Graphene-Based Sensing Platform for On-Chip Ochratoxin A Detection
Abstract
:1. Introduction
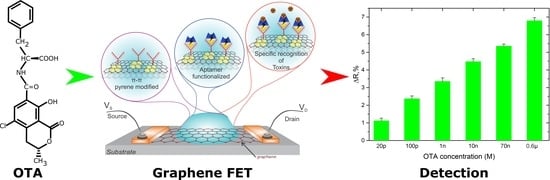
2. Results and Discussion
3. Materials and Methods
3.1. Graphene FET Fabrication
3.2. Aptamer Covalent Bonding
3.3. Ochratoxin A and Zearalenone Solution Preparation
3.4. Recovery Study
3.5. Graphene FET Characterization
Author Contributions
Funding
Conflicts of Interest
References
- Kabak, B.; Dobson, A.D.; Var, I.I.L. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Speijers, G.J.A.; van Egmond, H.P. Worldwide Ochratoxin A levels in food and feeds. In Human Ochratoxicosis and Its Pathologies; Creppy, E.E., Castegnaro, M., Dirheimer, G., Eds.; John Libbey Eurotext: London, UK, 1993; Volume 231, pp. 85–100. [Google Scholar]
- Vidic, J.; Vizzini, P.; Manzano, M.; Kavanaugh, D.; Ramarao, N.; Zivkovic, M.; Radonic, V.; Knezevic, N.; Giouroudi, I.; Gadjanski, I. Point-of-need DNA testing for detection of foodborne pathogenic bacteria. Sensors 2019, 19, 1100. [Google Scholar] [CrossRef] [PubMed]
- Ringot, D.; Chango, A.; Schneider, Y.J.; Larondelle, Y. Toxicokinetics and toxicodynamics of Ochratoxin A, an update. Chem. Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef] [PubMed]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Cramer, B.; Iha, M.H.; Krska, R.; Lattanzio, V.M.T.; Macdonald, S.; Malone, R.J.; Maragos, C.; Solfrizzo, M.; Stranska-Zachariasova, M. Developments in mycotoxin analysis: An update for 2016–2017. World Mycotoxin J. 2018, 11, 5–32. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, C.; Huang, Y.; Yan, J.; Chen, A.A. Lateral flow strip based aptasensor for detection of ochratoxin A in corn samples. Molecules 2018, 23, 291. [Google Scholar] [CrossRef] [PubMed]
- Rehmat, Z.; Mohammed, W.S.; Sadiq, M.B.; Somarapalli, M.; Anal, A.K. Ochratoxin A detection in coffee by competitive inhibition assay using chitosan-based surface plasmon resonance compact system. Colloid. Surf. B 2019, 174, 569–574. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Stepanova, V.; Kutyreva, M.; Gataulina, A.; Ulakhovich, N.; Evtugyn, V.; Hianik, T. Impedimetric aptasensor for ochratoxin A determination based on Au nanoparticles stabilized with hyper-branched polymer. Sensors 2013, 13, 16129–16145. [Google Scholar] [CrossRef]
- Radi, A.E.; Munoz-Berbel, X.; Lates, V.; Marty, J.L. Label-free impedimetric immunosensor for sensitive detection of ochratoxin A. Biosens. Bioelectron 2009, 24, 1888–1892. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Xie, H.; Sun, R.; Cao, T.; Paudyal, N.; Fang, W.; Song, H. Development of a magnetic nanoparticles-based screen-printed electrodes (MNPs-SPEs) biosensor for the quantification of Ochratoxin A in cereal and feed samples. Toxins 2018, 10, 317. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.I.; Knezevic, N.Z. Graphene-based biosensors for on-site detection of contaminants in food. Anal. Methods 2018, 10, 5061–5070. [Google Scholar] [CrossRef]
- Fu, W.; Feng, L.; Mayer, D.; Panaitov, G.; Kireev, D.; Offenhäusser, A.; Krause, H.J. Electrolyte-gated graphene ambipolar frequency multipliers for biochemical sensing. Nano Lett. 2016, 16, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Park, S.J.; Kwon, O.S.; Bae, J.; Jang, J. High-performance flexible graphene aptasensor for mercury detection in mussels. ACS Nano 2013, 7, 10563–10571. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhu, Y.; Wang, X.; Rotti, P.G.; DiMarco, C.; Tyler, S.R.; Zhao, X.; Engelhardt, J.F.; Hone, J.; Lin, Q. Real-time monitoring of insulin using a graphene field-effect transistor aptameric nanosensor. ACS Appl. Mater. Interfaces 2017, 9, 27504–27511. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Label-free biosensors based on aptamer-modified graphene field-effect transistors. J. Am. Chem. Soc. 2010, 132, 18012–18013. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Meshik, X.; Choi, M.; Farid, S.; Datta, D.; Lan, Y.; Poduri, S.; Sarkar, K.; Baterdene, U.; Huang, C.E.; et al. A graphene and aptamer based liquid gated FET-like electrochemical biosensor to detect adenosine triphosphate. IEEE Trans. Nanobiosci. 2015, 14, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Gao, T.; Yang, X.; Dai, X.; Zhou, W.; Zhang, A.; Lieber, C.M. Specific detection of biomolecules in physiological solutions using graphene transistor biosensors. Proc. Natl. Acad. Sci. USA 2016, 113, 14633–14638. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Xia, H.; Zauberman, J.; Tomaiuolo, M.; Ping, J.; Zhang, Q.; Ducos, P.; Ye, H.; Wang, S.; Yang, X.; et al. Detection of sub-fM DNA with target recycling and self-assembly amplification on graphene field-effect biosensors. Nano Lett. 2018, 18, 3509–3515. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Wang, C.; He, M.; Lin, Q. Selective detection of water pollutants using a differential aptamer-based graphene biosensor. Biosens. Bioelectron. 2019, 126, 59–67. [Google Scholar] [CrossRef]
- O’Sullivan, C.K. Aptasensors—The future of biosensing. Anal. Bioanal. Chem. 2002, 372, 44–48. [Google Scholar] [CrossRef]
- Hong, P.; Li, W.; Li, J. Applications of aptasensors in clinical diagnostics. Sensors 2012, 12, 1181–1193. [Google Scholar] [CrossRef]
- Barthelmebs, L.; Hayat, A.; Limiadi, A.W.; Marty, J.L.; Noguer, T. Electrochemical DNA aptamer-based biosensor for OTA detection, using superparamagnetic nanoparticles. Sens. Actuators B Chem. 2011, 156, 932–937. [Google Scholar] [CrossRef]
- Raz, S.R.; Haasnoot, W. Multiplex bioanalytical methods for food and environmental monitoring. TrAC Trends Anal. Chem. 2011, 30, 1526–1537. [Google Scholar]
- Kireev, D.; Sarik, D.; Wu, T.; Xie, X.; Wolfrum, B.; Offenhausser, A. High throughput transfer technique: Save your graphene. Carbon 2016, 107, 319–324. [Google Scholar] [CrossRef]
- Liang, X.; Sperling, B.A.; Calizo, I.; Cheng, G.; Hacker, C.A.; Zhang, Q.; Obeng, Y.; Yan, K.; Peng, H.; Li, Q.; et al. Toward clean and crackless transfer of graphene. ACS Nano 2011, 5, 9144–9153. [Google Scholar] [CrossRef] [PubMed]
- Kireev, D.; Brambach, M.; Seyock, S.; Maybeck, V.; Fu, W.Y.; Wolfrum, B.; Offenhausser, A. Graphene transistors for interfacing with cells: Towards a deeper understanding of liquid gating and sensitivity. Sci. Rep. 2017, 7, 6658. [Google Scholar] [CrossRef]
- Cruz-Aguado, J.A.; Penner, G. Determination of Ochratoxin A with a DNA aptamer. J. Agric. Food Chem. 2008, 56, 10456–10461. [Google Scholar] [CrossRef]
- Liu, F.; Ding, A.; Zheng, J.; Chen, J.; Wang, B. A label-free aptasensor for Ochratoxin a detection based on the structure switch of aptamer. Sensors 2018, 18, 1769. [Google Scholar] [CrossRef]
- Castillo, G.; Lamberti, I.; Mosiello, L.; Hianik, T. Impedimetric DNA aptasensor for sensitive detection of ochratoxin A in food. Electroanalysis 2012, 24, 512–520. [Google Scholar] [CrossRef]
- Ghosh, S.; Khan, N.I.; Tsavalas, J.G.; Song, E. Selective detection of lysozyme biomarker utilizing large area chemical vapor deposition-grown graphene-based field-effect transistor. Front. Bioeng. Biotechnol. 2018, 6, 29. [Google Scholar] [CrossRef]





| Samples | Spiked, pM | Detected, pM | Recovery, % |
|---|---|---|---|
| 1 | 100 | 105 | 105 |
| 2 | 500 | 600 | 120 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekrasov, N.; Kireev, D.; Emelianov, A.; Bobrinetskiy, I. Graphene-Based Sensing Platform for On-Chip Ochratoxin A Detection. Toxins 2019, 11, 550. https://doi.org/10.3390/toxins11100550
Nekrasov N, Kireev D, Emelianov A, Bobrinetskiy I. Graphene-Based Sensing Platform for On-Chip Ochratoxin A Detection. Toxins. 2019; 11(10):550. https://doi.org/10.3390/toxins11100550
Chicago/Turabian StyleNekrasov, Nikita, Dmitry Kireev, Aleksei Emelianov, and Ivan Bobrinetskiy. 2019. "Graphene-Based Sensing Platform for On-Chip Ochratoxin A Detection" Toxins 11, no. 10: 550. https://doi.org/10.3390/toxins11100550