Yang, Q.; Castoria, R.; Zhang, X.; Routledge, M.N.; Zhang, H. Recent Trends in Detecting, Controlling, and Detoxifying of Patulin Mycotoxin Using Biotechnology Methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2447–2472. [Google Scholar] [CrossRef]
- Riley, R.T.; Merrill, A.H. Ceramide Synthase Inhibition by Fumonisins: A Perfect Storm of Perturbed Sphingolipid Metabolism, Signaling, and Disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Singh, M.P.; Sharma, C.; Kang, S.C. Fumonisin B1 Actuates Oxidative Stress-Associated Colonic Damage via Apoptosis and Autophagy Activation in Murine Model. J. Biochem. Mol. Toxicol. 2018, 32, e22161. [Google Scholar] [CrossRef]
- Schertz, H.; Dänicke, S.; Frahm, J.; Schatzmayr, D.; Dohnal, I.; Bichl, G.; Schwartz-Zimmermann, H.; Colicchia, S.; Breves, G.; Teifke, J.; et al. Biomarker Evaluation and Toxic Effects of an Acute Oral and Systemic Fumonisin Exposure of Pigs with a Special Focus on Dietary Fumonisin Esterase Supplementation. Toxins 2018, 10, 296. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Zhang, R.-X.; Ge, S.; Zhou, T.; Liang, Y.-K. Sphingosine Kinase AtSPHK1 Functions in Fumonisin B1-Triggered Cell Death in Arabidopsis. Plant Physiol. Biochem. 2017, 119, 70–80. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid Metabolism in Cancer Signalling and Therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Trayssac, M.; Hannun, Y.A.; Obeid, L.M. Role of Sphingolipids in Senescence: Implication in Aging and Age-Related Diseases. J. Clin. Investig. 2018, 128, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Gelineau-van Waes, J.; Rainey, M.A.; Maddox, J.R.; Voss, K.A.; Sachs, A.J.; Gardner, N.M.; Wilberding, J.D.; Riley, R.T. Increased Sphingoid Base-1-Phosphates and Failure of Neural Tube Closure after Exposure to Fumonisin or FTY720. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Gardner, N.M.; Riley, R.T.; Showker, J.L.; Voss, K.A.; Sachs, A.J.; Maddox, J.R.; Gelineau-van Waes, J.B. Elevated Nuclear Sphingoid Base-1-Phosphates and Decreased Histone Deacetylase Activity after Fumonisin B1 Treatment in Mouse Embryonic Fibroblasts. Toxicol. Appl. Pharmacol. 2016, 298, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H.; Yoo, H.-S.; Lee, Y.-M.; Kie, J.-H.; Jang, S.; Oh, S. Elevation of Sphinganine 1-Phosphate as a Predictive Biomarker for Fumonisin Exposure and Toxicity in Mice. J. Toxicol. Environ. Health Part A 2006, 69, 2071–2082. [Google Scholar] [CrossRef]
- Voss, K.A.; Howard, P.C.; Riley, R.T.; Sharma, R.P.; Bucci, T.J.; Lorentzen, R.J. Carcinogenicity and Mechanism of Action of Fumonisin B1: A Mycotoxin Produced by Fusarium Moniliforme (=F. Verticillioides). Cancer Detect. Prev. 2002, 26, 1–9. [Google Scholar] [CrossRef]
- Enongene, E.N. Persistence and Reversibility of the Elevation in Free Sphingoid Bases Induced by Fumonisin Inhibition of Ceramide Synthase. Toxicol. Sci. 2002, 67, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Schwartz-Zimmermann, H.; Caha, S.; Moll, W.; Schatzmayr, G.; Applegate, T. Dose-Dependent Effects on Sphingoid Bases and Cytokines in Chickens Fed Diets Prepared with Fusarium Verticillioides Culture Material Containing Fumonisins. Toxins 2015, 7, 1253–1272. [Google Scholar] [CrossRef] [Green Version]
- Bracarense, A.-P.F.L.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Chronic Ingestion of Deoxynivalenol and Fumonisin, Alone or in Interaction, Induces Morphological and Immunological Changes in the Intestine of Piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef] [Green Version]
- Atroshi, F.; Rizzo, A.; Biese, I.; Veijalainen, P.; Saloniemi, H.; Sankari, S.; Andersson, K. Fumonisin B1-Introduce DNA Damage in Liver and Spleen: Effects of Pretreatment Wuth Coenzyme. Pharmacol. Res. 1999, 40, 9. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Wan, D.; Liu, Q.; Chen, D.; Liu, Z.; Martínez-Larrañaga, M.R.; Martínez, M.A.; Anadón, A.; Yuan, Z. Fumonisins: Oxidative Stress-Mediated Toxicity and Metabolism in Vivo and in Vitro. Arch. Toxicol. 2016, 90, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Sheik Abdul, N.; Marnewick, J.L. Fumonisin B1-induced Mitochondrial Toxicity and Hepatoprotective Potential of Rooibos: An Update. J. Appl. Toxicol. 2020, 40, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Stockmann-Juvala, H.; Mikkola, J.; Naarala, J.; Loikkanen, J.; Elovaara, E.; Savolainen, K. Oxidative Stress Induced by Fumonisin B1 in Continuous Human and Rodent Neural Cell Cultures. Free Radic. Res. 2004, 38, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.; Pillay, Y.; Ghazi, T.; Nagiah, S.; Abdul, N.S.; Chuturgoon, A.A. Fumonisin B1-Induced Oxidative Stress Triggers Nrf2-Mediated Antioxidant Response in Human Hepatocellular Carcinoma (HepG2) Cells. Mycotoxin Res. 2019, 35, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Kang, S.C. Endoplasmic Reticulum Stress-Mediated Autophagy Activation Attenuates Fumonisin B1 Induced Hepatotoxicity in Vitro and in Vivo. Food Chem. Toxicol. 2017, 110, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Domijan, A.; Želježić, D.; Peraica, M.; Kovačević, G.; Gregorović, G.; Krstanac, Ž.; Horvatin, K.; Kalafatić, M. Early Toxic Effects of Fumonisin B1 in Rat Liver. Hum. Exp. Toxicol. 2008, 27, 895–900. [Google Scholar] [CrossRef]
- Minervini, F.; Lacalandra, G.M.; Filannino, A.; Garbetta, A.; Nicassio, M.; Dell’Aquila, M.E.; Visconti, A. Toxic Effects Induced by Mycotoxin Fumonisin B1 on Equine Spermatozoa: Assessment of Viability, Sperm Chromatin Structure Stability, ROS Production and Motility. Toxicol. Vitr. 2010, 24, 2072–2078. [Google Scholar] [CrossRef]
- Mobio, T.A.; Tavan, E.; Baudrimont, I.; Anane, R.; Carratú, M.-R.; Sanni, A.; Gbeassor, M.F.; Shier, T.W.; Narbonne, J.-F.; Creppy, E.E. Comparative Study of the Toxic Effects of Fumonisin B1 in Rat C6 Glioma Cells and P53-Null Mouse Embryo Fibroblasts. Toxicology 2003, 183, 65–75. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef] [Green Version]
- Pilger, A.; Rüdiger, H.W. 8-Hydroxy-2′-Deoxyguanosine as a Marker of Oxidative DNA Damage Related to Occupational and Environmental Exposures. Int. Arch. Occup. Environ. Health 2006, 80, 1–15. [Google Scholar] [CrossRef]
- Yuan, Q.; Jiang, Y.; Fan, Y.; Ma, Y.; Lei, H.; Su, J. Fumonisin B1 Induces Oxidative Stress and Breaks Barrier Functions in Pig Iliac Endothelium Cells. Toxins 2019, 11, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Jia, B.; Liu, N.; Yu, D.; Zhang, S.; Wu, A. Fumonisin B1 Triggers Carcinogenesis via HDAC/PI3K/Akt Signalling Pathway in Human Esophageal Epithelial Cells. Sci. Total Environ. 2021, 787, 147405. [Google Scholar] [CrossRef] [PubMed]
- Gopee, N.V.; He, Q.; Sharma, R.P. Fumonisin B1-Induced Apoptosis Is Associated with Delayed Inhibition of Protein Kinase C, Nuclear Factor-ΚB and Tumor Necrosis Factor α in LLC-PK1 Cells. Chem. Biol. Interact. 2003, 146, 131–145. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Kang, W.; Lin, Z.; Gan, F.; Huang, K. Non-Cytotoxic Dosage of Fumonisin B1 Aggravates Ochratoxin A-Induced Nephrocytotoxicity and Apoptosis via ROS-Dependent JNK/MAPK Signaling Pathway. Toxicology 2021, 457, 152802. [Google Scholar] [CrossRef]
- Burke, P.J. Mitochondria, Bioenergetics and Apoptosis in Cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef]
- Khan, R.; Phulukdaree, A.; Chuturgoon, A. Concentration-Dependent Effect of Fumonisin B1 on Apoptosis in Oesophageal Cancer Cells. Hum. Exp. Toxicol. 2018, 37, 762–771. [Google Scholar] [CrossRef]
- Bucciantini, M.; Nosi, D.; Forzan, M.; Russo, E.; Calamai, M.; Pieri, L.; Formigli, L.; Quercioli, F.; Soria, S.; Pavone, F.; et al. Toxic Effects of Amyloid Fibrils on Cell Membranes: The Importance of Ganglioside GM1. FASEB J. 2012, 26, 818–831. [Google Scholar] [CrossRef]
- Du, P.; Li, S.-J.; Ojcius, D.M.; Li, K.-X.; Hu, W.-L.; Lin, X.; Sun, A.-H.; Yan, J. A Novel Fas-Binding Outer Membrane Protein and Lipopolysaccharide of Leptospira Interrogans Induce Macrophage Apoptosis through the Fas/FasL-Caspase-8/-3 Pathway. Emerg. Microbes Infect. 2018, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Bhandari, N.; Sharma, R.P. Fumonisin B1 Alters Sphingolipid Metabolism and Tumor Necrosis Factor a Expression in Heart and Lung of Mice. Life Sci. 2002, 9, 2015–2023. [Google Scholar] [CrossRef]
- Kócsó, D.J.; Szabó-Fodor, J.; Mézes, M.; Balogh, K.; Ferenczi, S.; Szabó, A.; Bóta, B.; Kovács, M. Fumonisin B1 Exposure Increases Hsp70 Expression in the Lung and Kidney of Rats without Inducing Significant Oxidative Stress. Acta Vet. Hung. 2018, 66, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, S.; Huang, S.; Yan, R.; Wang, M.; Chen, S.; Cai, J.; Long, M.; Li, P. Transcriptome Study Reveals Apoptosis of Porcine Kidney Cells Induced by Fumonisin B1 via TNF Signalling Pathway. Food Chem. Toxicol. 2020, 139, 111274. [Google Scholar] [CrossRef]
- Régnier, M.; Gourbeyre, P.; Pinton, P.; Napper, S.; Laffite, J.; Cossalter, A.; Bailly, J.; Lippi, Y.; Bertrand-Michel, J.; Bracarense, A.P.F.R.L.; et al. Identification of Signaling Pathways Targeted by the Food Contaminant FB1: Transcriptome and Kinome Analysis of Samples from Pig Liver and Intestine. Mol. Nutr. Food Res. 2017, 61, 1700433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgarbossa, A. Natural Biomolecules and Protein Aggregation: Emerging Strategies against Amyloidogenesis. IJMS 2012, 13, 17121–17137. [Google Scholar] [CrossRef] [PubMed]
- García-Seisdedos, D.; Babiy, B.; Lerma, M.; Casado, M.E.; Martínez-Botas, J.; Lasunción, M.A.; Pastor, Ó.; Busto, R. Curcumin Stimulates Exosome/Microvesicle Release in an in Vitro Model of Intracellular Lipid Accumulation by Increasing Ceramide Synthesis. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158638. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Evans, E.; Morgan, A.J.; He, X.; Smith, D.A.; Elliot-Smith, E.; Sillence, D.J.; Churchill, G.C.; Schuchman, E.H.; Galione, A.; Platt, F.M. Niemann-Pick Disease Type C1 Is a Sphingosine Storage Disease That Causes Deregulation of Lysosomal Calcium. Nat. Med. 2008, 14, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.M.; Griss, L.G.; Fortuoso, B.F.; Silva, A.D.; Fracasso, M.; Lopes, T.F.; Schetinger, M.R.S.; Gundel, S.; Ourique, A.F.; Carneiro, C.; et al. Feed Contaminated by Fumonisin (Fusarium Spp.) in Chicks Has a Negative Influence on Oxidative Stress and Performance, and the Inclusion of Curcumin-Loaded Nanocapsules Minimizes These Effects. Microb. Pathog. 2020, 148, 104496. [Google Scholar] [CrossRef]
- Ledur, P.C.; Santurio, J.M. Cytoprotective Effects of Curcumin and Silymarin on PK-15 Cells Exposed to Ochratoxin A, Fumonisin B1 and Deoxynivalenol. Toxicon 2020, 185, 97–103. [Google Scholar] [CrossRef]
- Sozmen, M.; Devrim, A.K.; Tunca, R.; Bayezit, M.; Dag, S.; Essiz, D. Protective Effects of Silymarin on Fumonisin B1-Induced Hepatotoxicity in Mice. J. Vet. Sci. 2014, 15, 51. [Google Scholar] [CrossRef] [Green Version]
- Marnewick, J.L.; van der Westhuizen, F.H.; Joubert, E.; Swanevelder, S.; Swart, P.; Gelderblom, W.C.A. Chemoprotective Properties of Rooibos (Aspalathus Linearis), Honeybush (Cyclopia Intermedia) Herbal and Green and Black (Camellia Sinensis) Teas against Cancer Promotion Induced by Fumonisin B1 in Rat Liver. Food Chem. Toxicol. 2009, 47, 220–229. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Bernillon, S.; Marchegay, G.; Lornac, A.; Pinson-Gadais, L.; Ponts, N.; Zehraoui, E.; Barreau, C.; Richard-Forget, F. Bioguided Isolation, Characterization, and Biotransformation by Fusarium Verticillioides of Maize Kernel Compounds That Inhibit Fumonisin Production. MPMI Mol. Plant Microbe Interact. 2014, 27, 1148–1158. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.M.; Abdel-Aziem, S.H.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Panax Ginseng Extract Modulates Oxidative Stress, DNA Fragmentation and up-Regulate Gene Expression in Rats Sub Chronically Treated with Aflatoxin B1 and Fumonisin B1. Cytotechnology 2015, 67, 861–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Wahhab, M.A.; Hassan, N.S.; El-Kady, A.A.; Khadrawy, Y.A.; El-Nekeety, A.A.; Mohamed, S.R.; Sharaf, H.A.; Mannaa, F.A. Red Ginseng Extract Protects against Aflatoxin B1 and Fumonisins-Induced Hepatic Pre-Cancerous Lesions in Rats. Food Chem. Toxicol. 2010, 48, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Konoshima, T.; Takasaki, M.; Tokuda, H. Anti-Carcinogenic Activity of the Roots of Panax Notoginseng. II. Biol. Pharm. Bull. 1999, 22, 1150–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrochio, L.; Cendoya, E.; Farnochi, M.C.; Massad, W.; Ramirez, M.L. Evaluation of Ability of Ferulic Acid to Control Growth and Fumonisin Production of Fusarium Verticillioides and Fusarium Proliferatum on Maize Based Media. Int. J. Food Microbiol. 2013, 167, 215–220. [Google Scholar] [CrossRef]
- Shin, H.-D.; McClendon, S.; Le, T.; Taylor, F.; Chen, R.R. A Complete Enzymatic Recovery of Ferulic Acid from Corn Residues with Extracellular Enzymes FromNeosartorya Spinosa NRRL185. Biotechnol. Bioeng. 2006, 95, 1108–1115. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Ferulic Acid: An Antioxidant Found Naturally in Plant Cell Walls and Feruloyl Esterases Involved in Its Release and Their Applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef]
- Mobio, T.A.; Baudrimont, I.; Sanni, A.; Shier, T.W.; Saboureau, D.; Dano, S.D.; Ueno, Y.; Steyn, P.S.; Creppy, E.E. Prevention by Vitamin E of DNA Fragmentation and Apoptosis Induced by Fumonisin B 1 in C6 Glioma Cells. Arch. Toxicol. 2000, 74, 112–119. [Google Scholar] [CrossRef]
- Jiang, Q.; Wong, J.; Fyrst, H.; Saba, J.D.; Ames, B.N. Tocopherol or Combinations of Vitamin E Forms Induce Cell Death in Human Prostate Cancer Cells by Interrupting Sphingolipid Synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 17825–17830. [Google Scholar] [CrossRef] [Green Version]
- Oginni, O.; Gbore, F.A.; Adewole, A.M.; Eniade, A.; Adebusoye, A.J.; Abimbola, A.T.; Ajumobi, O.O. Influence of Vitamins on Flesh Yields and Proximate Compositions of Clarias Gariepinus Fed Diets Contaminated with Increasing Doses of Fumonisin B1. J. Agric. Food Res. 2020, 2, 100079. [Google Scholar] [CrossRef]
- Sadler, T.W.; Merrill, A.H.; Stevens, V.L.; Sullards, M.C.; Wang, E.; Wang, P. Prevention of Fumonisin B1-Induced Neural Tube Defects by Folic Acid. Teratology 2002, 66, 169–176. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; El-Nekeety, A.A.; Hassan, N.S.; Gibriel, A.A.Y.; Abdel-Wahhab, K.G. Encapsulation of Cinnamon Essential Oil in Whey Protein Enhances the Protective Effect against Single or Combined Sub-Chronic Toxicity of Fumonisin B1 and/or Aflatoxin B1 in Rats. Environ. Sci. Pollut. Res. 2018, 25, 29144–29161. [Google Scholar] [CrossRef] [PubMed]
- López, A.G.; Theumer, M.G.; Zygadlo, J.A.; Rubinstein, H.R. Aromatic Plants Essential Oils Activity on Fusarium Verticillioides Fumonisin B1 Production in Corn Grain. Mycopathologia 2004, 158, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pante, G.C.; Castro, J.C.; Lini, R.S.; Romoli, J.C.Z.; de Almeida, R.T.R.; Garcia, F.P.; Nakamura, C.V.; Pilau, E.J.; de Abreu Filho, B.A.; Machinski, M. Litsea Cubeba Essential Oil: Chemical Profile, Antioxidant Activity, Cytotoxicity, Effect against Fusarium Verticillioides and Fumonisins Production. J. Environ. Sci. Health Part B 2021, 56, 387–395. [Google Scholar] [CrossRef] [PubMed]
- da Silva Bomfim, N.; Nakassugi, L.P.; Faggion Pinheiro Oliveira, J.; Kohiyama, C.Y.; Mossini, S.A.G.; Grespan, R.; Nerilo, S.B.; Mallmann, C.A.; Alves Abreu Filho, B.; Machinski, M. Antifungal Activity and Inhibition of Fumonisin Production by Rosmarinus Officinalis L. Essential Oil in Fusarium Verticillioides (Sacc.) Nirenberg. Food Chem. 2015, 166, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto-Ribeiro, M.M.G.; Grespan, R.; Kohiyama, C.Y.; Ferreira, F.D.; Mossini, S.A.G.; Silva, E.L.; de Abreu Filho, B.A.; Mikcha, J.M.G.; Machinski Junior, M. Effect of Zingiber Officinale Essential Oil on Fusarium Verticillioides and Fumonisin Production. Food Chem. 2013, 141, 3147–3152. [Google Scholar] [CrossRef] [Green Version]
- Bendaha, H.; Yu, L.; Touzani, R.; Souane, R.; Giaever, G.; Nislow, C.; Boone, C.; El Kadiri, S.; Brown, G.W.; Bellaoui, M. New Azole Antifungal Agents with Novel Modes of Action: Synthesis and Biological Studies of New Tridentate Ligands Based on Pyrazole and Triazole. Eur. J. Med. Chem. 2011, 46, 4117–4124. [Google Scholar] [CrossRef]
- Castro, J.C.; Pante, G.C.; Centenaro, B.M.; Almeida, R.T.R.D.; Pilau, E.J.; Dias Filho, B.P.; Mossini, S.A.G.; Abreu Filho, B.A.D.; Matioli, G.; Machinski Junior, M. Antifungal and Antimycotoxigenic Effects of Zingiber Officinale, Cinnamomum Zeylanicum and Cymbopogon Martinii Essential Oils against Fusarium Verticillioides. Food Addit. Contam. Part A 2020, 37, 1531–1541. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; Galli, G.M.; Griss, L.G.; Armanini, E.H.; Silva, A.D.; Fracasso, M.; Mostardeiro, V.; Morsch, V.M.; Lopes, L.Q.S.; Santos, R.C.V.; et al. Effects of Glycerol Monolaurate on Growth and Physiology of Chicks Consuming Diet Containing Fumonisin. Microb. Pathog. 2020, 147, 104261. [Google Scholar] [CrossRef]
- Domijan, A.-M.; Gajski, G.; Novak Jovanović, I.; Gerić, M.; Garaj-Vrhovac, V. In Vitro Genotoxicity of Mycotoxins Ochratoxin A and Fumonisin B1 Could Be Prevented by Sodium Copper Chlorophyllin—Implication to Their Genotoxic Mechanism. Food Chem. 2015, 170, 455–462. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Liu, Y.; Miao, H.; Cai, C.; Shao, Z.; Guo, R.; Sun, B.; Jia, C.; Zhang, L.; et al. Classic Myrosinase-Dependent Degradation of Indole Glucosinolate Attenuates Fumonisin B1-Induced Programmed Cell Death in Arabidopsis. Plant J. 2015, 81, 920–933. [Google Scholar] [CrossRef]
- Hassan, A.M.; Mohamed, S.R.; El-Nekeety, A.A.; Hassan, N.S.; Abdel-Wahhab, M.A. Aquilegia Vulgaris L. Extract Counteracts Oxidative Stress and Cytotoxicity of Fumonisin in Rats. Toxicon 2010, 56, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Gbore, F.A.; Adewumi, F.H. Ameliorative Potential of Moringa Leaf Meal on Nutrient Digestibility of Rabbits Fed Fumonisin B1-Contaminated Diets. Toxicon 2021, 201, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- El-Nekeety, A.A.; El-Kholy, W.; Abbas, N.F.; Ebaid, A.; Amra, H.A.; Abdel-Wahhab, M.A. Efficacy of Royal Jelly against the Oxidative Stress of Fumonisin in Rats. Toxicon 2007, 50, 256–269. [Google Scholar] [CrossRef]
- Wu, L.Y.; Xu, Y.D.; Wang, H.M.; Sun, X.L. Research Progress on Microbial Detoxification Mechanism of Mycotoxins. Food Res. Dev. 2018, 39, 192–199. [Google Scholar] [CrossRef]
- Heinl, S.; Hartinger, D.; Thamhesl, M.; Vekiru, E.; Krska, R.; Schatzmayr, G.; Moll, W.-D.; Grabherr, R. Degradation of Fumonisin B1 by the Consecutive Action of Two Bacterial Enzymes. J. Biotechnol. 2010, 145, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Heinl, S.; Hartinger, D.; Thamhesl, M.; Schatzmayr, G.; Moll, W.-D.; Grabherr, R. An Aminotransferase from Bacterium ATCC 55552 Deaminates Hydrolyzed Fumonisin B1. Biodegradation 2011, 22, 25–30. [Google Scholar] [CrossRef]
- Blackwell, B.A.; Gilliam, J.T.; Savard, M.E.; David Miller, J.; Duvick, J.P. Oxidative Deamination of Hydrolyzed Fumonisin B1 (AP1) by Cultures OfExophiala Spinifera. Nat. Toxins 1999, 7, 31–38. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Gong, A.; Liu, N.; Chen, S.; Zhao, X.; Li, X.; Chen, L.; Zhou, C.; Wang, J. Biodegradation of Mycotoxin Fumonisin B1 by a Novel Bacterial Consortium SAAS79. Appl. Microbiol. Biotechnol. 2019, 103, 7129–7140. [Google Scholar] [CrossRef]
- Martinez Tuppia, C.; Atanasova-Penichon, V.; Chéreau, S.; Ferrer, N.; Marchegay, G.; Savoie, J.-M.; Richard-Forget, F. Yeast and Bacteria from Ensiled High Moisture Maize Grains as Potential Mitigation Agents of Fumonisin B1: Maize Grain Silage as a Source of FB1 Degrading Microorganisms. J. Sci. Food Agric. 2017, 97, 2443–2452. [Google Scholar] [CrossRef]
- Benedetti, R.; Nazzi, F.; Locci, R.; Firrao, G. Degradation of Fumonisin B1 by a Bacterial Strain Isolated from Soil. Biodegradation 2006, 17, 31–38. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; et al. Safety and Efficacy of Fumonisin Esterase (FUMzyme®) as a Technological Feed Additive for All Avian Species. EFSA J. 2016, 14, e04617. [Google Scholar] [CrossRef]
- Deepthi, B.V.; Somashekaraiah, R.; Poornachandra Rao, K.; Deepa, N.; Dharanesha, N.K.; Girish, K.S.; Sreenivasa, M.Y. Lactobacillus Plantarum MYS6 Ameliorates Fumonisin B1-Induced Hepatorenal Damage in Broilers. Front. Microbiol. 2017, 8, 2317. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, B.V.; Poornachandra Rao, K.; Chennapa, G.; Naik, M.K.; Chandrashekara, K.T.; Sreenivasa, M.Y. Antifungal Attributes of Lactobacillus Plantarum MYS6 against Fumonisin Producing Fusarium Proliferatum Associated with Poultry Feeds. PLoS ONE 2016, 11, e0155122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Qiao, Y.; Wang, X.; Pei, J.; Zheng, J.; Zhang, B. Absorption of fumonisin B1 and B2 by Lactobacillus plantarum ZJ8. Acta Microbiol. Sin. 2014, 54, 1481–1488. [Google Scholar]
- Zhao, H.; Wang, X.; Zhang, J.; Zhang, J.; Zhang, B. The Mechanism of Lactobacillus Strains for Their Ability to Remove Fumonisins B1 and B2. Food Chem. Toxicol. 2016, 97, 40–46. [Google Scholar] [CrossRef]
- Ezdini, K.; Ben Salah-Abbès, J.; Belgacem, H.; Mannai, M.; Abbès, S. Lactobacillus Paracasei Alleviates Genotoxicity, Oxidative Stress Status and Histopathological Damage Induced by Fumonisin B1 in BALB/c Mice. Toxicon 2020, 185, 46–56. [Google Scholar] [CrossRef]
- Abbès, S.; Ben Salah-Abbès, J.; Jebali, R.; Younes, R.B.; Oueslati, R. Interaction of Aflatoxin B1 and Fumonisin B1 in Mice Causes Immunotoxicity and Oxidative Stress: Possible Protective Role Using Lactic Acid Bacteria. J. Immunotoxicol. 2016, 13, 46–54. [Google Scholar] [CrossRef]
- Armando, M.R.; Galvagno, M.A.; Dogi, C.A.; Cerrutti, P.; Dalcero, A.M.; Cavaglieri, L.R. Statistical Optimization of Culture Conditions for Biomass Production of Probiotic Gut-Borne Saccharomyces Cerevisiae Strain Able to Reduce Fumonisin B1. J. Appl. Microbiol. 2013, 114, 1338–1346. [Google Scholar] [CrossRef]







| Fumonisins | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| FB1 | CH3 | NH2 | H | OH | OH | TCA | TCA |
| Iso-FB1 | CH3 | NH2 | OH | H | OH | TCA | TCA |
| PHFB1a | CH3 | NH2 | H | OH | OH | OH | TCA |
| PHFB1b | CH3 | NH2 | H | OH | OH | TCA | OH |
| HFB1 | CH3 | NH2 | H | OH | OH | OH | OH |
| FB2 | CH3 | NH2 | H | OH | H | TCA | TCA |
| FB3 | CH3 | NH2 | H | H | OH | TCA | TCA |
| FB4 | CH3 | NH2 | H | H | H | TCA | TCA |
| FBK1 | CH3 | NH2 | H | H | OH | TCA | =O |
| FA1 | CH3 | NHCOCH3 | H | OH | OH | TCA | TCA |
| FA2 | CH3 | NHCOCH3 | H | OH | H | TCA | TCA |
| FA3 | CH3 | NHCOCH3 | H | H | OH | TCA | TCA |
| PHFA3a | CH3 | NHCOCH3 | H | H | OH | OH | TCA |
| PHFA3b | CH3 | NHCOCH3 | H | H | OH | TCA | OH |
| HFA3 | CH3 | NHCOCH3 | H | H | OH | OH | OH |
| FAK1 | CH3 | NHCOCH3 | H | OH | OH | TCA | =O |
| FC1 | H | NH2 | H | OH | OH | TCA | TCA |
| N-acetyl-FC1 | H | NHCOCH3 | H | OH | OH | TCA | TCA |
| Iso-FC1 | H | NH2 | OH | H | OH | TCA | TCA |
| N-acetyl-iso-FC1 | H | NHCOCH3 | OH | H | OH | TCA | TCA |
| OH-FC1 | H | NH2 | OH | OH | OH | TCA | TCA |
| N-acetyl-OH-FC1 | H | NHCOCH3 | OH | OH | OH | TCA | TCA |
| FC3 | H | NH2 | H | H | OH | TCA | TCA |
| FC4 | H | NH2 | H | H | H | TCA | TCA |
| FP1 | CH3 | 3HP | H | OH | OH | TCA | TCA |
| FP2 | CH3 | 3HP | H | OH | H | TCA | TCA |
| FP3 | CH3 | 3HP | H | H | OH | TCA | TCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, L.; Wang, L.; Ji, H.; Fang, Y.; Lei, P.; Zhang, X.; Jin, L.; Sun, D.; Dong, H. Toxic Mechanism and Biological Detoxification of Fumonisins. Toxins 2022, 14, 182. https://doi.org/10.3390/toxins14030182
Qu L, Wang L, Ji H, Fang Y, Lei P, Zhang X, Jin L, Sun D, Dong H. Toxic Mechanism and Biological Detoxification of Fumonisins. Toxins. 2022; 14(3):182. https://doi.org/10.3390/toxins14030182
Chicago/Turabian StyleQu, Linkai, Lei Wang, Hao Ji, Yimeng Fang, Pengyu Lei, Xingxing Zhang, Libo Jin, Da Sun, and Hao Dong. 2022. "Toxic Mechanism and Biological Detoxification of Fumonisins" Toxins 14, no. 3: 182. https://doi.org/10.3390/toxins14030182