Citrinin Mycotoxin Contamination in Food and Feed: Impact on Agriculture, Human Health, and Detection and Management Strategies
Abstract
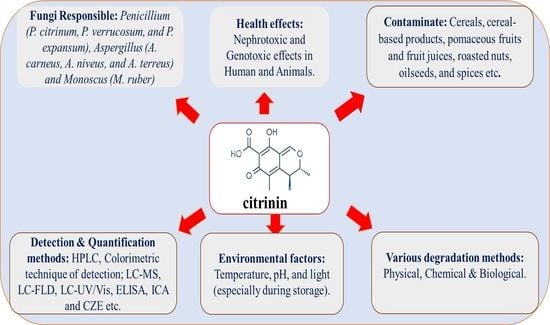
:1. Introduction
2. Major Source of Citrinin
3. Chemistry and Biosynthesis of Citrinin
4. Genes Responsible for Citrinin Production
5. Occurrence in Food and Feed
6. Effects on Agricultural Food and Feed
7. Mechanism of Toxicity and Health Effects of Citrinin
7.1. Mechanism of Toxicity
7.2. Health Effects of Citrinin
8. Effects of Processing on Citrinin
9. Effects of Environmental Factors on Citrinin Production
10. Detection Techniques
10.1. Sample Preparation
10.2. Detection and Quantification Methods
10.2.1. Thin-Layer Chromatography (TLC)
10.2.2. Colorimetric Technique of Detection
10.2.3. High-Performance Liquid Chromatography (HPLC)
10.2.4. Liquid Chromatography-Mass Spectroscopy (LC-MS)
10.2.5. Liquid Chromatography Fluorescence Detection (LC-FLD)
10.2.6. Liquid Chromatography UV/Visible Detection (LC-UV/Vis)
10.2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
10.2.8. Immunochromatographic Assay (ICA)
10.2.9. Capillary Zone Electrophoresis (CZE)
11. Masked Mycotoxins as a Major Concern in Detection
12. Degradation Kinetics
13. Management and Control Strategies
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalefield, R. Mycotoxins and mushrooms. In Veterinary Toxicology for Australia and New Zealand; Elsevier: Amsterdam, The Netherlands, 2017; pp. 373–419. [Google Scholar]
- Kováč, Š.; Nemec, P.; Betina, V.; Balan, J. Chemical structure of citrinin. Nature 1961, 190, 1104–1105. [Google Scholar] [CrossRef]
- Tangni, E.K.; Pussemier, L. Ochratoxin A and citrinin loads in stored wheat grains: Impact of grain dust and possible prediction using ergosterol measurement. Food Addit. Contam. 2006, 23, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostry, V.; Malir, F.; Ruprich, J. Producers and important dietary sources of ochratoxin A and citrinin. Toxins 2013, 5, 1574–1586. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Cox, R.J. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci. 2016, 7, 2119–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, J.I. Biology and ecology of toxigenic Penicillium species. Adv. Exp. Med. Biol. 2002, 504, 29–41. [Google Scholar]
- Pattanagul, P.; Pinthong, R.; Phianmongkhol, A.; Tharatha, S. Mevinolin, citrinin and pigments of adlay angkak fermented by Monascus sp. Int. J. Food Microbiol. 2008, 126, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Marič, A.; Skočaj, M.; Likar, M.; Sepčić, K.; Cigić, I.K.; Grundner, M.; Gregori, A. Comparison of lovastatin, citrinin and pigment production of different Monascus purpureus strains grown on rice and millet. J. Food Sci. Technol. 2019, 56, 3364–3373. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Mycotoxins toxicology. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 887–892. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [Green Version]
- Erdoğrul, Ö.; Azirak, S. Review of the studies on the red yeast rice (Monascus purpureus). Turk. Electron. J. Biotechnol. 2004, 2, 37–49. [Google Scholar]
- Chang, C.-H.; Yu, F.-Y.; Wang, L.-T.; Lin, Y.-S.; Liu, B.-H. Activation of ERK and JNK signaling pathways by mycotoxin citrinin in human cells. Toxicol. Appl. Pharmacol. 2009, 237, 281–287. [Google Scholar] [CrossRef]
- Nakajima, Y.; Iguchi, H.; Kamisuki, S.; Sugawara, F.; Furuichi, T.; Shinoda, Y. Low doses of the mycotoxin citrinin protect cortical neurons against glutamate-induced excitotoxicity. J. Toxicol. Sci. 2016, 41, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira Filho, J.W.G.; Islam, M.T.; Ali, E.S.; Uddin, S.J.; de Oliveira Santos, J.V.; de Alencar, M.V.O.B.; Júnior, A.L.G.; Paz, M.F.C.J.; de Brito, M.d.R.M.; de Sousa, J.M.d.C. A comprehensive review on biological properties of citrinin. Food Chem. Toxicol. 2017, 110, 130–141. [Google Scholar] [CrossRef]
- Nejati, P.; Chaychi Nosrati, A.; Bayat, M. An investigation on measurement means of Citrinin toxin quantity by toxigenic Aspergillus species in biomass, using ELISA. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 2466–2471. [Google Scholar]
- Jeswal, P.; Kumar, D. Mycobiota and natural incidence of aflatoxins, ochratoxin A, and citrinin in Indian spices confirmed by LC-MS/MS. Int. J. Microbiol. 2015, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.J.G.; Pereira, A.M.P.T.; Pena, A.; Lino, C.M. Citrinin in foods and supplements: A review of occurrence and analytical methodologies. Foods 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Hackbart, H.; Prietto, L.; Primel, E.G.; Garda-Buffon, J.; Badiale-Furlong, E. Simultaneous extraction and detection of ochratoxin A and citrinin in rice. J. Braz. Chem. Soc. 2012, 23, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, A.B.; Hirota, M.; Doi, E.; Kitabatake, N. Formation of a new toxic compound, citrinin H1, from citrinin on mild heating in water. J. Chem. Soc. Perkin Trans. 1 1993, 1, 2167–2171. [Google Scholar] [CrossRef]
- Li, Y.-P.; Xu, Y.; Huang, Z.-B. Isolation and characterization of the citrinin biosynthetic gene cluster from Monascus aurantiacus. Biotechnol. Lett. 2012, 34, 131–136. [Google Scholar] [CrossRef]
- Chai, X.; Ai, Z.; Liu, J.; Guo, T.; Wu, J.; Bai, J.; Lin, Q. Effects of pigment and citrinin biosynthesis on the metabolism and morphology of Monascus purpureus in submerged fermentation. Food Sci. Biotechnol. 2020, 29, 927–937. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Tseng, C.-P.; Chien, I.-L.; Wang, W.-Y.; Liaw, L.-L.; Yuan, G.-F. Exploring the distribution of citrinin biosynthesis related genes among Monascus species. J. Agric. Food Chem. 2008, 56, 11767–11772. [Google Scholar] [CrossRef]
- Zhang, H.; Ahima, J.; Yang, Q.; Zhao, L.; Zhang, X.; Zheng, X. A review on citrinin: Its occurrence, risk implications, analytical techniques, biosynthesis, physiochemical properties and control. Food Res. Int. 2020, 141, 110075. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kinoshita, H.; Ishihara, S.; Sakai, K.; Nagai, S.; Nihira, T. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2005, 71, 3453–3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, G.; Xu, Y.; Li, Y.; Tan, W. Construction of a replacement vector to disrupt pksCT gene for the mycotoxin citrinin biosynthesis in Monascus aurantiacus and maintain food red pigment production. Asia Pac. J. Clin. Nutr. 2007, 16, 137–142. [Google Scholar] [PubMed]
- Shimizu, T.; Kinoshita, H.; Nihira, T. Identification and in vivo functional analysis by gene disruption of ctnA, an activator gene involved in citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2007, 73, 5097–5103. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Du, X.-J.; Li, P.; Sun, C.-C.; Wang, S. Investigation of citrinin and pigment biosynthesis mechanisms in Monascus purpureus by transcriptomic analysis. Front. Microbiol. 2018, 9, 1374. [Google Scholar] [CrossRef]
- EFSA. European food safety authority panel on contaminants in the food chain. Scientific opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012, 10, 2605. [Google Scholar]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Bailly, J.D.; Querin, A.; Le Bars-Bailly, S.; Benard, G.; Guerre, P. Citrinin production and stability in cheese. J. Food Prot. 2002, 65, 1317–1321. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; García-Campaña, A.M.; Gámiz-Gracia, L. Simple methodology for the determination of mycotoxins in pseudocereals, spelt and rice. Food Control 2014, 36, 94–101. [Google Scholar] [CrossRef]
- Lhotská, I.; Šatínský, D.; Havlíková, L.; Solich, P. A fully automated and fast method using direct sample injection combined with fused-core column on-line SPE–HPLC for determination of ochratoxin A and citrinin in lager beers. Anal. Bioanal. Chem. 2016, 408, 3319–3329. [Google Scholar] [CrossRef]
- Martins, M.L.; Gimeno, A.; Martins, H.M.; Bernardo, F. Co-occurrence of patulin and citrinin in Portuguese apples with rotten spots. Food Addit. Contam. 2002, 19, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, N.; Xian, H.; Wei, D.; Shi, L.; Feng, X. A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1429, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Pepeljnjak, S.; Šegvic, M.; Ozegovic, L. Citrininotoxinogenicity of Penicillium spp. isolated from decaying apples. Braz. J. Microbiol. 2002, 33, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; Gámiz-Gracia, L.; García-Campaña, A.M. A new approach in sample treatment combined with UHPLC-MS/MS for the determination of multiclass mycotoxins in edible nuts and seeds. Talanta 2013, 115, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M. Quantitative estimation of aflatoxins, ochratoxin and citrinin in dried fruits and nuts samples from Indo-Gangetic region of Bihar. Int. J. Adv. Res. Innov. Ideas Educ. 2019, 5, 2395–4396. [Google Scholar]
- Polisenska, I.; Pfohl-Leszkowicz, A.; Hadjeba, K.; Dohnal, V.; Jirsa, O.; Denesova, O.; Jezkova, A.; Macharackova, P. Occurrence of ochratoxin A and citrinin in Czech cereals and comparison of two HPLC methods for ochratoxin A detection. Food Addit. Contam. 2010, 27, 1545–1557. [Google Scholar] [CrossRef] [Green Version]
- Heperkan, D.; Meric, B.E.; Sismanoglu, G.; Dalkiliç, G.; Güler, F.K. Mycobiota, mycotoxigenic fungi, and citrinin production in black olives. In Advances in Food Mycology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 203–210. [Google Scholar]
- El Adlouni, C.; Tozlovanu, M.; Naman, F.; Faid, M.; Pfohl-Leszkowicz, A. Preliminary data on the presence of mycotoxins (ochratoxin A, citrinin and aflatoxin B1) in black table olives “Greek style” of Moroccan origin. Mol. Nutr. Food Res. 2006, 50, 507–512. [Google Scholar] [CrossRef]
- Čulig, B.; Bevardi, M.; Bošnir, J.; Serdar, S.; Lasić, D.; Racz, A.; Galić, A.; Kuharić, Ž. Presence of citrinin in grains and its possible health effects. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Molinié, A.; Faucet, V.; Castegnaro, M.; Pfohl-Leszkowicz, A. Analysis of some breakfast cereals on the French market for their contents of ochratoxin A, citrinin and fumonisin B1: Development of a method for simultaneous extraction of ochratoxin A and citrinin. Food Chem. 2005, 92, 391–400. [Google Scholar] [CrossRef]
- Tabata, S.; Iida, K.; Kimura, K.; Iwasaki, Y.; Nakazato, M.; Kamata, K.; Hirokado, M. Investigation of ochratoxin A, B and citrinin contamination in various commercial foods. Shokuhin Eiseigaku Zasshi 2008, 49, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Markov, K.; Pleadin, J.; Bevardi, M.; Vahčić, N.; Sokolić-Mihalak, D.; Frece, J. Natural occurrence of aflatoxin B1, ochratoxin A and citrinin in Croatian fermented meat products. Food Control 2013, 34, 312–317. [Google Scholar] [CrossRef]
- Kiebooms, J.A.L.; Huybrechts, B.; Thiry, C.; Tangni, E.K.; Callebaut, A. A quantitative UHPLC-MS/MS method for citrinin and ochratoxin A detection in food, feed and red yeast rice food supplements. World Mycotoxin J. 2016, 9, 343–352. [Google Scholar] [CrossRef]
- Wawrzyniak, J.; Waśkiewicz, A. Ochratoxin A and citrinin production by Penicillium verrucosum on cereal solid substrates. Food Addit. Contam. Part A 2014, 31, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Odhav, B.; Naicker, V. Mycotoxins in South African traditionally brewed beers. Food Addit. Contam. 2002, 19, 55–61. [Google Scholar] [CrossRef]
- Oztas, E.; Ozden, H.; Ozhan, G. A preliminary survey of citrinin contamination in dried fruits, molasses and liquorice products in Turkey. J. Food Nutr. Res. 2020, 59, 81–86. [Google Scholar]
- Ojuri, O.T.; Ezekiel, C.N.; Eskola, M.K.; Šarkanj, B.; Babalola, A.D.; Sulyok, M.; Hajšlová, J.; Elliott, C.T.; Krska, R. Mycotoxin co-exposures in infants and young children consuming household-and industrially-processed complementary foods in Nigeria and risk management advice. Food Control 2019, 98, 312–322. [Google Scholar] [CrossRef]
- Ruan, C.; Diao, X.; Li, N.; Zhang, H.; Pang, Y.; Liu, C. Determination of ochratoxin A and citrinin in fruits using ultrasound-assisted solvent extraction followed by dispersive liquid–liquid microextraction with HPLC with fluorescence detection. Anal. Methods 2016, 8, 1586–1594. [Google Scholar] [CrossRef]
- Guo, B.Y.; Wang, S.; Ren, B.; Li, X.; Qin, F.; Li, J. Citrinin selective molecularly imprinted polymers for SPE. J. Sep. Sci. 2010, 33, 1156–1160. [Google Scholar] [CrossRef]
- Sato, T.; Higashihara, K.; Sasaki, A.; Toth, D.; Goto, T. Development and single laboratory validation of a method for citrinin. World Mycotoxin J. 2010, 3, 129–134. [Google Scholar] [CrossRef]
- Xue-Mei, L.; Xing-Hai, S.; Lan, X.; Zhen-Wen, D.; Shu-Ren, G. A validated RP-HPLC method for the determination of citrinin in xuezhikang capsule and other Monascus-fermented products. E-J. Chem. 2012, 9, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Hajnal, E.J.; Kos, J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Sulyok, M. Mycotoxins in maize harvested in Serbia in the period 2012–2015. Part 2: Non-regulated mycotoxins and other fungal metabolites. Food Chem. 2020, 317, 126409. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Xie, Z.; Liu, L.; Song, S.; Kuang, H. Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the detection of citrinin in cereals. Food Agric. Immunol. 2017, 28, 754–766. [Google Scholar] [CrossRef] [Green Version]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC-MS/MS multitoxin method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Marín, S.; Sanchis, V.; Ramos, A.J. Screening of mycotoxin multicontamination in medicinal and aromatic herbs sampled in Spain. J. Sci. Food Agric. 2009, 89, 1802–1807. [Google Scholar] [CrossRef]
- Ali, N. Co-occurrence of citrinin and ochratoxin A in rice in Asia and its implications for human health. J. Sci. Food Agric. 2018, 98, 2055–2059. [Google Scholar] [CrossRef]
- Samsudin, N.I.P.; Abdullah, N. A preliminary survey on the occurrence of mycotoxigenic fungi and mycotoxins contaminating red rice at consumer level in Selangor, Malaysia. Mycotoxin Res. 2013, 29, 89–96. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Dong, L.; Lu, L.; Chen, F.; Hu, D.; Wang, X. A study of fluorescence properties of citrinin in β-cyclodextrin aqueous solution and different solvents. J. Lumin. 2012, 132, 1437–1445. [Google Scholar] [CrossRef]
- Arévalo, F.J.; Granero, A.M.; Fernández, H.; Raba, J.; Zón, M.A. Citrinin (CIT) determination in rice samples using a micro fluidic electrochemical immunosensor. Talanta 2011, 83, 966–973. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Bing, X. Preparation of an immunoaffinity column for the clean-up of fermented food samples contaminated with citrinin. Food Addit. Contam. Part A 2013, 30, 389–394. [Google Scholar] [CrossRef]
- Gordon, R.Y.; Cooperman, T.; Obermeyer, W.; Becker, D.J. Marked variability of monacolin levels in commercial red yeast rice products: Buyer beware! Arch. Intern. Med. 2010, 170, 1722–1727. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-L.; Kuo, Y.-H.; Lee, C.-L.; Hsu, Y.-W.; Pan, T.-M. Synchronous high-performance liquid chromatography with a photodiode array detector and mass spectrometry for the determination of citrinin, monascin, ankaflavin, and the lactone and acid forms of monacolin K in red mold rice. J. AOAC Int. 2011, 94, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Xu, J.; Wang, X.; Qi, P.; Wei, W.; Chen, X.; Li, R.; Zhou, Y. Citrinin determination in red fermented rice products by optimized extraction method coupled to liquid chromatography tandem mass spectrometry (LC-MS/MS). J. Food Sci. 2015, 80, T1438–T1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, Q.; Zhang, X.; Zhang, H.; Huang, Q.; Li, D.; Yao, J. Comparison of extraction methods for analysis of citrinin in red fermented rice. Food Chem. 2014, 157, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Mornar, A.; Sertić, M.; Nigović, B. Development of a rapid LC/DAD/FLD/MS n method for the simultaneous determination of monacolins and citrinin in red fermented rice products. J. Agric. Food Chem. 2013, 61, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Guo, L.; Zheng, Y.; Guo, Y. Microsphere-based flow cytometric immunoassay for the determination of citrinin in red yeast rice. Food Chem. 2012, 134, 2540–2545. [Google Scholar] [CrossRef]
- Liu, R.; Xu, B. Optimization of extraction conditions of citrinin from red yeast rice by orthogonal design and quantification of citrinin by high-performance liquid chromatography. Food Anal. Methods 2013, 6, 677–682. [Google Scholar] [CrossRef]
- Meng, W.; Zhu, L.X.; Guo, X.M.; Li, K.H.; Liu, R.R. Determination of citrinin in red yeast rice by high performance liquid chromatography with immuno-affinity column. In Proceedings of Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland; pp. 353–356.
- Nigović, B.; Sertić, M.; Mornar, A. Simultaneous determination of lovastatin and citrinin in red yeast rice supplements by micellar electrokinetic capillary chromatography. Food Chem. 2013, 138, 531–538. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Tozlovanu, M.; Tran, T.L.; Pfohl-Leszkowicz, A. Occurrence of aflatoxin B1, citrinin and ochratoxin A in rice in five provinces of the central region of Vietnam. Food Chem. 2007, 105, 42–47. [Google Scholar] [CrossRef]
- Nazari, F.; Sulyok, M.; Yazdanpanah, H.; Kobarfard, F.; Krska, R. A survey of mycotoxins in domestic rice in Iran by liquid chromatography tandem mass spectrometry. Toxicol. Mech. Methods 2014, 24, 37–41. [Google Scholar] [CrossRef]
- Huertas-Pérez, J.F.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. High-throughput determination of citrinin in rice by ultra-high-performance liquid chromatography and fluorescence detection (UHPLC-FL). Food Addit. Contam. Part A 2015, 32, 1352–1357. [Google Scholar] [CrossRef]
- Urraca, J.L.; Huertas-Pérez, J.F.; Cazorla, G.A.; Gracia-Mora, J.; García-Campaña, A.M.; Moreno-Bondi, M.C. Development of magnetic molecularly imprinted polymers for selective extraction: Determination of citrinin in rice samples by liquid chromatography with UV diode array detection. Anal. Bioanal. Chem. 2016, 408, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Ferre, F.S. Worldwide occurrence of mycotoxins in rice. Food Control 2016, 62, 291–298. [Google Scholar] [CrossRef]
- Aziz, N.H.; Mattar, Z.A.; Mahrous, S.R. Contamination of grains by mycotoxin-producing molds and mycotoxins and control by gamma irradiation. J. Food Saf. 2006, 26, 184–201. [Google Scholar] [CrossRef]
- Zaied, C.; Zouaoui, N.; Bacha, H.; Abid, S. Natural occurrence of citrinin in Tunisian wheat grains. Food Control 2012, 28, 106–109. [Google Scholar] [CrossRef]
- Limay-Rios, V.; Miller, J.D.; Schaafsma, A.W. Occurrence of Penicillium verrucosum, ochratoxin A, ochratoxin B and citrinin in on-farm stored winter wheat from the Canadian Great Lakes Region. PLoS ONE 2017, 12, e0181239. [Google Scholar] [CrossRef]
- Dohnal, V.; Pavlikova, L.; Kuča, K. Rapid and sensitive method for citrinin determination using high-performance liquid chromatography with fluorescence detection. Anal. Lett. 2010, 43, 786–792. [Google Scholar] [CrossRef]
- Richard, E.; Heutte, N.; Bouchart, V.; Garon, D. Evaluation of fungal contamination and mycotoxin production in maize silage. Anim. Feed Sci. Technol. 2009, 148, 309–320. [Google Scholar] [CrossRef]
- Richard, E.; Heutte, N.; Sage, L.; Pottier, D.; Bouchart, V.; Lebailly, P.; Garon, D. Toxigenic fungi and mycotoxins in mature corn silage. Food Chem. Toxicol. 2007, 45, 2420–2425. [Google Scholar] [CrossRef]
- Garon, D.; Richard, E.; Sage, L.; Bouchart, V.; Pottier, D.; Lebailly, P. Mycoflora and multimycotoxin detection in corn silage: Experimental study. J. Agric. Food Chem. 2006, 54, 3479–3484. [Google Scholar] [CrossRef]
- Kononenko, G.P.; Burkin, A.A. A survey on the occurrence of citrinin in feeds and their ingredients in Russia. Mycotoxin Res. 2008, 24, 3–6. [Google Scholar] [CrossRef]
- Wang, M.L.; Lu, C.H.; Xu, Q.Y.; Song, S.Y.; Hu, Z.Y.; Zheng, Z.H. Four new citrinin derivatives from a marine-derived Penicillium sp. fungal strain. Molecules 2013, 18, 5723–5735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meerpoel, C.; Vidal, A.; Andjelkovic, M.; De Boevre, M.; Tangni, E.K.; Huybrechts, B.; Devreese, M.; Croubels, S.; De Saeger, S. Dietary exposure assessment and risk characterization of citrinin and ochratoxin A in Belgium. Food Chem. Toxicol. 2021, 147, 111914. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2019/1901 of 7 November 2019 amending Regulation (EC) No 1881/2006 as regards maximum levels of citrinin in food supplements based on rice fermented with red yeast Monascus purpureus. Off. J. Eur. Union 2019, 62, 2–4. [Google Scholar]
- European Commission. Commission Regulation (EU) No 212/2014 of 6 March 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of the contaminant citrinin in food supplements based on rice fermented with red yeast Monascus purpureus. EFSA J. 2014, 16, 3–4. [Google Scholar]
- Chagas, G.M.; Klüppel, M.L.W.; de Paiva Campello, A.; de Freitas Buchi, D.; de Oliveira, M.B.M. Alterations induced by citrinin in cultured kidney cells. Cell Struct. Funct. 1994, 19, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Chagas, G.M.; Oliveira, M.B.M.; Campello, A.P.; Kluppel, M.L.W. Mechanism of citrinin-induced dysfunction of mitochondria. IV—Effect on Ca2+ transport. Cell Biochem. Funct. 1995, 13, 53–59. [Google Scholar] [CrossRef]
- Yu, F.-Y.; Liao, Y.-C.; Chang, C.-H.; Liu, B.-H. Citrinin induces apoptosis in HL-60 cells via activation of the mitochondrial pathway. Toxicol. Lett. 2006, 161, 143–151. [Google Scholar] [CrossRef]
- Iwahashi, H.; Kitagawa, E.; Suzuki, Y.; Ueda, Y.; Ishizawa, Y.-h.; Nobumasa, H.; Kuboki, Y.; Hosoda, H.; Iwahashi, Y. Evaluation of toxicity of the mycotoxin citrinin using yeast ORF DNA microarray and Oligo DNA microarray. BMC Genom. 2007, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Vanacloig-Pedros, E.; Proft, M.; Pascual-Ahuir, A. Different toxicity mechanisms for citrinin and ochratoxin A revealed by transcriptomic analysis in yeast. Toxins 2016, 8, 273. [Google Scholar] [CrossRef]
- Ribeiro, S.M.R.; Chagas, G.M.; Campello, A.P.; Kluppel, M.L.W. Mechanism of citrinin-induced dysfunction of mitochondria. V. Effect on the homeostasis of the reactive oxygen species. Cell Biochem. Funct. 1997, 15, 203–209. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chan, W.-H. Inhibition of citrinin-induced apoptotic biochemical signaling in human hepatoma G2 cells by resveratrol. Int. J. Mol. Sci. 2009, 10, 3338–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Máté, G.; Gazdag, Z.; Mike, N.; Papp, G.; Pócsi, I.; Pesti, M. Regulation of oxidative stress-induced cytotoxic processes of citrinin in the fission yeast Schizosaccharomyces pombe. Toxicon 2014, 90, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Dwivedi, P.D.; Dhawan, A.; Das, M.; Ansari, K.M. Citrinin-generated reactive oxygen species cause cell cycle arrest leading to apoptosis via the intrinsic mitochondrial pathway in mouse skin. Toxicol. Sci. 2011, 122, 557–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mike, N.; Papp, G.; Čertik, M.; Czibulya, Z.; Kunsági-Máté, S.; Ember, I.; Vágvölgyi, C.; Pesti, M.; Gazdag, Z. Regulation of cytotoxic, non-estrogenic, oxidative stress-induced processes of zearalenone in the fission yeast Schizosaccharomyces pombe. Toxicon 2013, 73, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Kuroda, M. Citrinin, an inhibitor of cholesterol synthesis. J. Antibiot. 1976, 29, 841–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qingqing, H.; Linbo, Y.; Yunqian, G.; Shuqiang, L. Toxic effects of citrinin on the male reproductive system in mice. Exp. Toxicol. Pathol. 2012, 64, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.-j.; Jia, X.-q.; Gu, L.-j.; Sung, C.-k. Review on the qualitative and quantitative analysis of the mycotoxin citrinin. Food Control 2006, 17, 271–285. [Google Scholar] [CrossRef]
- Vrabcheva, T.; Usleber, E.; Dietrich, R.; Märtlbauer, E. Co-occurrence of ochratoxin A and citrinin in cereals from Bulgarian villages with a history of Balkan endemic nephropathy. J. Agric. Food Chem. 2000, 48, 2483–2488. [Google Scholar] [CrossRef]
- Bamias, G.; Boletis, J. Balkan nephropathy: Evolution of our knowledge. Am. J. Kidney Dis. 2008, 52, 606–616. [Google Scholar] [CrossRef]
- López Sáncheza, P.; de Nijsa, M.; Spanjerb, M.; Pietric, A.; Bertuzzic, T.; Starski, A.; Postupolski, J.; Castellari, M.; Hortós, M. Generation of occurrence data on citrinin in food. EFSA Support. Publ. 2017, 14, 1177E. [Google Scholar] [CrossRef] [Green Version]
- Lurá, M.C.; Fuentes, M.B.; Cabagna, M.; González, A.M.; Nepote, A.F.; Giugni, M.C.; Rico, M.; Latorre, M.G. Actividad de metabolitos de Penicillium citrinum sobre ratones Mus musculus. Rev. Iberoam. Micol. 2001, 18, 183–186. [Google Scholar] [PubMed]
- Chan, W.-H. Effects of citrinin on maturation of mouse oocytes, fertilization, and fetal development in vitro and in vivo. Toxicol. Lett. 2008, 180, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Thacker, H.L.; Carlton, W.W.; Sansing, G.A. Citrinin mycotoxicosis in the guinea-pig. Food Cosmet. Toxicol. 1977, 15, 553–561. [Google Scholar] [CrossRef]
- Jordan, W.H.; Carlton, W.W.; Sansing, G.A. Citrinin mycotoxicosis in the mouse. Food Cosmet. Toxicol. 1977, 15, 29–34. [Google Scholar] [CrossRef]
- Hanika, C.; Carlton, W.W.; Tuite, J. Citrinin mycotoxicosis in the rabbit. Food Chem. Toxicol. 1983, 21, 487–493. [Google Scholar] [CrossRef]
- Jordan, W.H.; Carlton, W.W.; Sansing, G.A. Citrinin mycotoxicosis in the rat. II. Clinicopathological observations. Food Cosmet. Toxicol. 1978, 16, 441–447. [Google Scholar] [CrossRef]
- Hanika, C.; Carlton, W.W.; Boon, G.D.; Tuite, J. Citrinin mycotoxicosis in the rabbit: Clinicopathological alterations. Food Chem. Toxicol. 1984, 22, 999–1008. [Google Scholar] [CrossRef]
- Kitabatake, N.; Trivedi, A.B.; Doi, E. Thermal decomposition and detoxification of citrinin under various moisture conditions. J. Agric. Food Chem. 1991, 39, 2240–2244. [Google Scholar] [CrossRef]
- Köppen, R.; Koch, M.; Siegel, D.; Merkel, S.; Maul, R.; Nehls, I. Determination of mycotoxins in foods: Current state of analytical methods and limitations. Appl. Microbiol. Biotechnol. 2010, 86, 1595–1612. [Google Scholar] [CrossRef]
- Clark, B.R.; Capon, R.J.; Lacey, E.; Tennant, S.; Gill, J.H. Citrinin revisited: From monomers to dimers and beyond. Org. Biomol. Chem. 2006, 4, 1520–1528. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Xie, J.; Li, X.; Huang, Z. Effects of some flavonoids on the mycotoxin citrinin reduction by Monascus aurantiacus Li AS3. 4384 during liquid-state fermentation. AMB Express 2020, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Liu, X.; Wang, Y.; Huang, Z.; Li, X. Addition of genistein to the fermentation process reduces citrinin production by Monascus via changes at the transcription level. Food Chem. 2021, 343, 128410. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, L.; Wang, Y.; Gao, H.; Li, X.; Huang, X.; Huang, T. Effects of rutin and its derivatives on citrinin production by Monascus aurantiacus Li AS3. 4384 in liquid fermentation using different types of media. Food Chem. 2019, 284, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Tokuşoğlu, Ö.; Alpas, H.; Bozoğlu, F. High hydrostatic pressure effects on mold flora, citrinin mycotoxin, hydroxytyrosol, oleuropein phenolics and antioxidant activity of black table olives. Innov. Food Sci. Emerg. Technol. 2010, 11, 250–258. [Google Scholar] [CrossRef]
- Heperkan, D.; Dazkır, G.S.; Kansu, D.Z.; Karbancıoglu Güler, F. Influence of temperature on citrinin accumulation by Penicillium citrinum and Peniccillium verrucosum in black table olives. Toxin Rev. 2009, 28, 180–186. [Google Scholar] [CrossRef]
- Zhou, G.; Fu, L.; Li, X. Optimisation of ultrasound-assisted extraction conditions for maximal recovery of active monacolins and removal of toxic citrinin from red yeast rice by a full factorial design coupled with response surface methodology. Food Chem. 2015, 170, 186–192. [Google Scholar] [CrossRef]
- Vaseghi, N.; Bayat, M.; Nosrati, A.C.; Ghorannevis, M.; Hashemi, S. Evaluation of the plasma jet effects on the Citrinin and Ochratoxin A producing species of the genus Penicillium. Bulg. Chem. Commun. 2018, 50, 383–392. [Google Scholar]
- Price, M.S.; Conners, S.B.; Tachdjian, S.; Kelly, R.M.; Payne, G.A. Aflatoxin conducive and non-conducive growth conditions reveal new gene associations with aflatoxin production. Fungal Genet. Biol. 2005, 42, 506–518. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Baxter, E.; Geisen, R.; Magan, N. Physiological relationship between food preservatives, environmental factors, ochratoxin and otapksPV gene expression by Penicillium verrucosum. Int. J. Food Microbiol. 2007, 119, 277–283. [Google Scholar] [CrossRef]
- O’Callaghan, J.; Coghlan, A.; Abbas, A.; García-Estrada, C.; Martín, J.-F.; Dobson, A.D.W. Functional characterization of the polyketide synthase gene required for ochratoxin A biosynthesis in Penicillium verrucosum. Int. J. Food Microbiol. 2013, 161, 172–181. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Li, Z.; Guo, Q.; Yang, M.; Chen, D.; Wang, C. The effect of blue light on the production of citrinin in Monascus purpureus M9 by regulating the mraox gene through lncRNA AOANCR. Toxins 2019, 11, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Dai, Y.; Chen, W.; Shao, Y.; Chen, F. Effects of light intensity and color on the biomass, extracellular red pigment, and citrinin production of Monascus ruber. J. Agric. Food Chem. 2016, 64, 9506–9514. [Google Scholar] [CrossRef] [PubMed]
- Atapattu, S.N.; Poole, C.F. Recent advances in analytical methods for the determination of citrinin in food matrices. J. Chromatogr. A 2020, 1627, 461399. [Google Scholar] [CrossRef]
- Klingelhöfer, I.; Morlock, G.E. Lovastatin in lactone and hydroxy acid forms and citrinin in red yeast rice powders analyzed by HPTLC-UV/FLD. Anal. Bioanal. Chem. 2019, 411, 6655–6665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Yao, S.; Zhu, P. Detection of trace amounts of citrinin in dried orange peel by using an optimized extraction method coupled with ultra-performance liquid chromatography–tandem mass spectrometry. Biomed. Chromatogr. 2018, 32, e4237. [Google Scholar] [CrossRef] [PubMed]
- Atapattu, S.N.; Rosenfeld, J.M. Micro scale analytical derivatizations on solid phase. Trends Anal. Chem. 2019, 113, 351–356. [Google Scholar] [CrossRef]
- Sajid, M.; Alhooshani, K. Dispersive liquid-liquid microextraction based binary extraction techniques prior to chromatographic analysis: A review. Trends Anal. Chem. 2018, 108, 167–182. [Google Scholar] [CrossRef]
- Lhotská, I.; Kholová, A.; Machyňáková, A.; Hroboňová, K.; Solich, P.; Švec, F.; Šatínský, D. Preparation of citrinin-selective molecularly imprinted polymer and its use for on-line solid-phase extraction coupled to liquid chromatography. Anal. Bioanal. Chem. 2019, 411, 2395–2404. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Cumova, M.; Kyrova, V.; Toman, J.; Grosse, Y.; Pospichalova, M.; Ruprich, J. Investigation of patulin and citrinin in grape must and wine from grapes naturally contaminated by strains of Penicillium expansum. Food Chem. Toxicol. 2018, 118, 805–811. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, M.; Chen, Q.; Huang, L.; Mao, Y.; Xia, N.; Teng, J.; Wei, B. Citrinin produced using strains of Penicillium citrinum from Liupao tea. Food Biosci. 2019, 28, 183–191. [Google Scholar] [CrossRef]
- Mandal, S.; Das, P. Ultrasensitive visual detection of mycotoxin citrinin with yellow-light emitting carbon dot and Congo red. Food Chem. 2020, 312, 126076. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Liu, X.; Liao, X.; Shi, L.; Zhang, S.; Lu, J.; Zhou, L.; Kong, W. Simultaneous determination of 19 mycotoxins in lotus seed using a multimycotoxin UFLC-MS/MS method. J. Pharm. Pharmacol. 2019, 71, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, Y.; Teng, J.; Xia, N.; Huang, L.; Wei, B.; Chen, Q. Evaluation of mycoflora and citrinin occurrence in Chinese liupao tea. J. Agric. Food Chem. 2020, 68, 12116–12123. [Google Scholar] [CrossRef] [PubMed]
- Tangni, E.K.; Van Hove, F.; Huybrechts, B.; Masquelier, J.; Vandermeiren, K.; Van Hoeck, E. Citrinin determination in food and food supplements by LC-MS/MS: Development and use of reference materials in an international collaborative study. Toxins 2021, 13, 245. [Google Scholar] [CrossRef]
- Twarużek, M.; Ałtyn, I.; Kosicki, R. Dietary supplements based on red yeast rice—A source of citrinin? Toxins 2021, 13, 497. [Google Scholar] [CrossRef] [PubMed]
- Twarużek, M.; Kosicki, R.; Kwiatkowska-Giżyńska, J.; Grajewski, J.; Ałtyn, I. Ochratoxin A and citrinin in green coffee and dietary supplements with green coffee extract. Toxicon 2020, 188, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of nano-ELISA in food analysis: Recent advances and challenges. Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Singh, G.; Velasquez, L.; Huet, A.-C.; Delahaut, P.; Gillard, N.; Koerner, T. Development of a sensitive polyclonal antibody-based competitive indirect ELISA for determination of citrinin in grain-based foods. Food Addit. Contam. Part A 2019, 36, 1567–1573. [Google Scholar] [CrossRef]
- Huang, W.; Tu, Z.; Ning, Z.; He, Q.; Li, Y. Development of real-time immuno-PCR based on phage displayed an anti-idiotypic nanobody for quantitative determination of citrinin in Monascus. Toxins 2019, 11, 572. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Duan, H.; Chen, R.; Ma, T.; Zeng, L.; Leng, Y.; Xiong, Y. Effect of different-sized gold nanoflowers on the detection performance of immunochromatographic assay for human chorionic gonadotropin detection. Talanta 2019, 194, 604–610. [Google Scholar] [CrossRef]
- Atar, N.; Yola, M.L.; Eren, T. Sensitive determination of citrinin based on molecular imprinted electrochemical sensor. Appl. Surf. Sci. 2016, 362, 315–322. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, B.; Chen, E.; Yu, X.; Ye, Z.; Sun, C.; Zhang, M. Dual fluorescent immunochromatographic assay for simultaneous quantitative detection of citrinin and zearalenone in corn samples. Food Chem. 2021, 336, 127713. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, Y.; Li, Y. Molecularly imprinted polymer-decorated signal on-off ratiometric electrochemical sensor for selective and robust dopamine detection. Biosens. Bioelectron. 2019, 135, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Y.; Xia, Y.; Zhao, F.; Zeng, B. A novel ratiometric electrochemical sensor for the selective detection of citrinin based on molecularly imprinted poly (thionine) on ionic liquid decorated boron and nitrogen co-doped hierarchical porous carbon. Food Chem. 2021, 363, 130385. [Google Scholar] [CrossRef]
- Shekhar, M.; Singh, N.; Dutta, R.; Kumar, S.; Mahajan, V. Comparative study of qualitative and quantitative methods to determine toxicity level of Aspergillus flavus isolates in maize. PLoS ONE 2017, 12, e0189760. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Tong, Z.; Gao, X.; Zhang, L.; Li, S. Simultaneous detection of zearalenone, citrinin, and ochratoxin A in pepper by capillary zone electrophoresis. Food Addit. Contam. Part A 2020, 37, 1388–1398. [Google Scholar] [CrossRef]
- Ranasinghe, M.; Quirino, J.P. Can we replace liquid chromatography with the greener capillary electrophoresis? Curr. Opin. Green Sustain. Chem. 2021, 31, 100515. [Google Scholar] [CrossRef]
- Kuchenbuch, H.S.; Cramer, B.; Humpf, H.-U. Matrix binding of T-2 toxin: Structure elucidation of reaction products and indications on the fate of a relevant food-borne toxin during heating. Mycotoxin Res. 2019, 35, 261–270. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Yoshinari, T.; Szymczyk, K.; Podolska, G.; Gwiazdowski, R.; Kubiak, K. Transformations of selected Fusarium toxins and their modified forms during malt loaf production. Toxins 2020, 12, 385. [Google Scholar] [CrossRef]
- Gonçalves, C.; Mischke, C.; Stroka, J. Determination of deoxynivalenol and its major conjugates in cereals using an organic solvent-free extraction and IAC clean-up coupled in-line with HPLC-PCD-FLD. Food Addit. Contam. Part A 2020, 37, 1765–1776. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, J.; Shao, Y.; Peng, X.; Zhang, D.; Hu, L.; Chen, F.; Zhou, Y. Evaluation of the underestimation of citrinin content in Hongqu using hydrolysis treatments and UPLC-FLD. Food Control 2021, 130, 108245. [Google Scholar] [CrossRef]
- Vidal, A.; Marín, S.; Sanchis, V.; De Saeger, S.; De Boevre, M. Hydrolysers of modified mycotoxins in maize: α-Amylase and cellulase induce an underestimation of the total aflatoxin content. Food Chem. 2018, 248, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Beloglazova, N.V.; De Boevre, M.; Goryacheva, I.Y.; Werbrouck, S.; Guo, Y.; De Saeger, S. Immunochemical approach for zearalenone-4-glucoside determination. Talanta 2013, 106, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Qin, J.-A.; Fu, Y.-W.; Luo, J.-Y.; Lu, J.-H.; Logrieco, A.F.; Yang, M.-H. Modified mycotoxins in foodstuffs, animal feed, and herbal medicine: A systematic review on global occurrence, transformation mechanism and analysis methods. Trends Anal. Chem. 2020, 133, 116088. [Google Scholar] [CrossRef]
- Gupta, R.C.; Srivastava, A.; Lall, R. Ochratoxins and citrinin. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1019–1027. [Google Scholar]
- Wang, K.; Lin, Z.; Zhang, H.; Zhang, X.; Zheng, X.; Zhao, L.; Yang, Q.; Ahima, J.; Boateng, N.A.S. Investigating proteome and transcriptome response of Cryptococcus podzolicus Y3 to citrinin and the mechanisms involved in its degradation. Food Chem. 2019, 283, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.B.; Doi, E.; Kitabatake, N. Toxic compounds formed on prolonged heating of citrinin under watery conditions. J. Food Sci. 1993, 58, 229–232. [Google Scholar] [CrossRef]
- Hirota, M.; MENTA, A.; Yoneyama, K.; Kitabatake, N. A major decomposition product, citrinin H2, from citrinin on heating with moisture. Biosci. Biotechnol. Biochem. 2002, 66, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Chen, W.-P.; Wang, J.-J.; Pan, T.-M. A simple and rapid approach for removing citrinin while retaining monacolin K in red mold rice. J. Agric. Food Chem. 2007, 55, 11101–11108. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Cramer, B.; Graf, I.; Lerch, S.; Humpf, H.-U.; Geisen, R. Wavelength-dependent degradation of ochratoxin and citrinin by light in vitro and in vivo and its implications on Penicillium. Toxins 2012, 4, 1535–1551. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Fu, Z.-L.; Chen, M.-H.; Ban, Z.; Wang, Y.-R.; Zhang, X.-W. Blue light effects on pigment and citrinin production in Monascus. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 1–4. [Google Scholar]
- Hajjaj, H.; Klaebe, A.; Goma, G.; Blanc, P.J.; Barbier, E.; François, J. Medium-chain fatty acids affect citrinin production in the filamentous fungus Monascus ruber. Appl. Environ. Microbiol. 2000, 66, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshini, C.H.; Narasareddy, G.V. Amelioration of toxic effects of aflatoxin and citrinin by adsorbents in broilers. Indian Vet. J. 2010, 87, 23–25. [Google Scholar]
- Panda, P.; Aiko, V.; Mehta, A. Effect of aqueous extracts of Mentha arvensis (mint) and Piper betle (betel) on growth and citrinin production from toxigenic Penicillium citrinum. J. Food Sci. Technol. 2015, 52, 3466–3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lin, Z.; Apaliya, M.T.; Gu, X.; Zheng, X.; Zhao, L.; Abdelhai, M.H.; Zhang, H.; Hu, W. The possible mechanisms involved in citrinin elimination by Cryptococcus podzolicus Y3 and the effects of extrinsic factors on the degradation of citrinin. J. Microbiol. Biotechnol. 2017, 27, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Ahima, J.; Zhang, H.; Apaliya, M.T.; Zhang, X.; Yang, Q.; Zhao, L. The effect of Rhodotorula mucilaginosa on degradation of citrinin production by Penicillium digitatum and its toxin in vitro. J. Food Meas. Charact. 2019, 13, 2998–3004. [Google Scholar] [CrossRef]
- Abd-Allah, E.F.; Ezzat, S.M. Natural occurrence of citrinin in rice grains and its biocontrol by Trichoderma hamatum. Phytoparasitica 2005, 33, 73–84. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Sheu, S.-C.; Mau, J.-L.; Hsieh, P.-C. Isolation and characterization of a strain of Klebsiella pneumoniae with citrinin-degrading activity. World J. Microbiol. Biotechnol. 2011, 27, 487–493. [Google Scholar] [CrossRef]
- Kanpiengjai, A.; Mahawan, R.; Lumyong, S.; Khanongnuch, C. A soil bacterium Rhizobium borbori and its potential for citrinin-degrading application. Ann. Microbiol. 2016, 66, 807–816. [Google Scholar] [CrossRef]
- Magro, M.; Moritz, D.E.; Bonaiuto, E.; Baratella, D.; Terzo, M.; Jakubec, P.; Malina, O.; Čépe, K.; De Aragao, G.M.F.; Zboril, R. Citrinin mycotoxin recognition and removal by naked magnetic nanoparticles. Food Chem. 2016, 203, 505–512. [Google Scholar] [CrossRef]
- Savi, G.D.; Piacentini, K.C.; Scussel, V.M. Ozone treatment efficiency in Aspergillus and Penicillium growth inhibition and mycotoxin degradation of stored wheat grains (Triticum aestivum L.). J. Food Process. Preserv. 2015, 39, 940–948. [Google Scholar] [CrossRef]
- He, S.; Liu, X.; Wang, Y.; Xie, J.; Gao, H.; Li, X.; Huang, Z. Metabolomics analysis based on UHPLC-Q-TOF-MS/MS reveals effects of genistein on reducing mycotoxin citrinin production by Monascus aurantiacus Li AS3 4384. LWT—Food Sci. Technol. 2020, 130, 109613. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Mahato, D.K.; Sharma, B.; Borah, R.; Haque, S.; Mahmud, M.M.C.; Shah, A.K.; Rawal, D.; Bora, H.; Bui, S. Ochratoxins in food and feed: Occurrence and its impact on human health and management strategies. Toxicon 2020, 187, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K. Occurrence, impact on agriculture, human health, and management strategies of zearalenone in food and feed: A review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S. Patulin in food: A mycotoxin concern for human health and its management strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Stoll, D.; Geisen, R. Fungicides effectively used for growth inhibition of several fungi could induce mycotoxin biosynthesis in toxigenic species. Int. J. Food Microbiol. 2013, 166, 407–412. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Bi, Y.; Luo, Y. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- Kinay, P.; Mansour, M.F.; Gabler, F.M.; Margosan, D.A.; Smilanick, J.L. Characterization of fungicide-resistant isolates of Penicillium digitatum collected in California. Crop Prot. 2007, 26, 647–656. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Nicosia, M.G.L.D.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Droby, S.; Schena, L. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 2016, 114, 54–61. [Google Scholar] [CrossRef]
- Trabelsi, D.; Hamdane, A.M.; Said, M.B.; Abdrrabba, M. Chemical composition and antifungal activity of essential oils from flowers, leaves and peels of Tunisian Citrus aurantium against Penicillium digitatum and Penicillium italicum. J. Essent. Oil Bear. Plants 2016, 19, 1660–1674. [Google Scholar] [CrossRef]
- Shao, X.; Cao, B.; Xu, F.; Xie, S.; Yu, D.; Wang, H. Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol. Technol. 2015, 99, 37–43. [Google Scholar] [CrossRef]
- Jeong, R.-D.; Chu, E.-H.; Lee, G.W.; Cho, C.; Park, H.-J. Inhibitory effect of gamma irradiation and its application for control of postharvest green mold decay of Satsuma mandarins. Int. J. Food Microbiol. 2016, 234, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.T.; Alférez, F. Effect of LED Blue Light on Penicillium digitatum and Penicillium italicum Strains. Photochem. Photobiol. 2015, 91, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Argudo, C.; Palou, L.; Bermejo, A.; Cano, A.; del Río, M.A.; González-Mas, M.C. Effect of X-ray irradiation on nutritional and antifungal bioactive compounds of ‘Clemenules’ clementine mandarins. Postharvest Biol. Technol. 2012, 68, 47–53. [Google Scholar] [CrossRef]
- Yamaga, I.; Kuniga, T.; Aoki, S.; Kato, M.; Kobayashi, Y. Effect of ultraviolet-B irradiation on disease development caused by Penicillium italicum in satsuma mandarin fruit. Hortic. J. 2015, 85, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Ben-Yehoshua, S. Effects of postharvest heat and UV applications on decay, chilling injury and resistance against pathogens of citrus and other fruits and vegetables. In International Conference: Postharvest Unlimited; ISHS: Leuven, Belgium, 2002; Volume 599, pp. 159–173. [Google Scholar]
- Montesinos-Herrero, C.; Moscoso-Ramírez, P.A.; Palou, L. Evaluation of sodium benzoate and other food additives for the control of citrus postharvest green and blue molds. Postharvest Biol. Technol. 2016, 115, 72–80. [Google Scholar] [CrossRef]
- Moscoso-Ramírez, P.A.; Montesinos-Herrero, C.; Palou, L. Control of citrus postharvest Penicillium molds with sodium ethylparaben. Crop Prot. 2013, 46, 44–51. [Google Scholar] [CrossRef]
- Palou, L.; Usall, J.; Munoz, J.A.; Smilanick, J.L.; Vinas, I. Hot water, sodium carbonate, and sodium bicarbonate for the control of postharvest green and blue molds of clementine mandarins. Postharvest Biol. Technol. 2002, 24, 93–96. [Google Scholar] [CrossRef]
- Youssef, K.; Ligorio, A.; Nigro, F.; Ippolito, A. Activity of salts incorporated in wax in controlling postharvest diseases of citrus fruit. Postharvest Biol. Technol. 2012, 65, 39–43. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, K.; Ming, J. Control of blue and green mold decay of citrus fruit by Pichia membranefaciens and induction of defense responses. Sci. Hortic. 2012, 135, 120–127. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Mandal, G.; Singh, D.; Sharma, R.R. Effect of hot water treatment and biocontrol agent (Debaryomyces hansenii) on shelf-life of peach. Indian J. Hortic. 2007, 64, 25–28. [Google Scholar]
- Mossini, S.A.G.; Kemmelmeier, C. Inhibition of citrinin production in Penicillium citrinum cultures by neem [Azadirachta indica A. Juss (Meliaceae)]. Int. J. Mol. Sci. 2008, 9, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, F.A.; Misaghi, A.; Gheisari, H.; Basti, A.A.; Amiri, A.; Ghalebi, S.R.; Derakhshan, Z.; Tafti, R.D. The effect of Zataria multiflora Boiss Essential oil on the growth and citrinin production of Penicillium citrinum in culture media and cheese. Food Chem. Toxicol. 2018, 118, 691–694. [Google Scholar] [CrossRef]
- Noori, N.; Yahyaraeyat, R.; Khosravi, A.; Atefi, P.; Akhondzadeh Basti, A.; Akrami, F.; Bahonar, A.; Misaghi, A. Effect of Zataria multiflora boiss. Essential oil on growth and citrinin production by Penicillium citrinum in culture media and mozzarella cheese. J. Food Saf. 2012, 32, 445–451. [Google Scholar] [CrossRef]
- Vazquez, B.I.; Fente, C.; Franco, C.M.; Vazquez, M.J.; Cepeda, A. Inhibitory effects of eugenol and thymol on Penicillium citrinum strains in culture media and cheese. Int. J. Food Microbiol. 2001, 67, 157–163. [Google Scholar] [CrossRef]


| Genera | Subgenus | Series | Species |
|---|---|---|---|
| Penicillium | Furcatum | - | P. citrinum Thom |
| Penicillium | Expansa | P. expansum Link | |
| Penicillium | Corymbifera | P. radicicola Overy & Frisvad | |
| Penicillium | Verrucosa | P. verrucosum Dierckx | |
| Penicillium | - | P. viridicatum Westling | |
| Penicillium | - | P. camemberti Sopp | |
| Aspergillus | - | - | A. carneus Tiegh |
| - | - | A. niveus Blochwitz | |
| - | - | A. oryzae | |
| Circumdati | - | A. terreus Thom | |
| Monascus | - | - | M. purpureus Went |
| - | - | M. ruber Tiegh |
| Food Matrix | Country | Range (μg/kg) | Detection Technique | References |
|---|---|---|---|---|
| Amaranth | Spain | 1.8–5.9 | QuEChERS | [31] |
| Apples | Portugal | 320–920 | SPE-HPLC | [32] |
| Portugal | 3.06–5.37 | TLC | [33] | |
| China | 1.7–16.3 | UPLC-MS/MS | [34] | |
| Croatia | 240 | TLC | [35] | |
| Almond | Spain | 3.0–7.4 | UHPLC-MS/MS | [36] |
| India | 2.80–18.20 | ELISA | [37] | |
| Barley | Czech Republic | 93.64 | HPLC | [38] |
| Black Pepper | India | 17.8 | LC-MS/MS | [16] |
| Black olives | Turkey | 350 | TLC | [39] |
| Morocco | 0.2–0.5 | HPLC | [40] | |
| Breakfast cereals | France | 1.5–42 | HPLC-FD | [41] |
| France | 0.5–1.5 | HPLC | [42] | |
| Brown rice | Spain | 6.4–10 | QuEChERS | [31] |
| Buckwheat | Spain | 1.5–6.9 | QuEChERS | [31] |
| Spain | 0.62 | LC-MS/MS | [43] | |
| Cashew | India | 4.70–9.80 | ELISA | [37] |
| Cajna salami | Croatia | <1.0–1.0 | HPLC | [44] |
| Cereals | Belgium | 14.3 | UHPLC-MS/MS | [45] |
| Croatia | 19.63 | HPLC-FD | [41] | |
| Cereal solid substrates | Poland | 5.7–74.8 | HPLC-FLD | [46] |
| Cereals and derivatives | Germany | <1–2.7 | HPLC-FD | [41] |
| Cocoa | Belgium | 3.4 | UHPLC-MS/MS | [45] |
| Coriander | India | 23.0 | LC-MS/MS | [16] |
| Commercial beers | South Africa | 6 | TLC | [47] |
| Cumin | India | 14.7 | LC-MS/MS | [16] |
| Dried grape | Turkey | 5.56 | HPLC-FD | [48] |
| Dried white mulberry | Turkey | 4.26–5.29 | HPLC-FD | [48] |
| Dry ginger | India | 19.4 | LC-MS/MS | [16] |
| Family Cereal | Nigeria | 1.2–151 | LC-MS/MS | [49] |
| Fermented dry meat products | Croatia | <1.0–1.3 | ELISA | [44] |
| Fenugreek | India | 17.2 | LC-MS/MS | [16] |
| Fruits | China | 0.06–0.10 | QuEChERS-HPLC-FLD | [50] |
| Grape | China | 0.16 | USAE-DLLME-HPLC-FLD | [50] |
| Ground rice | China | 5–100 | HPLC-DAD | [51] |
| Hazelnut | Spain | 3.1–8.0 | UHPLC-MS/MS | [36] |
| Industrially-processed complementary foods | Nigeria | 1.2–151 | LC-MS/MS | [49] |
| Infant formula | Nigeria | 3.6 | LC-MS/MS | [49] |
| Koji rice | USA | 50–1000 | IAC-HPLC | [52] |
| Lager beer | Czech Republic | 0.2–10 | SPE-HPLC | [32] |
| Liquorice root | Turkey | 14.66–19.14 | HPLC-FD | [48] |
| Monascus pigment powder | China | 122–594 | RP-HPLC | [53] |
| Maize | Serbia | 5–547 | LC-MS/MS | [54] |
| China | 4.71–18.49 | ic-ELISA | [55] | |
| Mozambique/Burkina Faso | 531–5074 | LC-MS/MS | [56] | |
| Macadamia nut | Spain | 3.3–7.3 | UHPLC-MS/MS | [36] |
| Medicinal and aromatic herbs | Spain | 16.5 | ELISA | [57] |
| Mushroom | USA | 400 | IAC-HPLC | [52] |
| Ogi | Nigeria | 0.8–159 | LC-MS/MS | [49] |
| Olive | China | 0.05 | IAC-HPLC-FLD | [50] |
| Orange | China | 40.3 | UPLC-MS/MS | [34] |
| Parboiled rice | India | 12–55 | HPLC | [58] |
| Pear | China | 0.16 | USAE-DLLME-HPLC-FLD | [50] |
| Peanut | Spain | 2.9–8.9 | UHPLC-MS/MS | [36] |
| Pine nuts | Spain | 5.5–9.0 | UHPLC-MS/MS | [36] |
| Pumpkin seed | Spain | 2.6–7.3 | UHPLC-MS/MS | [36] |
| Pistachio | Spain | 4.4–8.5 | UHPLC-MS/MS | [36] |
| India | 4.57–15.80 | ELISA | [37] | |
| Quinoa | Spain | 5.3–6.9 | QuEChERS | [31] |
| Raisin | India | 2.84–17.40 | ELISA | [37] |
| Red chilli | India | 12.5 | LC-MS/MS | [16] |
| Red rice | Spain | 2.8–6.2 | QuEChERS | [31] |
| Malaysia | 0.23–20.65 | ELISA | [59] | |
| Red kojic rice | China | 50 | HPLC-FD | [60] |
| Japan | 200 | MFEI | [61] | |
| China | 100 | IAC | [62] | |
| Red mold rice | USA | 50–2500 | IAC-HPLC | [52] |
| Malaysia | 0.23–20.65 | HPLC | [59] | |
| USA | 24–189 | HPLC-UV | [63] | |
| Taiwan | 5742–27,000 | HPLC-FLD | [64] | |
| China | 49–13,550 | HPLC-FLD | [64] | |
| China | 7.5–120 | HPLC | [64] | |
| Red fermented rice | China | 140–44,240 | LC-MS/MS | [65] |
| China | 0.12–5.71 | HPLC | [66] | |
| Croatia | 95–98 | Rapid LC/DAD/FLD/MS | [67] | |
| China | 0.14–44.24 | LC-MS/MS | [65] | |
| China | 250–825 | HPLC-FLD | [65] | |
| Red yeast rice | China | 2.33–32.47 | MFCI | [68] |
| Belgium | 3.6–121,097 | UHPLC-MS/MS | [45] | |
| China | 57.28 | HPLC-FLD | [69] | |
| China | 100.6–443.6 | IAC-HPLC | [70] | |
| China | 16.6–5253 | LC-MS/MS | [68] | |
| Croatia | 98 | LC-MS | [71] | |
| Red yeast rice powder | China | 0.10–5.41 | RP-HPLC | [53] |
| Red yeast powder | China | 55 | HPLC-FD | [62] |
| Red yeast rice food additives | China | 127–4960 | LC-MS/MS | [68] |
| Red yeast rice functional food and medicine products | China | 16.6–62.5 | LC-MS/MS | [68] |
| Rice | Argentina | 0.5–50 | ELISA | [61] |
| Vietnam | 0.42 | HPLC-FLD | [72] | |
| Iran | 5–21.05 | LC-MS/MS | [73] | |
| Vietnam | 0.38–0.42 | UHPLC-FL | [74] | |
| China | 0.11 | LLE-HPLC-FLD | [50] | |
| China | 0.7–1.0 | SPME-LC-FLD | [50] | |
| Spain | 5–200 | HPLC-DAD | [75] | |
| Japan | 49–92 | HPLC | [76] | |
| Canada | 700–1130 | HPLC | [76] | |
| China | 9.65–19.85 | ic-ELISA | [55] | |
| Iran | 5–21.05 | HPLC | [58] | |
| India | 49–92 | HPLC | [58] | |
| Sausages | Croatia | <1.0–1.0 | ELISA | [44] |
| Semi-dry sausages | Croatia | <1.0 | HPLC | [44] |
| Croatia | <1.0 | ELISA | [44] | |
| Spices | Belgium | 1.4–19.8 | UHPLC-MS/MS | [45] |
| Spelt | Spain | 2.6–10.4 | QuEChERS | [31] |
| Soybean | Egypt | 270 | HPLC | [77] |
| Sunflower seed | Spain | 4.6–10.2 | UHPLC-MS/MS | [36] |
| Sweet cherries | China | 2.2–7.9 | UPLC-MS/MS | [34] |
| Tomato | China | 1.1–8.4 | UPLC-MS/MS | [34] |
| Tom bran | Nigeria | 1.7–1173 | LC-MS/MS | [49] |
| Tom bran | Nigeria | 0.8–1173 | LC-MS/MS | [49] |
| Walnut | Spain | 4.6–7.7 | UHPLC-MS/MS | [36] |
| White rice | Spain | 4.0–6.4 | UHPLC-MS/MS | [31] |
| Wheat | Tunisia | 0.1–170 | HPLC | [78] |
| Canada | 175.2 | HPLC | [79] | |
| China | 4.77–19.49 | ic-ELISA | [55] | |
| Czech Republic | 0.09 | HPLC-FD | [80] | |
| Wheat flour | Belgium | 0.1 | UHPLC-MS/MS | [45] |
| Czech Republic | 19.2–2068.6 | HPLC-FD | [80] | |
| Winter salami | Croatia | <1.0–1.3 | HPLC | [44] |
| Feed | ||||
| Feed | Burkina Faso | 341 | LC-MS/MS | [56] |
| Complete animal feeds | Belgium | 1.9–2.0 | UHPLC-MS/MS | [45] |
| Maize silage | France | 1.5–5.0 | LC-MS | [81] |
| Maize silage | France | 5–25 | LC-MS | [82] |
| Maize silage | France | 2–1.5 | LC-MS | [83] |
| Compounded feeds | Russia | 10–182 | ELISA | [84] |
| Maize gluten | Russia | 62 | ELISA | [84] |
| Wheat bran | Russia | 397 | ELISA | [84] |
| Soy-bean oilseed meal | Russia | 30 | ELISA | [84] |
| Degradation Methods | Experimental Details | Key Findings | References |
|---|---|---|---|
| Physical | |||
| Light (Blue light) | Monascus production | Decreased CIT by 79%; 28.5% increase in pigment production | [165] |
| Blue light | In vivo | Blue light completely degraded the CIT | [164] |
| Temperature/Heat | Heating under aquous condition Temperature: 90–130 °C Time: 10–20 min | Partial degradation and formation of low cytotoxic substances; increase in temperature and time above 120 °C to form another less cytotoxic substance | [161] |
| Heating/boiling | Heating at 100–140 °C in aqueous medium | High-temperature treatment degraded CIT into CIT H1 and H2 | [162] |
| High hydrostatic pressure (HHP) | Time: 5 min Pressure: 250 MPa Temperature: 35 ± 1 °C | 90–100% of the microbial population was reduced; the CIT level was reduced up to 100%; increased phenolic compounds; enhanced antioxidant activity | [118] |
| Cold atmospheric pressure plasma | Power output: 50 kV, 100 watts Electron frequency: 30 kHz Gas flow: 6 L/min | Reduced 50% of CIT; no negative effect on nutrients | [121] |
| Magnetic nanoparticles | Formation of a CIT–nanoparticle complex; effective in CIT removal; can be used in the food industry; is difficult to operate on a large scale | [174] | |
| Ultrasonication | Power: 250 W Liquid: solid ratio 40:1 Time: 50.7 min, temperature: 20 °C | Removed up to 87.7% CIT from red yeast rice | [120] |
| Chemical | |||
| Ozone | O3 treatment: (40 and 60 μmol/mol Time: 180 min | CIT level reduced from 173.51 μg/kg to 42.90 μg/kg 180 min after treatment | [175] |
| Medium-chain fatty acids | In vivo Monascus ruber | Improved pigment formation; reduced CIT production in the process | [166] |
| Flavanoids | Monascus aurantiacus Li AS3.4384 | Inhibition of CIT formation up to 87.9% | [117] |
| Monascus species-fermented red mold rice | 45% ethanol, 1.5% phosphate, and extraction for 70 min | Reduced CIT level by 91.6%; maintained 79.5% monacolin K | [163] |
| Biological | |||
| Genistein | Monascus mold (used to produce Monascus pigments, monacolin K, and ergosterol) | Suppressed acetyl- CoA formation; reduced CIT content; reduced significant differential metabolites | [176] |
| Cryptococcus podzolicus Y-3 cells | - | In response to CIT stress, DNA repair, antioxidative activity, and the TCA cycle were activated; degradation of CIT | [160] |
| Cryptococcuspodzolicus Y3 | - | Degradation up to 98%; intracellular enzyme caused degradation; degradation into less toxic compounds; degradation was factor-dependent | [169] |
| Rhodotorula mucilaginosa | - | Degradaded CIT by 91.67% at pH 4.0 and 28 °C; degradation was factor-dependent | [170] |
| Klebsiella pneumoniae strain NPUST-B11 | - | Ful degradation of CIT after 10 h of incubation. | [172] |
| Rhizobium borborid | Temperature: 30 °C Time: 120 h | R. borbori PS45 and E. cloacae PS21 were found to be the most promising among the collected strains; they caused 63.4% and 43.6% reduction, respectively | [173] |
| Adsorbents | Activated charcoal and 0.4% lyophilized yeast culture (0.2%) with feed | Ameliorated toxic effect of mycotoxin to broilers | [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, N.; Sharma, B.; Mishra, S.; Arora, S.; Selvakumar, R.; Saurabh, V.; et al. Citrinin Mycotoxin Contamination in Food and Feed: Impact on Agriculture, Human Health, and Detection and Management Strategies. Toxins 2022, 14, 85. https://doi.org/10.3390/toxins14020085
Kamle M, Mahato DK, Gupta A, Pandhi S, Sharma N, Sharma B, Mishra S, Arora S, Selvakumar R, Saurabh V, et al. Citrinin Mycotoxin Contamination in Food and Feed: Impact on Agriculture, Human Health, and Detection and Management Strategies. Toxins. 2022; 14(2):85. https://doi.org/10.3390/toxins14020085
Chicago/Turabian StyleKamle, Madhu, Dipendra Kumar Mahato, Akansha Gupta, Shikha Pandhi, Nitya Sharma, Bharti Sharma, Sadhna Mishra, Shalini Arora, Raman Selvakumar, Vivek Saurabh, and et al. 2022. "Citrinin Mycotoxin Contamination in Food and Feed: Impact on Agriculture, Human Health, and Detection and Management Strategies" Toxins 14, no. 2: 85. https://doi.org/10.3390/toxins14020085