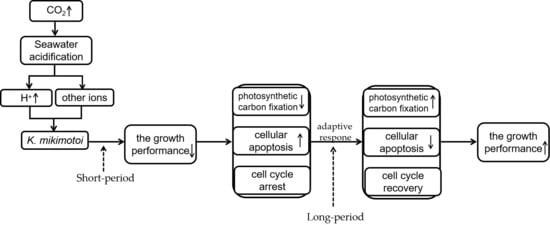
The Bloom-Forming Dinoflagellate Karenia mikimotoi Adopts Different Growth Modes When Exposed to Short or Long Period of Seawater Acidification
Abstract
:1. Introduction
2. Results
2.1. Changes of Population Dynamics to Different pCO2 Levels
2.2. Alteration in the Photosynthetic Carbon Fixation of K. mikimotoi Exposed to Different pCO2 Levels
2.3. Alteration of Cellular Apoptosis of K. mikimotoi When Exposed to Different pCO2 Levels
2.4. Alteration of the Cell Cycle of K. mikimotoi Due to Elevated pCO2
2.5. Discrimination of H+ and Carbon System Alterations in Apoptosis and the Cell Cycle
3. Discussion
3.1. What’s the Possible Explanation for the Different Growth Modes of K. mikimotoi Exposed to Short or Long Period of Seawater Acidification
3.2. What’s the Possible Explanation Leading to the Different Physiological Responses of K. mikimotoi When Exposed to HCl or CO2-Induced Acidifying Conditions?
4. Conclusions
5. Materials and Methods
5.1. Microalgal Cultivation
5.2. Acidifying System Set-Up and Experimental Design
5.3. Population Dynamics
5.4. Determination of Photosynthetic Carbon Fixation
5.5. Estimation of the Key Index in the Apoptotic Pathway
5.6. Determination of the Cell Cycle
5.7. Discrimination of H+ and Ions in Carbon System Alteration on Apoptosis and Cell Cycle Based on Acute Toxicity Experiment
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griggs, D.J.; Noguer, M. Climate change 2001: The scientific basis. Contribution of working group I to the third assessment report of the intergovernmental panel on climate change. Weather 2002, 57, 267–269. [Google Scholar] [CrossRef]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidification: The other CO2 problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [Green Version]
- Caldeira, K.; Wickett, M.E. Oceanography: Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.; Ball, L.; Beardall, J.; Giordano, M.; Maberly, S.C. Algae lacking carbon-concentrating mechanisms. Can. J. Bot. 2005, 83, 879–890. [Google Scholar] [CrossRef]
- IPCC. Climate change 2014: Synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Fabry, V.J.; Seibel, B.A.; Feely, R.A.; Orr, J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008, 65, 414–432. [Google Scholar] [CrossRef]
- Kurihara, H. Effects of CO2-driven ocean acidifcation on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 2008, 373, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Wittmann, A.C.; Pörtner, H.O. Sensitivities of extant animal taxa to ocean acidification. Nat. Clim. Chang. 2013, 3, 995–1001. [Google Scholar] [CrossRef]
- Bressan, M.; Chinellato, A.; Munari, M.; Matozzo, V.; Manci, A.; Marčeta, T.; Finos, L.; Moro, I.; Pastore, P.; Badocco, D.; et al. Does seawater acidification affect survival, growth and shell integrity in bivalve juveniles? Mar. Environ. Res. 2014, 99, 136–148. [Google Scholar] [CrossRef]
- Gazeau, F.; Alliouane, S.; Bock, C.; Bramanti, L.; López Correa, M.; Gentile, M.; Ziveri, P. Impact of ocean acidification and warming on the Mediterranean mussel (Mytilus galloprovincialis). Front. Mar. Sci. 2014, 1, 62. [Google Scholar] [CrossRef] [Green Version]
- Matoo, O.B. Interactive Effects of Ocean Acidification and Multiple Stressors on Physiology of Marine Bivalves. Doctoral Dissertation, The University of North Carolina at Charlotte, Charlotte, NC, USA, 2013. [Google Scholar]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Freitas, R.; Pires, A.; Velez, C.; Almeida, A.; Moreira, A.; Wrona, F.J.; Soares, A.M.V.M.; Figueira, E. Effects of seawater acidification on Diopatra neapolitana (Polychaete, Onuphidae): Biochemical and regenerative capacity responses. Ecol. Indic. 2016, 60, 152–161. [Google Scholar] [CrossRef]
- Pörtner, H. Contribution to the theme section ‘effects of ocean acidification on marine ecosystems’ ecosystem effects of ocean acidification in times of ocean warming: A physiologist’s view. Mar. Ecol. Prog. Ser. 2008, 373, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Kroeker, K.J.; Micheli, F.; Gambi, M.C. Ocean acidification causes ecosystem shifts via altered species interactions. Nat. Clim. Chang. 2013, 3, 156–159. [Google Scholar] [CrossRef]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant. Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef] [Green Version]
- Burkhardt, S.; Amoroso, G.; Riebesell, U.; Sultemeyer, D. CO2 and HCO3− uptake in marine diatoms acclimated to different CO2 concentrations. Limnol. Oceanogr. 2001, 46, 1378–1391. [Google Scholar] [CrossRef] [Green Version]
- Trimborn, S.; Lundholm, N.; Thoms, S.; Richter, K.U.; Krock, B.; Hansen, P.J.; Rost, B. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: The effect of pH-induced changes in seawater carbonate chemistry. Physiol. Plant. 2008, 133, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low-Décarie, E.; Fussmann, G.F.; Bell, G. The effect of elevated CO2 on growth and competition in experimental phytoplankton communities. Glob. Chang. Biol. 2011, 17, 2525–2535. [Google Scholar] [CrossRef]
- Torstensson, A.; Chierici, M.; Wulff, A. The influence of increased temperature and carbon dioxide levels on the benthic/sea ice diatom Navicula directa. Polar Biol. 2012, 35, 205–214. [Google Scholar] [CrossRef]
- Eberlein, T.; Van de Waal, D.B.; Brandenburg, K.M.; John, U.; Voss, M.; Achterberg, E.P.; Rost, B. Interactive effects of ocean acidification and nitrogen limitation on two bloom-forming dinoflagellate species. Mar. Ecol. Prog. Ser. 2016, 543, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Tortell, P.D.; Morel, F.M.M. Sources of inorganic carbon for phytoplankton in the eastern Subtropical and Equatorial Pacific Ocean. Limnol. Oceanogr. 2002, 47, 1012–1022. [Google Scholar] [CrossRef]
- Van De Waal, D.B.; Eberlein, T.; John, U.; Wohlrab, S.; Rost, B. Impact of elevated pCO2 on paralytic shellfish poisoning toxin content and composition in Alexandrium tamarense. Toxicon 2014, 78, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Hein, M.; Sand-Jensen, K. CO2 increases oceanic primary production. Nature 1997, 388, 526–527. [Google Scholar] [CrossRef]
- Riebesell, U.; Aberle-Malzahn, N.; Achterberg, E.P.; Algueró-Muñiz, M.; Alvarez-Fernandez, S.; Arístegui, J.; Bach, L.T.; Boersma, M.; Boxhammer, T.; Guan, W.; et al. Toxic algal bloom induced by ocean acidification disrupts the pelagic food web. Nat. Clim. Chang. 2018, 8, 1082–1086. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Bolch, C.J.; Bryan, J.; Koerbin, B. Microalgal spores in ship’s ballast water: A danger to aquaculture. In Toxic Marine Phytoplankton; Graneii, E., Sundstrom, B., Edler, L., Anderson, D.M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1990; pp. 475–480. [Google Scholar]
- Dang, L.X.; Li, Y.; Liu, F.; Zhang, Y.; Yang, W.D.; Li, H.Y.; Liu, J.S. Chemical response of the toxic dinoflagellate Karenia mikimotoi against grazing by three species of Zooplankton. J. Eukaryot. Microbiol. 2015, 62, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, T.; Ling, J.; Yu, R.; Zhou, M. Detrimental impacts of the dinoflagellate Karenia mikimotoi, in Fujian coastal waters on typical marine organisms. Harmful Algae 2017, 61, 1–12. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, J.; Xu, S.; Tian, Q.; Zhang, Y.; He, P. Retracted: Effects of seaweed gracilaria verrucosa, on the growth of microalgae: A case study in the laboratory and in an enclosed sea of Hangzhou bay, China. Harmful Algae 2011, 10, 411–418. [Google Scholar] [CrossRef]
- Neely, T.; Campbell, L. A modified assay to determine hemolytic toxin variability among Karenia clones isolated from the Gulf of Mexico. Harmful Algae 2006, 5, 592–598. [Google Scholar] [CrossRef]
- Satake, M.; Shoji, M.; Oshima, Y.; Naoki, H.; Fujita, T.; Yasumoto, T. Gymnocin-A, a cytotoxic polyether from the notorious red tide dinoflagellate, Gymnodinium mikimotoi. Tetrahedron Lett. 2002, 43, 5829–5832. [Google Scholar] [CrossRef]
- Yang, C.Z.; Albright, L.J.; Yousif, A.N. Oxygen-radical-mediated effects of the toxic phytoplankter Heterosigma carterae on juvenile rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1995, 23, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.A.; Ross, T.; Pyecroft, S.; Hallegraeff, G. Superoxide production by marine microalgae. Mar. Biol. 2005, 147, 533–540. [Google Scholar] [CrossRef]
- Tatters, A.O.; Schnetzer, A.; Fu, F.; Lie, A.Y.A.; Hutchins, D.A. Short-versus long-term responses to changing CO2 in a coastal dinoflagellate bloom: Implications for interspecific competitive interactions and community structure. Evolution 2013, 67, 1879–1891. [Google Scholar] [CrossRef]
- Riebesell, U.; Tortell, P.D. Effects of ocean acidification on pelagic organisms and ecosystems. In Ocean Acidification; Gattuse, J.P., Hansson, L., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 99–121. [Google Scholar]
- Brading, P.; Warner, M.E.; Davey, P.; Smith, D.J.; Achterberg, E.P.; Suggett, D.J. Differential effects of ocean acidification on growth and photosynthesis among phylotypes of Symbiodinium (Dinophyceae). J. Limnol. Oceanogr. 2011, 56, 927–938. [Google Scholar] [CrossRef] [Green Version]
- Van de Waal, D.B.; John, U.; Ziveri, P.; Reichart, G.J.; Hoins, M.; Sluijs, A.; Rost, B. Ocean acidification reduces growth and calcification in a marine dinoflagellate. PLoS ONE 2013, 8, e65987. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Zhou, B.; Wang, Y.; Wang, Y.; Zhang, X.; Zhao, Y.; Zhao, X.; Tang, X. Effect of CO2-induced seawater acidification on growth, photosynthesis and inorganic carbon acquisition of the harmful bloom-forming marine microalga, Karenia mikimotoi. PLoS ONE 2017, 12, e0183289. [Google Scholar] [CrossRef] [Green Version]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKibben, S.M.; Peterson, W.; Wood, A.M.; Trainer, V.L.; Hunter, M.; White, A.E. Climatic regulation of the neurotoxin domoic acid. Proc. Natl. Acad. Sci. USA 2017, 114, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Hutchins, D.A.; Feng, Y.; Seubert, E.L.; Caron, D.A.; Fu, F.X. Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnol. Oceanogr. 2011, 56, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Zhang, Y.; Warner, M.E.; Feng, Y.; Sun, J.; Hutchins, D.A. A comparison of future increased CO2 and temperature effects on sympatric Heterosigma akashiwo and Prorocentrum minimum. Harmful Algae 2008, 7, 76–90. [Google Scholar] [CrossRef]
- Fu, F.; Mulholland, M.R.; Garcia, N.S.; Beck, A.; Bernhardt, P.W.; Warner, M.E.; SañudoWilnelmy, S.; Hutchins, D.A. Interactions between changing pCO2, N2 fixation, and Fe limitation in the marine unicellular cyanobacterium Crocosphaera. Limnol. Oceanogr. 2008, 53, 2472–2484. [Google Scholar] [CrossRef] [Green Version]
- Hattenrath-Lehmann, T.K.; Smith, J.L.; Wallace, R.B.; Merlo, L.R.; Koch, F.; Mittelsdorf, H.; Goleski, J.A.; Anderson, D.M.; Gobler, C.J. The effects of elevated CO2 on the growth and toxicity of field populations and cultures of the saxitoxin-producing dinoflagellate, Alexandrium fundyense. Limnol. Oceanogr. 2015, 60, 198–214. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Place, A.R.; Garcia, N.S.; Hutchins, D.A. CO2 and phosphate availability control the toxicity of the harmful bloom dinoflagellate Karlodinium veneficum. Aquat. Microb. Ecol. 2010, 59, 55–65. [Google Scholar] [CrossRef]
- Sobrino, C.; Neale, P.J.; Lubian, L.M. Interaction of UV radiation and inorganiccarbon supply in the inhibition of photosynthesis: Spectral and temporal response of twomarine picoplankton. J. Photoch. Photobio. 2005, 81, 384–393. [Google Scholar] [CrossRef]
- Chen, S.; Beardall, J.; Gao, K. A red tide alga grown under ocean acidification upregulates its tolerance to lower pH by increasing its photophysiological functions. Biogeosciences 2014, 11, 4829–4837. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Tang, X.; Zhou, B.; Xu, N.; Wang, Y. Mechanism of Deca-BDE-induced apoptosis in Neuro-2a cells: Role of death-receptor pathway and reactive oxygen species-mediated mitochondrial pathway. J. Environ. Sci. 2016, 46, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Tortell, P.D.; Rau, G.H.; Morel, F.M.M. Inorganic carbon acquisition in coastal Pacific phytoplankton communities. Limnol. Oceanogr. 2000, 45, 1485–1500. [Google Scholar] [CrossRef]
- Rost, B.; Riebesell, U.; Burkhardt, S.; Sültemeyer, D. Carbon acquisition of bloom-forming marine phytoplankton. Limnol. Oceanogr. 2003, 48, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Rost, B.; Richter, K.U.; Riebesell, U.; Hansen, P.J. Inorganic carbon acquisition in red tide dinoflagellates. Plant. Cell Environ. 2006, 29, 810–822. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Warner, M.E.; Zhang, Y.; Feng, Y.; Hutchins, D.A. Effects of increased temperature and CO2 on photsynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (cyanobacteria). J. Phycol. 2007, 43, 485–496. [Google Scholar] [CrossRef]
- Mackey, K.R.M.; Morris, J.J.; Morel, F.M.M.; Kranz, A.S.A. Response of photosynthesis to ocean acidification. Oceanography 2015, 28, 74–91. [Google Scholar] [CrossRef]
- Riebesell, U.; Schulz, K.G.; Bellerby, R.G.J.; Botros, M.; Fritsche, P.; Meyerhöfer, M.; Neill, C.; Nondal, G.; Oschlies, A.; Wohlers, J.; et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature 2007, 450, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gao, K.; Riebesell, U. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 2010, 7, 2915–2923. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.R.; Moore, S.K.; Tomlinson, H.C.; Silke, J.; Cusack, C.K. Living with harmful algal blooms in a changing world: Strategies for modelling and mitigating their effects in coastal marine ecosystems. In Coastal and Marine Hazards, Risks, and Disasters; Elsevier: Amsterdam, The Netherlands, 2015; pp. 495–561. [Google Scholar]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.V.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raven, J.; Beardall, J.; Sánchez-Baracaldo, P. The possible evolution, and future, of CO2 concentrating mechanisms. J. Exp. Bot. 2017, 68, 3701–3716. [Google Scholar] [CrossRef] [Green Version]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Fu, F.X.; Tatters, A.O.; Hutchins, D.A. Global change and the future of harmful algal blooms in the ocean. Mar. Ecol. Prog. Ser. 2012, 470, 207–233. [Google Scholar] [CrossRef] [Green Version]
- Beardall, J.; Stojkovic, S.; Larsen, S. Living in a high CO2 world: Impacts of global climate change on marine phytoplankton. Plant. Ecol. Divers. 2009, 2, 191–205. [Google Scholar] [CrossRef]
- Raven, J.; Beardall, J. CO2 concentrating mechanisms and environmental change. Aquat. Bot. 2014, 118, 24–37. [Google Scholar] [CrossRef]
- Tatters, A.O.; Flewelling, L.J.; Fu, F.; Granholm, A.A.; Hutchins, D.A. High CO2 promotes the production of paralytic shellfish poisoning toxins by alexandrium catenella from southern california waters. Harmful Algae 2013, 30, 37–43. [Google Scholar] [CrossRef]
- Pang, M.; Xu, J.; Qu, P.; Mao, X.; Wu, Z.; Xin, M.; Sun, p.; Wang, Z.; Zhang, X.; Chen, H. Effect of CO2 on growth and toxicity of alexandrium tamarense from the east china sea, a major producer of paralytic shellfish toxins. Harmful Algae 2017, 68, 240–247. [Google Scholar] [CrossRef]
- Kasuba, K.C.; Vavilala, S.L.; D’Souza, J.S. Apoptosis-like cell death in unicellular photosynthetic organisms—A review. Algal. Res. 2015, 12, 126–133. [Google Scholar] [CrossRef]
- Hopkinson, B.M.; Dupont, C.L.; Allen, A.E.; Morel, F.M. Efficiency of the CO2-concentrating mechanism of diatoms. Proc. Natl. Acad. Sci. USA 2011, 108, 3830–3837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinfelder, J.R. Carbon Concentrating Mechanisms in Eukaryotic Marine Phytoplankton. Annu. Rev. Mar. Sci. 2011, 3, 291–315. [Google Scholar] [CrossRef] [Green Version]
- Portner, H.O.; Langenbuch, M.; Reipschlager, A. Biological impact of elevated ocean CO2 concentrations: Lessons from animal physiology and earth history. J. Oceanogr. 2004, 60, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Tang, X.; Wang, Y.; Yang, Y.; Zhang, W.; Zhao, Y.; Zhang, X. ROS changes are responsible for tributyl phosphate (TBP)-induced toxicity in the alga Phaeodactylum tricornutum. Aquat. Toxicol. 2019, 208, 168–178. [Google Scholar] [CrossRef]
- Xu, M.; Sun, T.; Tang, X.; Lu, K.; Jiang, Y.; Cao, S.; Wang, Y. CO2 and HCl-induced seawater acidification impair the ingestion and digestion of blue mussel Mytilus edulis. Chemosphere 2020, 240, 124821. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms: I. cyclotella nana hustedt, and detonula confervacea (cleve) gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Tang, X.; Jiang, Y.; Wang, Y. Seawater acidification induced immune function changes of haemocytes in Mytilus edulis: A comparative study of CO2 and HCl enrichment. Sci. Rep. 2017, 7, 41488. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace, D.; Allison, L.J. Program Developed for CO2 System Calculations; Carbon Dioxide Information Analysis Center, Managed by Lockheed Martin Energy Research Corporation for the US Department of Energy: New York, NY, USA, 1998. [Google Scholar]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Springer: Berlin/Heidelberg, Germany, 1975; pp. 29–60. [Google Scholar]
- Bradford, M.M. A Rapid Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. J. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, B.; Chen, H.; Tang, X.; Wang, Y. Reactive oxygen species (ROS) and the calcium-(Ca2+) mediated extrinsic and intrinsic pathways underlying BDE-47-induced apoptosis in rainbow trout (Oncorhynchus mykiss) gonadal cells. Sci. Total Environ. 2019, 656, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, J.; Haszeldine, S.; Holloway, S.; Pearce, J.; Oakey, J.; Shackley, S.; Turley, C. Scope for future CO2 emission reductions from electricity generation through the deployment of carbon capture and storage technologies. In Avoiding Dangerous Climate Change; Schellnhuber, H.J., Cramer, W., Nakicenovic, N., Wigley, T., Yohe, G., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 379–383. [Google Scholar]
- Hawkins, D.G. No exit: Thinking about leakage from geologic carbon storage sites. Energy 2004, 29, 1571–1578. [Google Scholar] [CrossRef]











| Groups | Equation: | |||
|---|---|---|---|---|
| K (ind mL−1) | a | r (d−1) | Tp (d) | |
| 390 ppmv | 177.69 ± 5.63 | 7.10 ± 0.22 | 0.44 ± 0.02 | 16.14 ± 0.23 |
| 1000 ppmv | 188.45 ± 5.07 | 6.82 ± 0.20 | 0.46 ± 0.03 | 14.85 ± 0.53 |
| 2000 ppmv | 178.37 ± 2.97 | 6.68 ± 0.13 | 0.46 ± 0.02 | 14.53 ± 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhou, Z.; Li, Y.; Wang, Y.; Xu, M.; Zhou, B.; Lu, K.; Wang, Y. The Bloom-Forming Dinoflagellate Karenia mikimotoi Adopts Different Growth Modes When Exposed to Short or Long Period of Seawater Acidification. Toxins 2021, 13, 629. https://doi.org/10.3390/toxins13090629
Li Y, Zhou Z, Li Y, Wang Y, Xu M, Zhou B, Lu K, Wang Y. The Bloom-Forming Dinoflagellate Karenia mikimotoi Adopts Different Growth Modes When Exposed to Short or Long Period of Seawater Acidification. Toxins. 2021; 13(9):629. https://doi.org/10.3390/toxins13090629
Chicago/Turabian StyleLi, Yuanyuan, Zhengli Zhou, Yijun Li, Yanqun Wang, Mengxue Xu, Bin Zhou, Keyu Lu, and You Wang. 2021. "The Bloom-Forming Dinoflagellate Karenia mikimotoi Adopts Different Growth Modes When Exposed to Short or Long Period of Seawater Acidification" Toxins 13, no. 9: 629. https://doi.org/10.3390/toxins13090629