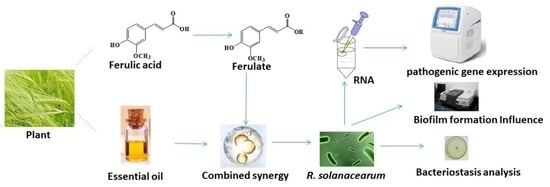
Antibacterial Activity of Ferulic Acid Ester against Ralstonia solanacearum and Its Synergy with Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Bacterial
2.2. Gas Chromatography Analysis
2.3. Synthesis of Ferulic Acid Ester
2.4. Determination of the Inhibition Rate (IR)
2.5. Determination of the MIC Values
2.6. Determination of Combined Antimicrobial Efficiency
2.7. Determination of the Minimum Bactericidal Concentration (MBC)
2.8. Growth Curve Assay
2.9. Biofilm Assay
2.10. Influence of Pathogenic Gene Expression
2.11. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Ferulic Acid Ester
3.2. Antimicrobial Activity of Ferulic Acid Ester against R. solanacearum
3.3. Combined Antimicrobial Efficiency of 2e and EO
3.4. The Growth Curve of R. solanacearum
3.5. Biofilm Assay of R. solanacearum
3.6. Influence of Pathogenic Gene Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyska, M.; Cunniffe, N.; Gilligan, C. Trade-off between disease resistance and crop yield: A landscape-scale mathematical modelling perspective. J. R. Soc. Interface 2016, 13, 20160451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurabachew, H.; Ayana, G. Bacterial Wilt caused by Ralstonia solanacearum in Ethiopia: StatusaAnd Management Approaches: A Review. Int. J. Phytopathol. 2017, 5, 107–119. [Google Scholar] [CrossRef]
- Mansfeld, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicker, E.; Grassart, L.; Coransonbeaudu, R.; Mian, D.; Guilbaud, C.; Fegan, M.; Prior, P. Ralstonia solanacearum strains from Martinique (French West Indies) exhibiting a new pathogenic potential. Appl. Environ. Microb. 2007, 73, 6790–6801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuliar; Nion, Y.A.; Toyota, K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Eeviron. 2015, 30, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Genin, S.; Denny, T.P. Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 2012, 50, 67–89. [Google Scholar] [CrossRef]
- Schreinemachers, P.; Tipraqsa, P. Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 2012, 37, 616–626. [Google Scholar] [CrossRef]
- Fu, L.; Penton, C.R.; Ruan, Y.Z.; Shen, Z.Z.; Xue, C. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil. Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Chellemi, D.O.; Rosskopf, E.N.; Kokalis-Burelle, N. The Effect of Transitional Organic Production Practices on Soilborne Pests of Tomato in a Simulated Microplot Study. Phytopathology 2013, 103, 792–801. [Google Scholar] [CrossRef]
- Teixeira, F.R.; Lima, M.C.O.P.; Almeida, H.O.; Romeiro, R.S.; Silva, D.J.H.; Pereira, P.R.G.; Fontes, E.P.B.; Baracat-Pereira, M.C. Bioprospection of cationic and anionic antimicrobial peptides from bell pepper leaves for inhibition of Ralstonia solanacearum and Clavibacter michiganensis ssp. michiganensis growth. J. Phytopathol. 2006, 154, 418–421. [Google Scholar] [CrossRef]
- Yuan, G.Q.; Li, Q.Q.; Qin, J.; Ye, Y.F. Isolation of methyl gallate from Toxicodendron sylvestre and its effect on tomato bacterial wilt. Plant Dis. 2012, 96, 1143–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorim, E.P.D.R.; De-Andrade, F.W.R.; Moraes, E.M.D.S.; Silva, J.C.D.S.; Lima, R.D.S.; Lemos, E.E.P.D. Antibacterial activity of essential oils and extracts on the development of Ralstonia solanacearum in banana seedlings/Atividade antibacteriana de oleos essenciais e extratos vegetais sobre o desenvolvimento de Ralstonia solanacearum em mudas de bananeira. Rev. Bras. Frutic. 2011, 33, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, S.C.; Soares, A.C.F.; Brito, A.S.; Laranjeira, F.F.; Ledo, C.A.S.; Santos, A.P. Control of tomato bacterial wilt through the incorporation of aerial part of pigeon pea and crotalaria to soil. Summa Phytopathol. 2006, 32, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.; Dutta, V.; Arora, N.K. Biopesticides in India: Technology and sustainability linkages. 3 Biotech 2020, 10, 210. [Google Scholar] [CrossRef]
- Ashmawy, N.A.; Behiry, S.I.; Al-Huqail, A.A.; Ali, H.M.; Salem, M.Z.M. Bioactivity of selected phenolic acids and hexane extracts from Bougainvilla spectabilis and Citharexylum spinosum on the growth of Pectobacterium carotovorum and Dickeya solani Bacteria: An opportunity to save the environment. Processes 2020, 8, 482. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Hamayun, M.; Iqbal, A. Novel antimicrobial and antioxidative activity by endophytic Penicillium roqueforti and Trichoderma reesei isolated from Solanum surattense. Acta Physiol. Plant. 2019, 41, 164. [Google Scholar] [CrossRef]
- Li, L.; Feng, X.; Tang, M.; Hao, W.; Han, Y.; Zhang, G.; Wan, S. Antibacterial activity of Lansiumamide B to tobacco bacterial wilt (Ralstonia solanacearum). Microbiol. Res. 2014, 169, 522–526. [Google Scholar] [CrossRef]
- Vu, T.T.; Choi, G.J.; Kim, J.C. Plant-derived Antibacterial Metabolites Suppressing Tomato Bacterial Wilt Caused by Ralstonia solanacearum. Res. Plant Dis. 2017, 23, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Abo-Elyousr, K.A.M.; Seleim, M.A.A.; Abd-El-Moneem, K.M.H.; Saead, F.A. Integrated effect of glomus mosseae and selected plant oils on the control of bacterial wilt disease of tomato. Crop Prot. 2014, 66, 67–71. [Google Scholar] [CrossRef]
- Alves, A.O.; Santos, M.M.B.; Santos, T.C.G.; Souza, E.B.; Mariano, R.L.R. Biofumigation with essential oils for managing bacterial wilt of sweet peppers. J. Plant Pathol. 2014, 96, 363–367. [Google Scholar]
- Ferraz, C.A.; Pastorinho, M.R.; Palmeira-de-Oliveira, A.; Sousa, A.C. Ecotoxicity of plant extracts and essential oils: A review. Environ. Pollut. 2022, 292, 118319. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.T.; Mollaei, S.; Khurizadeh, S. Evaluation of volatile and phenolic compounds, and antioxidant activity of different parts of Ferulago angulata (schlecht.) Boiss. Ind. Crop. Prod. 2019, 140, 111589. [Google Scholar]
- Wallis, C.M.; Chen, J. Grapevine phenolic compounds in xylem sap and tissues are significantly altered during infection by Xylella fastidiosa. Phytopathology. 2012, 102, 816–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.M.; Yu, J.P. Chemical Composition, Antimicrobial Activity and Mechanism of Action of Essential Oil from the Leaves of Macleaya Cordata (Willd.) R. B r. J. Food Safety 2015, 35, 227–236. [Google Scholar] [CrossRef]
- Miyague, L.; Macedo, R.E.F.; Meca, G.; Holley, R.A.; Luciano, F.B. Combination of phenolic acids and essential oils against Listeria monocytogenes. LWT Food Sci. Technol. 2015, 64, 333–336. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Tasir, S.; Seven, A.; Akgun, N.; Karbancioglu-Guler, F. Evaluation of the single and combined antibacterial efficiency of essential oils for controlling Campylobacter coli, Campylobacter jejuni, Escherichia coli, Staphylococcus aureus, and mixed cultures. Flavour Fragr. J. 2019, 34, 280–287. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, C.S.; Shin, H.K. Simultaneous Determination of (-)-Menthone and (-)-Menthol in Menthae Herba by Gas Chromatography and Principal Component Analysis. Nat. Prod. Sci. 2010, 16, 180–184. [Google Scholar]
- Nadal, J.; Pedroso, F.B.; Minozzo, B.R.; Brito, P.S.D.; Farago, P.V.; Vellosa, J.C.R.; Miyoshi, E. A simple and high-yield synthesis of hexadecyl ferulate and its in vitro antioxidant potential. Braz. Arch. Biol. Technol. 2018, 61, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, S.; Liu, X.; Wang, C.; Xiao, W.; Wang, J.; Wu, F.A. Cooperative reinforcement of ionic liquid and reactive solvent on enzymatic synthesis of cafeic acid phenethyl ester as an in vitro inhibitor of plant pathogenic bacteria. Molecules 2017, 22, 72. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Ding, W.; Xu, Y.Q.; Wu, D.S.; Li, S.L.; Chen, J.N.; Guo, B. New insights into the antibacterial activity of hydroxycoumarins against Ralstonia solanacearum. Molecules 2016, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Ayari, S.; Shankar, S.; Follett, P.; Hossain, F.; Lacroix, M. Potential synergistic antimicrobial efficiency of binary combinations of essential oils against Bacillus cereus and Paenibacillus amylolyticus—Part A. Microb. Pathog. 2020, 141, 104008. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Meth. 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Sowndarya, J.; Rubini, D.; Sinsinwar, S.; Senthilkumar, M.; Nithyanand, P.; Vadivel, V. Gallic Acid an Agricultural Byproduct Modulates the Biofilm Matrix Exopolysaccharides of the Phytopathogen Ralstonia solanacearum. Curr. Microbiol. 2020, 77, 3339–3354. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Pratt, L.A.; Watnick, P.I.; Newman, D.K.; Weaver, V.B.; Koberto, R. Genetic approaches to study of biofilms. Methods Enzymol. 1999, 310, 91–109. [Google Scholar] [PubMed]
- Wang, J.Z.; Yan, C.H.; Zhang, X.R.; Tu, Q.B.; Xu, Y.; Sheng, S.; Wu, F.A.; Wang, J. A novel nanoparticle loaded with methyl caffeate and caffeic acid phenethyl ester against Ralstonia solanacearum—A plant pathogenic bacteria. RSC Adv. 2020, 10, 3978–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatkar, A.; Nanda, A.; Kumar, P.; Narasimhan, B. Synthesis and antimicrobial evaluation of ferulic acid derivatives. Res. Chem. Intermed. 2015, 41, 299–309. [Google Scholar] [CrossRef]
- Waters, B.W.; Hung, Y. The Effect of pH and Chloride Concentration on the Stability and Antimicrobial Activity of Chlorine-Based Sanitizers. J. Food Sci. 2014, 79, 1750. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem. Interactions 2019, 308, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Yap, P.S.; Krishnan, T.; Yusoff, K.; Chan, K.; Yap, W.; Lai, K.; Lim, S.E. Mode of Action: Synergistic Interaction of Peppermint (Mentha × piperita L. Carl) Essential Oil and Meropenem Against Plasmid-Mediated Resistant E. coli. Rec. Nat. Prod. 2018, 12, 582–594. [Google Scholar] [CrossRef]
- Sajna, K.V.; Sukumaran, R.K.; Gottumukkala, L.D.; Jayamurthy, H.; Dhar, K.S.; Pandey, A. Studies on structural and physical characteristics of a novel exopolysaccharide from Pseudozyme sp. NII 08165. Int. J. Biol. Macromol. 2013, 59, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Shoda, M. Bacterial control of plant diseases. J. Biosci. Bioeng. 2000, 89, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Pan, Z.C.; Prior, P.; Xu, J.S.; Zhang, Z.; Zhang, H.; Zhang, L.Q.; He, L.Y.; Feng, J. Genetic diversity of Ralstonia solanacearum strains from China. Eur. J. Plant Pathol. 2009, 125, 641–653. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Y.F.; Wang, Z.Y.; Fan, Y.; Yang, X.; Wang, Z.G.; Yu, S.; Pang, Q.X.; Cao, A.C. Deoxymikanolide adversely altered physiology and ultrastructure of Ralstonia solanacearum. Pestic. Biochem. Phys. 2010, 174, 104803. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control. 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Cunnac, S.; Boucher, C.; Genin, S. Characterization of the cis-acting regulatory element controlling HrpB-mediated activation of the type III secretion system and effector genes in Ralstonia solanacearum. J. Bacteriol. 2004, 186, 2309–2318. [Google Scholar] [CrossRef]







| Primer Name | Nucleotide Sequence(5′→3′) | Size (bp) |
|---|---|---|
| hrpB | F: TTCTCGATGATGTAGCGATAGG R: GCTGGAATTTTCGACTTCCTCTA | 238 |
| pehC | F: GTTGTTCGGATTGCTGTACG R: AGTCAAACGATTGCCTGAACTA | 227 |
| pilT | F: AAGAACAAAGCGTCTGATCTGC R: CTTCCAGGTTTTCTTCGTAATGCT | 175 |
| polA | F: GGAATGTCGGAAAGTCAAGAAA R: CTTGTAGGCGGGGTACAGTTC | 238 |
| ace | F: GCCTATGTGCGTGAGTTCTTCT R: CTTCGAACTTGACGTACGGAAC | 338 |
| egl | F: CAGCGCGACCTACTACAAGA R: TCATCAGCCCGAAGATGAC | 299 |
| phcA | F: GGACATGATCTTCACGGTCAACT R: GACTCATCCTCCTTTTCTGCATC | 298 |
| 16S rRNA | F: CTAGAGTGTGTCAGAGGGAGGTAGA R: ATGTCAAGGGTAGGTAAGGTTTTTC | 349 |
| mg/mL | 2e + EO1 | 2e + EO2 | 2e + EO3 | 2e + EO4 | 2e + EO5 | 2e + EO6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | 0.64 ± 0.03 | 2.02 ± 0.08 | 0.64 ± 0.03 | 2.42 ± 0.09 | 0.64 ± 0.03 | 1.20 ± 0.08 | 0.64 ± 0.03 | 4.51 ± 0.09 | 0.64 ± 0.03 | 4.44 ± 0.07 | 0.64 ± 0.03 | 4.38 ± 0.05 |
| MICmix | 0.16 ± 0.02 | 0.24 ± 0.03 | 0.32 ± 0.04 | 0.32 ± 0.04 | 0.32 ± 0.06 | 0.13 ± 0.04 | 0.32 ± 0.04 | 1.32 ± 0.04 | 0.32 ± 0.04 | 2.23 ± 0.07 | 0.32 ± 0.04 | 2.25 ± 0.05 |
| FIC | 0.37 ± 0.09 | 0.63 ± 0.06 | 0.61 ± 0.23 | 0.79 ± 0.18 | 1.00 ± 0.07 | 1.01 ± 0.17 | ||||||
| Effect | S | AD | AD | AD | AD | AD | ||||||
| Main Components | Retention Time (min) | Retention Indices | Percentages of the Main Constituents (%) | |
|---|---|---|---|---|
| SI | RSI | |||
| (+)-Dipentene | 15.97 | 874 | 877 | 3.00 |
| L-menthone | 20.13 | 942 | 958 | 8.07 |
| Menthone | 20.49 | 858 | 865 | 5.44 |
| Menthol | 20.76 | 947 | 952 | 65.38 |
| Isomenthol acetate | 24.09 | 946 | 954 | 2.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Q.-B.; Shi, H.-C.; Li, P.; Sheng, S.; Wu, F.-A. Antibacterial Activity of Ferulic Acid Ester against Ralstonia solanacearum and Its Synergy with Essential Oils. Sustainability 2022, 14, 16348. https://doi.org/10.3390/su142416348
Tu Q-B, Shi H-C, Li P, Sheng S, Wu F-A. Antibacterial Activity of Ferulic Acid Ester against Ralstonia solanacearum and Its Synergy with Essential Oils. Sustainability. 2022; 14(24):16348. https://doi.org/10.3390/su142416348
Chicago/Turabian StyleTu, Qing-Bo, Hui-Cong Shi, Ping Li, Sheng Sheng, and Fu-An Wu. 2022. "Antibacterial Activity of Ferulic Acid Ester against Ralstonia solanacearum and Its Synergy with Essential Oils" Sustainability 14, no. 24: 16348. https://doi.org/10.3390/su142416348