Recent Discovery of Amaranthus palmeri S. Watson in Italy: Characterization of ALS-Resistant Populations and Sensitivity to Alternative Herbicides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Herbicide Bioassays
2.3. DNA Extraction and Detection of Mutated ALS Alleles
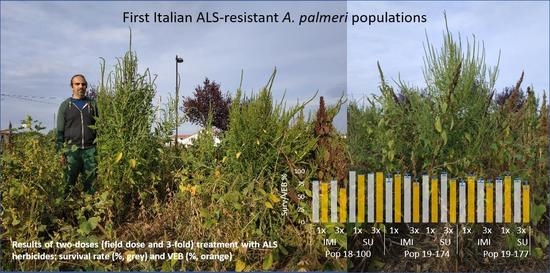
3. Results
3.1. Resistance to ALS Inhibitors
3.2. Response to Non-ALS Herbicides
3.3. ALS Gene Mutation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosyakin, S.L.; Robertson, K.R. Amaranthus. In Flora of North America North of Mexico Vol. 4 Magnoliophyta: Caryophillidae; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA, 2003; pp. 410–435. [Google Scholar]
- Iamonico, D. Amaranthus palmeri S. Watson. In: Euro+Med Plantbase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/PTaxonDetail.asp?NameCache=Amaranthuspalmeri&PTRefFk=7300000 (accessed on 12 November 2020).
- Iamonico, D.; Ardenghi, N.M.G.; Faggi, G. Euro+Med–Cheklist notulae, 4. Willdenowia 2015, 45, 119–120. [Google Scholar] [CrossRef] [Green Version]
- Verloove, F.; Ardenghi, N. New distributional records of non-native vascular plants in northern Italy. Nat. Hist. Sci. 2015, 2, 5. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Adorni, M.; Angiolini, C.; Apruzzese, M.; Ardenghi, N.M.G.; Assini, S.; Aversa, M.; Bacchetta, G.; Banfi, E.; et al. Notulae to the Italian alien vascular flora: 9. Ital. Bot. 2020, 9, 71–86. [Google Scholar] [CrossRef]
- Colombo, S. Noterella 0274: Amaranthus palmeri S.Watson. Acta Plant. Notes 2020, 7, 258. [Google Scholar]
- Heap, I. Herbicide Resistant Weeds by Species and Site of Action. Available online: http://weedscience.org/Summary/SpeciesbySOATable.aspx (accessed on 25 January 2021).
- Shyam, C.; Borgato, E.A.; Peterson, D.E.; Dille, J.A.; Jugulam, M. Predominance of metabolic resistance in a six-way-resistant Palmer amaranth (Amaranthus palmeri) population. Front. Plant Sci. 2021, 11, 614618. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: http://www.weedscience.org/ (accessed on 1 October 2020).
- Carvalho, S.J.P.; Gonçalves Netto, A.; Nicolai, M.; Cavenaghi, A.L.; López-Ovejero, R.F.; Christoffoleti, P.J. Detection of glyphosate-resistant Palmer Amaranth (Amaranthus palmeri) in agricultural areas of Mato Grosso, Brazil. Planta Daninha 2015, 33, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Larran, A.S.; Palmieri, V.E.; Perotti, V.E.; Lieber, L.; Tuesca, D.; Permingeat, H.R. Target-site resistance to acetolactate synthase (ALS)-inhibiting herbicides in Amaranthus palmeri from Argentina. Pest Manag. Sci. 2017, 73, 2578–2584. [Google Scholar] [CrossRef] [Green Version]
- Torra, J.; Royo-Esnal, A.; Romano, Y.; Osuna, M.D.; León, R.G.; Recasens, J. Amaranthus palmeri a new invasive weed in Spain with herbicide resistant biotypes. Agronomy 2020, 10, 993. [Google Scholar] [CrossRef]
- Tranel, P.J.; Wright, T.R.; Heap, I. Mutations in Herbicide-Resistant Weeds to ALS Inhibitors. Available online: http://weedscience.org/mutations/mutationdisplayall.aspx (accessed on 19 January 2021).
- Nakka, S.; Thompson, C.R.; Peterson, D.E.; Jugulam, M. Target site-based and non-target site based resistance to ALS inhibitors in Palmer amaranth (Amaranthus palmeri). Weed Sci. 2017, 65, 681–689. [Google Scholar] [CrossRef]
- Milani, A.; Scarabel, L.; Sattin, M. A family affair: Resistance mechanism and alternative control of three Amaranthus species resistant to acetolactate synthase inhibitors in Italy. Pest Manag. Sci. 2020, 76, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Priess, G.L.; Norsworthy, J.K.; Roberts, T.L.; Gbur, E.E. Impact of postemergence herbicides on soybean injury and canopy formation. Weed Technol. 2020, 34, 727–734. [Google Scholar] [CrossRef]
- González-Torralva, F.; Norsworthy, J.K.; Piveta, L.B.; Varanasi, V.K.; Barber, T.; Brabham, C. Susceptibility of Arkansas Palmer amaranth accessions to common herbicide sites of action. Weed Technol. 2020, 34, 770–775. [Google Scholar] [CrossRef]
- Chaudhari, S.; Varanasi, V.K.; Nakka, S.; Bhowmik, P.C.; Thompson, C.R.; Peterson, D.E.; Currie, R.S.; Jugulam, M. Evolution of target and non-target based multiple herbicide resistance in a single Palmer amaranth (Amaranthus palmeri) population from Kansas. Weed Technol. 2020, 34, 447–453. [Google Scholar] [CrossRef]
- Culpepper, A.S.; Grey, T.L.; Vencill, W.K.; Kichler, J.M.; Webster, T.M.; Brown, S.M.; York, A.C.; Davis, J.W.; Hanna, W.W. Glyphosate-resistant Palmer amaranth (Amaranthus palmeri) confirmed in Georgia. Weed Sci. 2006, 54, 620–626. [Google Scholar] [CrossRef]
- Scarabel, L.; Varotto, S.; Sattin, M. A European biotype of Amaranthus retroflexus cross-resistant to ALS inhibitors and response to alternative herbicides. Weed Res. 2007, 47, 527–533. [Google Scholar] [CrossRef]
- Hess, M.; Barralis, G.; Bleiholder, H.; Buhr, L.; Eggers, T.; Hack, H.; Stauss, R. Use of the extended BBCH scale-general for the descriptions of the growth stages of mono- and dicotyledonous weed species. Weed Res. 1997, 37, 433–441. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus (Madison) 1990, 12, 39–40. [Google Scholar]
- Milani, A.; Panozzo, S.; Farinati, S.; Scarabel, L. Sanger sequences for “Recent discovery of Amaranthus palmeri in Italy: Characterization of ALS-resistant populations and sensitivity to alternative herbicides”. Mendeley Data 2021. [Google Scholar] [CrossRef]
- Singh, S.; Singh, V.; Salas-Perez, R.A.; Bagavathiannan, M.V.; Lawton-Rauh, A.; Roma-Burgos, N. Target-site mutation accumulation among ALS inhibitor-resistant Palmer amaranth. Pest Manag. Sci. 2019, 75, 1131–1139. [Google Scholar] [CrossRef]
- Küpper, A.; Manmathan, H.K.; Giacomini, D.; Patterson, E.L.; McCloskey, W.B.; Gaines, T.A. Population genetic structure in glyphosate-resistant and -susceptible Palmer amaranth (Amaranthus palmeri) populations using genotyping-by-sequencing (GBS). Front. Plant Sci. 2018, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milani, A.; Lutz, U.; Galla, G.; Scarabel, L.; Weigel, D.; Sattin, M. Population structure and evolution of resistance to acetolactate synthase (ALS)-inhibitors in Amaranthus tuberculatus in Italy. Pest Manag. Sci. 2021, 77, 2971–2980. [Google Scholar] [CrossRef]
- Chahal, P.S.; Aulakh, J.S.; Jugulam, M.; Jhala, A.J. Herbicide-Resistant Palmer amaranth (Amaranthus palmeri S. Wats) in the United States—Mechanisms of Resistance, Impact, and Management. In Herbicides, Agronomic Crops and Weed Biology; InTech: London, UK, 2015; pp. 1–30. [Google Scholar]
- Meyers, S.L.; Jennings, K.M.; Monks, D.W. Sweetpotato tolerance and Palmer amaranth control with metribuzin and oryzalin. Weed Technol. 2017, 31, 903–907. [Google Scholar] [CrossRef]
- Meyers, S.L.; Jennings, K.M.; Monks, D.W. Herbicide-based weed management programs for Palmer amaranth (Amaranthus palmeri) in sweetpotato. Weed Technol. 2013, 27, 331–340. [Google Scholar] [CrossRef]
- Gonçalves Netto, A.; Nicolai, M.; Carvalho, S.J.P.; Malardo, M.R.; López-Ovejero, R.F.; Christoffoleti, P.J. Control of ALS- and EPSPS-resistant Amaranthus palmeri by alternative herbicides applied in PRE- and POST-emergence. Planta Daninha 2019, 37, 1–8. [Google Scholar] [CrossRef]
- Iamonico, D. Taxonomic revision of the genus Amaranthus (Amaranthaceae) in Italy. Phytotaxa 2015, 199, 001–084. [Google Scholar] [CrossRef] [Green Version]
- Norsworthy, J.K.; Griffith, G.; Griffin, T.; Bagavathiannan, M.; Gbur, E.E. In-field movement of glyphosate-resistant Palmer amaranth (Amaranthus palmeri) and its impact on cotton lint yield: Evidence supporting a zero-threshold strategy. Weed Sci. 2014, 62, 237–249. [Google Scholar] [CrossRef]
- Davis, A.S.; Schutte, B.J.; Hager, A.G.; Young, B.G. Palmer Amaranth (Amaranthus palmeri) damage niche in Illinois soybean is seed limited. Weed Sci. 2015, 63, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Schwartz-Lazaro, L.M.; Norsworthy, J.K.; Walsh, M.J.; Bagavathiannan, M.V. Efficacy of the integrated harrington seed destructor on weeds of soybean and rice production systems in the southern United States. Crop Sci. 2017, 57, 2812–2818. [Google Scholar] [CrossRef] [Green Version]
- Green, J.K.; Norsworthy, J.K.; Walsh, M.J. Distribution of common cocklebur and palmer amaranth seed exiting the combine for harvest weed seed control in soybean. Crop Forage Turfgrass Manag. 2020, 6, e20064. [Google Scholar] [CrossRef]
- FAOSTAT Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/#home (accessed on 14 January 2021).



| Population | ||||
|---|---|---|---|---|
| 20-179 | 18-100 | 19-174 | 19-177 | |
| Experiment 1 | 69 (±3) | 49 (±5) | 32 (±4) | 35 (±1) |
| Experiment 2 | 98 (±7) | 83 (±4) | 23 (±1) | 59 (±4) |
| Population | ||||
|---|---|---|---|---|
| 20-179 | 18-100 | 19-174 | 19-177 | |
| Experiment 1 | 3 (±1) | 51 (±7) | 17 (±7) | 100 (±7) |
| Experiment 2 | 22 (±14) | 24 (±7) | 65 (±15) | 39 (±13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milani, A.; Panozzo, S.; Farinati, S.; Iamonico, D.; Sattin, M.; Loddo, D.; Scarabel, L. Recent Discovery of Amaranthus palmeri S. Watson in Italy: Characterization of ALS-Resistant Populations and Sensitivity to Alternative Herbicides. Sustainability 2021, 13, 7003. https://doi.org/10.3390/su13137003
Milani A, Panozzo S, Farinati S, Iamonico D, Sattin M, Loddo D, Scarabel L. Recent Discovery of Amaranthus palmeri S. Watson in Italy: Characterization of ALS-Resistant Populations and Sensitivity to Alternative Herbicides. Sustainability. 2021; 13(13):7003. https://doi.org/10.3390/su13137003
Chicago/Turabian StyleMilani, Andrea, Silvia Panozzo, Silvia Farinati, Duilio Iamonico, Maurizio Sattin, Donato Loddo, and Laura Scarabel. 2021. "Recent Discovery of Amaranthus palmeri S. Watson in Italy: Characterization of ALS-Resistant Populations and Sensitivity to Alternative Herbicides" Sustainability 13, no. 13: 7003. https://doi.org/10.3390/su13137003