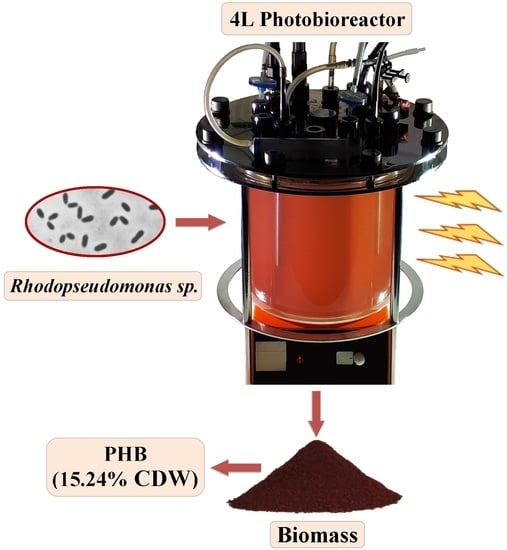
Poly-β-Hydroxybutyrate Production by Rhodopseudomonas sp. Grown in Semi-Continuous Mode in a 4 L Photobioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organism and Culture Conditions
2.2. Culture System
2.3. Analytical Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.K.; Otari, S.V.; Jeon, J.M.; Gurav, R.; Choi, Y.K.; Bhatia, R.K.; Pugazhendhi, A.; Kumar, V.; Rajesh Banu, J.; Yoon, J.J.; et al. Biowaste-to-bioplastic (polyhydroxyalkanoates): Conversion technologies, strategies, challenges, and perspective. Bioresour. Technol. 2021, 326, 124733. [Google Scholar] [CrossRef]
- Carlozzi, P.; Touloupakis, E. Bioplastic production by feeding the marine Rhodovulum sulfidophilum DSM-1374 with four different carbon sources under batch, fed-batch and semi-continuous growth regimes. New Biotechnol. 2021, 62, 10–17. [Google Scholar] [CrossRef]
- Touloupakis, E.; Carlozzi, P. Growth of photosynthetic microorganisms in different photobioreactors operated outdoors. In Cyanobacteria Biotechnology; Advanced Biotechnology, Series; Hudson, P., Lee, S.Y., Nielsen, J., Stephanopoulos, G., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2021; pp. 505–519. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Giardi, M.T.; Zappi, D.; Touloupakis, E.; Antonacci, A. Photoautotrophs-bacteria co-cultures: Advances, challenges and applications. Materials 2021, 14, 3027. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Faraloni, C.; Silva Benavides, A.M.; Masojídek, J.; Torzillo, G. Sustained photobiological hydrogen production by Chlorella vulgaris without nutrient starvation. Int. J. Hydrogen Energy 2021, 46, 3684–3694. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Moon, Y.M.; Song, H.S.; Jeon, J.M.; Choi, K.Y.; Yang, Y.H. Bioconversion of plant biomass hydrolysate into bioplastic (polyhydroxyalkanoates) using Ralstonia eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://ec.europa.eu/transparency/regdoc/rep/1/2018/EN/COM-2018-28-F1-EN-MAIN-PART-1.PDF (accessed on 10 August 2021).
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Vieira, J.A.; Druzian, J.I. Microalgae as source of polyhydroxyalkanoates (PHAs)—A review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar] [CrossRef]
- Koch, M.; Doello, S.; Gutekunst, K.; Forchhammer, K. PHB is produced from glycogen turn-over during nitrogen starvation in Synechocystis sp. PCC6803. Int. J. Mol. Sci. 2019, 20, 1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touloupakis, E.; Poloniataki, E.G.; Ghanotakis, D.F.; Carlozzi, P. Production of biohydrogen and/or poly-β-hydroxybutyrate by Rhodopseudomonas sp. using various carbon sources as substrate. Appl. Biochem. Biotechnol. 2021, 193, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Samui, A.B.; Kanai, T. Polyhydroxyalkanoates based copolymers. Int. J. Biol. Macromol. 2019, 140, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Talan, A.; Tyagi, R.D.; Drogui, P. Concomitant production of value- added products with polyhydroxyalkanoate (PHA) synthesis: A review. Bioresour. Technol. 2021, 337, 125419. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Song, H.S.; Moon, Y.M.; Hong, Y.G.; Bhatia, S.K.; Jung, H.R.; Choi, T.R.; Yang, S.Y.; Park, H.Y.; Choi, Y.K.; et al. Polyhydroxybutyrate production in halophilic marine bacteria Vibrio proteolyticus isolated from the Korean peninsula. Bioprocess Biosyst. Eng. 2019, 42, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Sathiyanarayanan, G.; Bhatia, S.K.; Song, H.S.; Jeon, J.M.; Kim, J.; Lee, Y.K.; Kim, Y.G.; Yang, Y.H. Production and characterization of medium-chain-length polyhydroxyalkanoate copolymer from Arctic psychrotrophic bacterium Pseudomonas sp. PAMC28620. Int. J. Biol. Macromol. 2017, 97, 710–720. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Moon, Y.-M.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Song, H.-S.; Jeon, J.-M.; Yoon, J.-J.; Kim, Y.-G. One-pot exploitation of chitin biomass for simultaneous production of electricity, n-acetylglucosamine and polyhydroxyalkanoate sin microbial fuel cell using novel marine bacterium Arenibacter palladensis YHY2. J. Clean Prod. 2019, 209, 324–332. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Jeon, J.M.; Yoon, J.J.; Bhatia, S.K.; Gurav, R.; et al. Screening of the strictly xylose-utilizing Bacillus sp. SM01 for polyhydroxybutyrate and its co-culture with Cupriavidus necator NCIMB11599 for enhanced production of PHB. Int. J. Biol. Macromol. 2021, 181, 410–417. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Song, H.S.; Bhatia, S.K.; Gurav, R.; Jeon, J.M.; et al. Engineering of Shewanella marisflavi BBL25 for biomass-based polyhydroxybutyrate production and evaluation of its performance in electricity production. Int. J. Biol. Macromol. 2021, 183, 1669–1675. [Google Scholar] [CrossRef]
- Carlozzi, P.; Di Lorenzo, T.; Ghanotakis, D.F.; Touloupakis, E. Effects of pH, temperature and salinity on P3HB synthesis culturing the marine Rhodovulum sulfidophilum DSM-1374. Appl. Microbiol. Biotechnol. 2020, 104, 2007–2015. [Google Scholar] [CrossRef]
- Amadu, A.A.; Qiu, S.; Ge, S.; Addico, G.N.D.; Ameka, G.K.; Yu, Z.; Xia, W.; Abbew, A.W.; Shao, D.; Champagne, P.; et al. A review of biopolymer (Poly-β-hydroxybutyrate) synthesis in microbes cultivated on wastewater. Sci. Total Env. 2021, 756, 143729. [Google Scholar] [CrossRef]
- Fernández-Dacosta, C.; Posada, J.A.; Kleerebezem, R.; Cuellar, M.C.; Ramirez, A. Microbial community-based polyhydroxyalkanoates (PHAs) production from wastewater: Techno-economic analysis and ex-ante environmental assessment. Bioresour. Technol. 2015, 185, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Higuchi-Takeuchi, M.; Numata, K. Marine purple photosynthetic bacteria as sustainable microbial production hosts. Front. Bioeng. Biotechnol. 2019, 7, 258. [Google Scholar] [CrossRef]
- Hrabak, O. Industrial production of poly-/3-hydroxybutyrate. FEMS Microbiol. Rev. 1992, 103, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Page, W.J.; Knosp, O. Hyperproduction of poly-/3-hydroxybutyrate during exponential growth of Azotobacter vinelandii. Appl. Env. Microbiol. 1989, 55, 1334–1339. [Google Scholar] [CrossRef] [Green Version]
- Shabina, M.; Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoatese co-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77. [Google Scholar] [CrossRef] [Green Version]
- Panuschka, S.; Drosg, B.; Ellersdorfer, M.; Meixner, K.; Fritz, I. Photoautotrophic production of poly-hydroxybutyrate—First detailed cost estimations. Algal Res. 2019, 41, 101558. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [Green Version]
- Carlozzi, P.; Touloupakis, E.; Di Lorenzo, T.; Giovannelli, A.; Seggiani, M.; Cinelli, P.; Lazzeri, A. Whey and molasses as inexpensive raw materials for parallel production of biohydrogen and polyesters via a two-stage bioprocess: New routes towards a circular bioeconomy. J. Biotechnol. 2019, 303, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.-H.; Kwon, E.E.; Jeon, Y.J. Production of bioplastic through food waste valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mawatari, Y.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y.-C. Bacterial conversion of waste into polyhydroxybutyrate (PHB): A new approach of bio-circular economy for treating waste and energy generation. Bioresour. Technol. Rep. 2019, 7, 100246. [Google Scholar] [CrossRef]
- Pratt, S.; Vandi, L.J.; Gapes, D.; Werker, A.; Oehmen, A.; Laycock, B. Polyhydroxyalkanoate (PHA) bioplastics from organic waste. In Biorefinery; Bastidas-Oyanedel, J.R., Schmidt, J., Eds.; Springer: Cham, Switzerland, 2019; pp. 615–638. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, Y.L.; Li, Z.; Loh, X.J. Recent progress in polyhydroxyalkanoates-based copolymers for biomedical applications. Biotechnol. J. 2019, 14, e1900283. [Google Scholar] [CrossRef]
- Moorkoth, D.; Nampoothiri, K.M. Production and characterization of poly(3-hydroxybutyrate-co-3hydroxyvalerate) (PHBV) by a novel halotolerant mangrove isolate. Bioresour. Technol. 2016, 201, 253–260. [Google Scholar] [CrossRef]
- Chanprateep, S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef]
- Ong, S.Y.; Chee, J.Y.; Sudesh, K. Degradation of polyhydroxyalkanoate (PHA): A Review. J. Sib. Fed. Univ. 2017, 10, 211–225. [Google Scholar] [CrossRef]
- Jendrossek, D.; Schirmer, A.; Schlegel, H.G. Biodegradation of polyhydroxyalkanoic acids. Appl. Microbiol. Biotechnol. 1996, 46, 451–463. [Google Scholar] [CrossRef]
- Fernandes, M.; Salvador, A.; Alves, M.M.; Vicente, A.A. Factors affecting polyhydroxyalkanoates biodegradation in soil. Polym. Degrad. Stab. 2020, 182, 109408. [Google Scholar] [CrossRef]
- Suzuki, M.; Tachibana, Y.; Kasuya, K. Biodegradability of poly(3-hydroxyalkanoate) and poly(ε-caprolactone) via biological carbon cycles in marine environments. Polym. J. 2021, 53, 47–66. [Google Scholar] [CrossRef]
- Suyama, T.; Tokiwa, Y.; Ouichanpagdee, P.; Kanagawa, T.; Kamagata, Y. Phylogenetic affiliation of soil bacteria that degrade aliphatic polyesters available commercially as biodegradable plastics. Appl. Env. Microbiol. 1998, 64, 5008–5011. [Google Scholar] [CrossRef] [Green Version]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Cho, J.Y.; Lee Park, S.; Lee, H.J.; Kim, S.H.; Suh, M.J.; Ham, S.; Bhatia, S.K.; Gurav, R.; Park, S.H.; Park, K.; et al. Polyhydroxyalkanoates (PHAs) degradation by the newly isolated marine Bacillus sp. JY14. Chemosphere 2021, 283, 131172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, G.-Q. Polyhydroxyalkanoates: Sustainability, production, and industrialization. In Sustainable Polymers from Biomass; Tang, C., Ryu, C.Y., Eds.; Wiley-VCH Verlag: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.-M.; Zaharia, C.; Andrei, E.R. Methods of synthesis, properties and biomedical applications of polyhydroxyalkanoates: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 695–712. [Google Scholar] [CrossRef]
- Ang, S.L.; Sivashankari, R.; Shaharuddin, B.; Chuah, J.-A.; Tsuge, T.; Abe, H.; Sudesh, K. Potential applications of polyhydroxyalkanoates as a biomaterial for the aging population. Polym. Degrad. Stab. 2020, 181, 109371. [Google Scholar] [CrossRef]
- Puyol, D.; Barry, E.M.; Hülsen, T.; Batstone, D.J. A mechanistic model for anaerobic phototrophs in domestic wastewater applications: Photo-anaerobic model (PAnM). Water Res. 2017, 116, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Fradinho, J.; Allegue, L.D.; Ventura, M.; Melero, J.A.; Reis, M.A.M.; Puyol, D. Up-scale challenges on biopolymer production from waste streams by purple phototrophic bacteria mixed cultures: A critical review. Bioresour. Technol. 2021, 327, 124820. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, J.; Ma, C.; Yang, Z.; Li, Z.; Yang, X.; Wang, M.; Zhang, H.; Hu, J.; Zhang, C. Photosynthetic bacteria-based technology is a potential alternative to meet sustainable wastewater treatment requirement? Env. Int. 2020, 137, 105417. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zhi, R.; Zhang, G. Photosynthetic bacteria wastewater treatment with the production of value-added products: A review. Bioresour. Technol. 2020, 299, 122648. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Khatipov, E.; Miyake, M.; Miyake, J.; Asada, Y. Accumulation of poly-hydroxybutyrate by Rhodobacter sphaeroides on various carbon and nitrogen substrates. FEMS Microbiol. Lett. 1998, 162, 39–45. [Google Scholar] [CrossRef]
- Carlozzi, P.; Giovannelli, A.; Traversi, M.L.; Touloupakis, E.; DiLorenzo, T. Poly-3-hydroxybutyrate and H2 production by Rhodopseudomonas sp. S16-VOGS3 grown in a new generation photobioreactor under single or combined nutrient deficiency. Int. J. Biol. Macromol. 2019, 135, 821–828. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Sustainable PHA production in integrated lignocellulose biorefineries. New Biotechnol. 2019, 49, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Petushkova, E.; Iuzhakov, S.; Tsygankov, A. Differences in possible TCA cycle replenishing pathways in purplenon-sulfur bacteria possessing glyoxylate pathway. Photosyn. Res. 2019, 139, 523–537. [Google Scholar] [CrossRef]
- Carlozzi, P.; Sacchi, A. Biomass production and studies on Rhodopseudomonas palustris grown in an outdoor, temperature controlled, underwater tubular photobioreactor. J. Biotechnol. 2001, 88, 239–249. [Google Scholar] [CrossRef]
- Padovani, G.; Emiliani, G.; Giovanelli, A.; Traversi, M.L.; Carlozzi, P. Assessment of glycerol usage by five different purple non-sulfur bacterial strains for bioplastic production. J. Environ. Chem. Eng. 2018, 6, 616–622. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, L.; Mallick, N.; Mala, J. Progress and challenges in producing polyhydroxyalkanoate biopolymers from cyanobacteria. J. Appl. Phycol. 2017, 29, 1213–1232. [Google Scholar] [CrossRef]
- Lee, Y.R.; Fitriana, H.N.; Lee, S.Y.; Kim, M.-S.; Moon, M.; Lee, W.-H.; Lee, J.-S.; Lee, S. Molecular profiling and optimization studies for growth and PHB production conditions in Rhodobacter sphaeroides. Energies 2020, 13, 6471. [Google Scholar] [CrossRef]
- Ienczak, J.L.; Schmidell, W.; de Aragão, G.M.F. High-cell-density culture strategies for polyhydroxyalkanoate production: A review. J. Ind. Microbiol. Biotechnol. 2013, 40, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Adessi, A.; De Philippis, R. Photobioreactor design and illumination systems for H2 production with anoxygenic photosynthetic bacteria: A review. Int. J. Hydrogen Energy 2014, 39, 3127–3141. [Google Scholar] [CrossRef]
- Elkahlout, K.; Sagir, E.; Alipour, S.; Koku, H.; Gunduz, U.; Eroglu, I.; Yucel, M. Long-term stable hydrogen production from acetate using immobilized Rhodobacter capsulatus in a panel photobioreactor. Int. J. Hydrogen Energy 2019, 44, 18801–18810. [Google Scholar] [CrossRef]
- Kayahan, E.; Eroglu, I.; Koku, H. A compact tubular photobioreactor for outdoor hydrogen production from molasses. Int. J. Hydrogen Energy 2017, 42, 2575–2582. [Google Scholar] [CrossRef]
- Wu, S.C.; Liou, S.Z.; Lee, C.M. Correlation between bio-hydrogen production and polyhydroxybutyrate (PHB) synthesis by Rhodopseudomonas palustris WP3-5. Bioresour. Technol. 2012, 113, 44–50. [Google Scholar] [CrossRef]
- Gosse, J.L.; Engel, B.J.; Hui, J.C.; Harwood, C.S.; Flickinger, M.C. Progress toward a biomimetic leaf: 4,000h of hydrogen production by coating-stabilized non growing photosynthetic Rhodopseudomonas palustris. Biotechnol. Prog. 2010, 26, 907–918. [Google Scholar] [CrossRef]
- Melnicki, M.R.; Eroglu, E.; Melis, A. Changes in hydrogen production and polymer accumulation upon sulfur-deprivation in purple photosynthetic bacteria. Int. J. Hydrogen Energy 2009, 34, 6157–6170. [Google Scholar] [CrossRef]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Lloyd, R.; Fuller, R.C. The accumulation of poly(3-hydroxyalkanoates) in Rhodobacter sphaeroides. Arch. Microbiol. 1991, 155, 337–340. [Google Scholar] [CrossRef]
- Carlozzi, P.; Seggiani, M.; Cinelli, P.; Mallegni, N.; Lazzeri, A. Photofermentative poly-3-hydroxybutyrate production by Rhodopseudomonas sp.S16-VOGS3 in a novel outdoor 70-L photobioreactor. Sustainability 2018, 10, 3133. [Google Scholar] [CrossRef] [Green Version]
- Ranaivoarisoa, T.O.; Singh, R.; Rengasamy, K.; Guzman, M.S.; Bose, A. Towards sustainable bioplastic production using the photoautotrophic bacterium Rhodopseudomonas palustris TIE-1. J. Ind. Microbiol. Biotechnol. 2019, 46, 1401–1417. [Google Scholar] [CrossRef] [Green Version]
- Foong, C.P.; Higuchi-Takeuchi, M.; Numata, K. Optimal iron concentrations for growth associated polyhydroxyalkanoate biosynthesis in the marine photosynthetic purple bacterium Rhodovulum sulfidophilum under photoheterotrophic condition. PLoS ONE 2019, 14, e0212654. [Google Scholar] [CrossRef] [Green Version]
- Lenz, R.W.; Marchessault, R.H. Bacterial polyesters: Biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 2005, 6, 1–8. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touloupakis, E.; Poloniataki, E.G.; Casciana, M.; Ghanotakis, D.F.; Carlozzi, P. Poly-β-Hydroxybutyrate Production by Rhodopseudomonas sp. Grown in Semi-Continuous Mode in a 4 L Photobioreactor. Symmetry 2021, 13, 1609. https://doi.org/10.3390/sym13091609
Touloupakis E, Poloniataki EG, Casciana M, Ghanotakis DF, Carlozzi P. Poly-β-Hydroxybutyrate Production by Rhodopseudomonas sp. Grown in Semi-Continuous Mode in a 4 L Photobioreactor. Symmetry. 2021; 13(9):1609. https://doi.org/10.3390/sym13091609
Chicago/Turabian StyleTouloupakis, Eleftherios, Eleni G. Poloniataki, Martina Casciana, Demetrios F. Ghanotakis, and Pietro Carlozzi. 2021. "Poly-β-Hydroxybutyrate Production by Rhodopseudomonas sp. Grown in Semi-Continuous Mode in a 4 L Photobioreactor" Symmetry 13, no. 9: 1609. https://doi.org/10.3390/sym13091609